 Research Article
Research Article
External and Internal Conditions Affecting Fertilization and Hatching of Avian Eggs
Ziad Ahmad Alabdallah*
Department of Anatomy -Histology and Embryology, Al Furat University, Syria
ZZiad Ahmad Alabdallah, Department of Anatomy -Histology and Embryology, Al Furat University, Deirez-ZOR, College of Veterinary, Syria.
Received Date:March 26, 2023; Published Date:April 24, 2023
Abstract
Consumer acceptance of table and hatching eggs is largely determined by the qualitative features of eggs, including their exterior and interior properties. It also has a significant impact on the technology used to produce egg products, including powdered, frozen, and liquid eggs. Egg weight, freshness, eggshell integrity, and cleanliness are among the exterior qualities. Whereas an egg’s interior characteristics include its chemical makeup, yolk index, Haugh unit, and other factors. Extrinsic and intrinsic features are related to housing, storage, stocking density, nutrition, housing conditions, the handling of eggs, and the stage of the production cycle. These egg properties can also be strongly impacted by shelf life.
Keywords:Characteristics; External; Internal; Weight; Shape; Shell
Introduction
Hatchability, fertility, number of eggs laid, and quality attributes of the eggs are often factors that affect the productivity and profitability of poultry species [80,8]. However, a number of other variables, including body weight, breed, strain, season, rearing techniques, and relative humidity, can also have an impact on egg quality [45,36,55,48,66,78,47,87,89,39]. Also, scientists have noted that a number of egg characteristics have a genetic basis and depend on the genetic diversity of females, which is primarily accountable for the ability of fertilized eggs to hatch [79,85,3,86,88]. The genetic quality of eggs can be improved by comprehending genetic heterogeneity [69].
Main Text
External egg characteristics:
i. Egg weight:
The egg of a bird serves as both a means of reproduction and an
important source of food for humans. Many bird species have differ
ent egg sizes and shapes. Both table eggs and hatching eggs need to
be of a certain size and exterior quality. Egg weight affects both the
nutritional content of eggs and the weight of day-old chicks [58].
A laying hen’s egg weight is influenced by a variety of variables,
including inheritance [19], breed, age, body size, feed and water
consumption, environmental temperature, and disease [24,4,5,6].
Egg weight has a significant impact on egg quality and classification
[23]. Without cracking any eggs, it is possible to figure out this parameter
[3]. Protein, yolk, and shell make up a direct proportion of
an egg’s mass. Significant variability in egg size between white leghorn
lines was documented by Marion et al. larger eggs often have
a higher yolk-to-protein ratio, but light-colored eggs have a lower
protein-to-yolk ratio [34]. Moreover, the egg’s weight has an impact
on the shell’s quality. Compared to little eggs, large eggs have more
cracks [3]. It has been found that there is a correlation between
egg weight and the percentage of cracked eggs [1]. The importance
of the relative proportion of egg components has significantly increased
with the development of the egg-breaking industry [2]. The
albumen and yolk, which are used for distinct markets and have
different commercial values, are separated using thinners. The attribute
of the egg that is most frequently connected to the durability
of the shell is its weight. Shell thickness and egg size are tightly connected
[27]. The size of the egg grows larger as the hen ages, while
the hardness of the shell weakens [57,12]. There is a substantial
correlation between egg mass and protein height (-0.021) and between
egg mass and Haugh units, according to studies by Iposu et
al. and Silversides (-0.198).
Ekeroglu et al. found similar findings, citing substantial correlation coefficients between weight and shape index (0.227), shell strength (-0.207), yolk width (0.759), yolk height (0.589), yolk color (-0.461), yolk index (-0.177), and protein index (0.345). Japanese quail eggs ranged in mass from 8.31 to 13.00 g. The weight categories of quail eggs affected the fertility percentage considerably (P < 0.001); the greatest value was 91.06% with the heaviest group > 13.00 g. The hatchery sector also greatly benefits from the optimization of egg storage times and conditions [11,82,83,84]. Around the midpoint of the production phase, Iqbal et al. discovered a significant difference (P ≤ 0.05) in the weight loss of the eggs in groups of various sizes. