 Research Article
Research Article
Histological Comparison of Kidneys Between Female and Male Quail Birds at Different Age Stages
Ziad Alabdallah1,2*
1Department of Anatomy-Histology and Embryology, Al Furat University, Deirez-Zor, College of Veterinary, Syria
2Peoples Friendship University of Russia (RUDN University) Moscow, Miklukho-Maklaya Street, Russia
Ziad Alabdallah, Department of Anatomy-Histology and Embryology, Al Furat University, Deirez-Zor, College of Veterinary, Syria.
Received Date:November 21,2022; Published Date:December 09,2022
Abstract
Introduction: Kidneys comprise the paired organ essentially responsible for excreting nitrogenous wastes, excessive water, inorganic salts, and
toxic substances produced during the process of body metabolism as well as maintenance of osmotic regulation and homeostatic fluid balance of
the body.
Aim: Histological study of the capsule between female and male quail birds.
Material & Methods: A total of thirty-six adult male and female Japanese quail were allocated randomly into four different age groups of 30, 90,
180, and 270 days. Data analysis was performed using SPSS-22. The results showed a 24% increase in Bowman’s space in male quail compared with
females after 30 days. Moreover, a 10% increase in Bowman’s space was recorded at 270 days in male quail compared with females.
Results: showed a 12% increase in glomerular diameter in females compared with males. The data also showed a 12% increase in the diameter
of Bowman’s capsule in females compared to males. The outer diameter of the thin tubule in the loop of Henle in females increased by 4% compared
to males. A 12% increase was noted in the outer diameter of the thickened tubule & the collecting tubes in male quail compared to the females at
the age 30 days.
Keywords: Histological; Baumann’s capsule; Loop of Henle; Quail; Kidney
Introduction
A quail is a bird of the order Galliformes, which are considered rather primitive birds, and most of the species in this order are medium-sized birds. Their body form & behavioral characteristics are similar to those of domestic chickens. In birds, the urinary system consists of elongated, paired kidneys and muscular ureters which drain each kidney & open into the urodeum of the cloaca [1]. Birds have no renal pelvis or urinary bladder [2,3]. Undoubtedly, the kidneys play numerous vital roles in birds. One primary role of the kidney is the elimination of metabolic wastes as well as excess water [4,5]. Avian kidneys also aid the liver in detoxification. Differing renal anatomies and physiologies lead to different renal diseases in birds compared to mammals.
The avian kidney has two types of nephrons: the cortical type which is reptilian in form, devoid of a nephron Henle loop (loop of henle), & confined to the cortical region of lobule; and the medullary type which has a nephron loop that penetrates the conical medullary region of the lobule similar to that in mammals [1,6]. One of the most unique characteristics of avian kidneys is the presence of 2 types of nephrons, one with and other without a loop of Henle [5,7]. Most avian nephrons are loopless or cortical [8], and thus the capacity of the avian kidney to concentrate urine is limited and less than that of mammals [9]. In mammals, all nephrons contain a loop of Henle; some of them are longer than others. The ability to conserve ions & water may be correlated with the structure of nephrons. The surface of the kidney is covered by a large number of small rough structures with shallow depressions between them. Each of these structures are a unit of the cortical kidney. The avian glomerulus is similar to its mammalian counterpart, but it is smaller and has a simpler system of capillary loops arranged around the core of mesangial cells. The distal convoluted tubule (DCT) is characteristically different from the proximal convoluted tubule (PCT) in that the cells of the lining epithelium possess no brush border, and the epithelial cells are approximately cuboidal in shape [1,4,10].
Materials and Methods of Research
Japanese quail (Coturnix japonica) were raised in the Bird
Research Unit of the Agrarian and Technological Institute, People’s
Friendship Russian University, Moscow, Russia. The experiments
were approved by the Animal Experiments Committee of the
Agrarian and Technological Institute in December 2019. Thirtysix
healthy 30-, 90-, 180-, & 270-day-old male & female Japanese
quail were used for the experiment & classified into three groups
according to their age. The experiments were carried out in
February 2020. Quail were kept at a temperature of about 20°C and
given a program of about 14 h light/day. The birds were housed with
food and water. On the day of analysis at 9:00 am, bird body masses
and histological kidney analyses were performed. Morphometric
analysis: after the birds reached 30, 90, 180, & 270 days of age,
the body mass of birds from each group was measured & the birds
were dissected. The kidneys were excised and their masses were
estimated. The kidneys were dissected free of synsacral fossae.
Tissue samples from the cranial, middle, and caudal divisions of
each kidney were fixed in 10% neutral buffered formaldehyde for
24H and processed to be embedded in paraffin in routine manner.
The transverse serial sections (5 μm) were stained with H & E for
general histological observation and a variety of techniques for types
of fibers in the connective tissues: 1) Verhoeff’s for elastic fibers, 2)
Masson’s trichrome for collagen fibers, and 3) Gomori’s method for
reticulum. To investigate the pH of the secretion material, Alcian
blue (AB) (pH 2.5) was used to determine acidic mucosubstances,
& periodic acid-Schiff (PAS) reaction was employed to determine
neutral mucosubstances [11,12]. Histological studies on stained
sections were carried out by light microscopy.
Results and Discussion
When examined under a light microscope, it turned out that the kidney of a Japanese quail is surrounded by a moderately thick capsule. Abdul-Gahaffor et al, [13] reported that the kidneys of young pigeons were covered with a thin capsule, in adult pigeons the capsule looked very thin. The kidney capsule in Japanese quails is composed of dense connective tissue composed of irregular elastic, reticular and collagen fibers and blood vessels, Mobini and Abdollahi [14], Al-Ajeely R, Mohammed [5] in Racing Pigeon. Sreeranjini et al, [15] and Al-Azawy [16] also reported reticular fibers and smooth muscle in the kidney capsule of poultry and geese, while Abdul Gahaffor et al, [13] reported collagen and reticular fibers in pigeons. Smooth muscle fibers were absent in the renal capsule of Japanese quail, while these fibers were observed in the renal capsule of poultry and geese [16].
It is represented by renal lobules, easily determined by the location of the renal corpuscles, which are distributed in the form of a regular circle along the periphery of the lobules, convoluted and straight tubules, and interlobular collecting ducts. Between all these structures are thin layers of connective tissue. In the juvenile period of bird development, the smallest values were noted for the thickness of the renal capsule [14,17]. The minimum thickness value of the kidney capsule in males & females was observed in 30-day-old quails, & maximum in quails of 270 days of age. Over the entire period of research, the thickness of the capsule in males asynchronously increased by an average of 1.57 times, in females by 3.45 times.
In diurnal quails, the boundaries of the lobules are poorly visible, the renal lobules are easily distinguishable by the localization of the renal corpuscles and interlobular collecting ducts [17,18]. The number of renal corpuscles in the field of the microscope view in the left kidney of a day old is 5.67 ± 0.67 pcs., The maximum number of renal corpuscles was recorded at 150 days of age - 12.33 ± 1.45 pcs.
From the juvenile period (young animals) to the period of morphological maturity (adult herd) there is an increase in the height of the epithelial cells of the convoluted tubules in the left kidney of males and females by 3.8 μm and 1.8 μm, respectively, compared with individuals of a daily age (Table 1).
Table 1:Histological changes in Bowman’s space diameter, glomerular diameter, Bowman’s capsule diameter and renal capsule thickness.
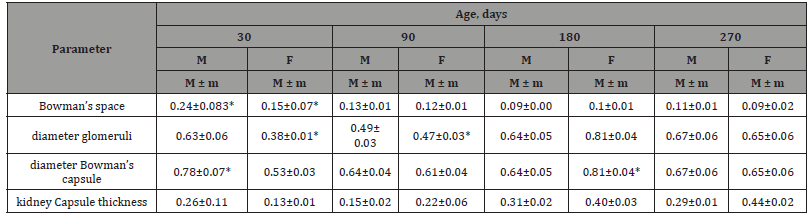
In post-incubation ontogeny, an asynchronous increase in the diameter of the convoluted tubules of the thick (ascending) part of the loop of Henle of the kidneys of males & females occurs similarly. In the postnatal period, it increases on average 1.2 times in males & 1.4 times in females. While some researchers report that the proximal convoluted tubules are lined with a single layer of cuboidal cells [8,13,19,17], others have noted that they appear to be simple columnar [18]. The Japanese quail had one layer of large cuboidal cells. Recorded accumulations of apical microvilli of the proximal convoluted tubules of the kidneys in other bird species are consistent with our results [8,13,17-19]. The PAS-positive responses of all epithelial cells in the Japanese quail proximal convoluted tubule reflect the results of Abdul-Gahaffor et al., [13] in pigeons, but Nabipour et al, [19] reported no PAS reactions in pigeons and owls. The lumenal surface of proximal convoluted cells of Japanese quail responded positively to acid mucopolysaccharides, while Abdul Gahaffor et al, [13] and Nabipour et al., [19] reported no AB reactions in pigeons (pigeons), pigeons (doves) & owls. Casotti and Braun [20] demonstrated that the presence of acid mucopolysaccharides is involved in the prevention of tubal obstruction. The distal convoluted tubules of the kidneys in quail were consistent with previous studies [1,8,13,17-19].
In our study, the collecting ducts were lined with a single layer of cuboidal or low columnar cells. In contrast, Abdul-Gahaffor et al, [13] and Batah [8] reported only simple cuboid lining in pigeons & coots. Thus, it can be concluded that the cellular activity of collecting tubules in quails is higher than in other studied bird species. The positive reactions of Japanese quail renal collecting duct cells reflect the data of Nabipour et al., [19] in pigeons. This researcher concluded that the collecting tubules play a role in the production of concentrated urine by reabsorbing water from the tubule lumen. In addition, the presence of these mucus substances may be important for the excretion of uric acid from the kidneys [1,21].
The minimum value of the diameter of the proximal convoluted tubules of the kidneys of male & females falls on the 270-day age of the third technological period, amounting to 16.4±1.31 μm and 17.2±1.43 μm, respectively. During life, the diameter of the proximal convoluted tubules of the kidney changes asynchronously. In post incubation ontogenesis, an asynchronous decrease in the diameter of the cavity of the proximal convoluted tubules of the kidneys of males & females occurs. At the age of 270 days, they are 7.6 ± 0.28 μm in both males & females.
When studying the change in the height of the lining epithelium of the proximal convoluted tubules, it was noted that throughout almost the entire period of research, this indicator was higher in males, on average, by 5.6%. It can be noted that this indicator reaches its maximum value in females at the age of 30 days (5.6 ± 0.38 μm). A tendency towards a decrease in the diameter of the lumen & the height of the lining epithelium of the proximal convoluted tubules was observed towards the end of the period of morphological maturity.
The diameter of the distal convoluted tubules of the kidney reached its maximum value in males at the age of one day (17.8±1.17 μm), and in females at 180 days of age (19.2±1.89 μm). The values of the lumen diameter varied from 6.8 to 7.6 μm in males & from 6.4 to 8.8 μm in females. A tendency to a decrease in the height of the lining epithelium as the birds mature was noted, in males the decrease in height averaged 57.5%, in females 82.5%.
With regard to the height of the lining epithelium, we found a decrease in the height of the lining epithelium in female quails more than in males at the age of 180 days. The largest value of the diameter of the thick section of the collecting duct was recorded in female quails than in males at all ages, except for 30 days of age, where reverse values were found. As for the diameter of the thin section of the collecting duct, we noticed an increase in its diameter in male quails at the age of 30 days and in females at the age of 180 days.
In our study, we found an excess of the outer diameter of the proximal tubules in female quails compared to males at all studied ages, except for 90 days, where we noted the equality of the outer diameter in females and males.
The collecting ducts were lined with simple columnar epithelium, which was similar to Nabipour et al. [19], but Dhyaa et al. [17] reported that these tubules are lined with simple cuboidal epithelium. Unlike other birds [8,13,17-19, 22], the characteristic border microvilli that projected into the terminal network were observed on the luminal surfaces of epithelial cells of the collecting ducts of the kidneys in Japanese quail. These new data again indicate that cell collection activity is higher in quails than in other birds studied.
In the present study, the epithelial histochemical reactions of the collecting ducts were similar to those in other poultry [13,19]. The collecting ducts reabsorb water & sodium from the renal tubules. In addition, they secrete mucus to prevent the precipitation of uric acid, thus they are responsible for keeping the tubular lumen open. When studying the diameter of the collecting tubules of the kidney, it can be noted that this indicator reached its maximum value at 270 days of age, amounting to 68.8±4.22 μm in males & 86.0.8±7.35 μm in females. The values of the diameter of the collecting ducts changed asynchronously in males, and progressively in females, with an increase in size from a day old to 270 days of age (Table 2).
Table 2:Histological Changes in Collecting Ducts.
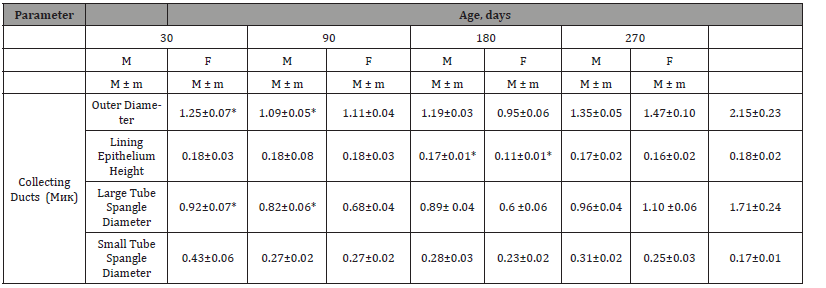
In the course of the study, we found an excess in the outer diameter of the collecting ducts in female quails compared to males at all ages, except for the 30-day age, where we observed the opposite picture. We also found an increase in the height of the lining epithelium in male quails compared to females at all ages except at 90 days of age, where we found a trend. As for the inner diameter of the collecting ducts, we noticed an excess of its values in female quails in comparison with males at all ages, except for 30 days of age, where we observed the opposite phenomenon. In the course of our research, we stated a greater value of the outer diameter of the collecting ducts in female quails compared to males starting from the age of 90 days.
Conclusion
During the growing period, the absolute weight of the kidneys increases by 9.4 times, including 5.9 times for the first 30 days, 1.2 times from 30 to 90 days, and 1.1 times from 90 to 270 days. The highest relative total weight of the kidneys (2.63 ± 0.024%) in relation to body weight was observed at day old, which was 2.9 times higher than the average value for the subsequent period of postnatal ontogenesis (P<0.001). The diameter of the convoluted tubules of the thin (descending) and thick (ascending) branches of the loop of Henle in the kidneys of both males & females asynchronously increases by 1.18 times & 1.37 times, respectively, and 1.2 and 1.4 times, respectively.
Acknowledgement
None.
Disclosures
Lectures and advisory fees from Takeda and BMS. Investigational fee from Celgene.
Conflict of interest
No conflict of interest.
References
- Alabdallah A, Norezzine A, Anatolyevich Vatnikov Y, Alekseevich Nikishov A, Vladimirovich Kulikov E, et al. (2021) Influence of Different Genders of Japanese Quail on the Functional State of Kidneys. Archives of Razi Institute 76(3): 667-680.
- Nikishov AA, Alabdallakh Z, Vetoshkina GA, Kulikov Ye V (2020) Morphometric Characteristic of Kidneys in the Japanese Quail. 157 (2-3): 154-155.
- Nikishov AA, Ziad A, Seleznev SB (2019) The Topographic and Anatomical Characterization of Kidneys in Japanese Quail. 155 (2): 215-215.
- Alabdallah ZA, Nikishov AA, Karamyan AS (2021) Sex-related of some hematological and serum biochemical changes, fed high-protein diet in Japanese quail (Coturnix japonica). Iranian Journal of Ichthyology 8: 150-154.
- Al-Ajeely R, Mohammed FS (2012) Morpho-histological study on the development of kidney and ureter in hatching and adulthood racing pigeon (Columba livia domestica). Int J Sci Nature 3: 665-677.
- Ziad A (2021) Changes in the morphological and anatomical structures of kidney in birds. Innovative approaches in modern science pp: 134-139.
- Ziad A (2020) Histological structure differences of kidney in birds. Innovative approaches in modern science pp: 72-76.
- Batah AL (2012) Morphological and histological study for the kidneys of coot bird (Fulica atra). Basrah J Vet Res 11(1).
- Alabdallah Z, Nikishov A, Vatnikov Y, Al-Ragawi A, Seleznev S (2021) The Effect of High Protein in the Feed Mixture on the Morphological Changes in the Kidneys of Quail Birds. Journal of Chemical Health Risks 11(4): 383-392.
- Ziad A (2021) Biochemical Parameters Associated with Kidney Injury in Birds. Innovative approaches in modern science pp: 130-134.
- Kiernan JA (1999) Histological and histochemical methods: theory and practice. Shock 12(6): 479.
- Alabdallach AZ et al. (2020) Histological and morphometric characteristics of chicken embryos with different genotypes. Eur Asian Journal of Bio Sciences 14(1): 719-725.
- Abdul-Gahaffor R, Al-Ajeely A, Mohammed FS (2012) Morpho- histological study on the development of kidney and ureter in hatching and adult hood racing pige on (Columbaliviadomestica). Int J Sci Nat3(3): 665-677.
- Mobini B, Abdollahi M (2016) Effect of sexon histological and histochemical structures of different parts of the kidney in Japanese quail. Poult Sci 95(9): 2145-2150.
- Sreeranjini AR, lyyangar MP, Prarnodkumar D (2010) Histological study on the fibrous architecture of kidney and ureter of japanese quail (coturnixcotumixs Tamilnadu). J Veterinary and Animal Science 6(2): 107-110.
- Al- Azawy NH (2005) Comparative anatomical and histological study of kidney in domestic fowls and geese (Gallus domesticus and Anser anser). M Sc Thesis College of Veterinary Medicine Baghdad University.
- Dhyaa Ab Abood, Ali F Reshag, Azhar SK, Myson A Ahmed (2014) Comparative anatomical and histological features of the kidney in Harrier (Circusaueroginosus), Chicken (Gallus domesticus) and Mallard duck (Anasplatyrhynchos). The Iraqi Journal of Veterinary Medicine 38(1): 017-003.
- Bacha WJ, Bacha LM (2012) Color Atlas of Veterinary Histology. 3rd (Edn), Philadelphia Wiley Black well pp:184-185.
- Nabipour A, Alishahi E, Asadian M (2009) Some histological and physiological features of avian kidney. J Appl Anim Res 36: 195-198.
- Casotti G, Braun E (2000) Renal of anatomy in sparrows from differents environments. J Morphology 243(3): 283-291.
- Casotti G (2001) Effects of season on kidney morphology in house sparrows. J Exp Biol 204(6): 1201-1206.
- Aughey E, Frye FL (2001) Comparative Veterinary Histology with Clinical Correlates. 1st (Edn), Manson Publishing London pp: 143-148.
-
Ziad Alabdallah*. Histological Comparison of Kidneys Between Female and Male Quail Birds at Different Age Stages. Open J Pathol Toxicol Res. 1(3): 2022. OJPTR.MS.ID.000512.
-
Lining epithelium, Morphological maturity, Post incubation ontogenesis, Proximal convoluted tubules, Pigeons, Apical microvilli, Juvenile period, Lobules
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






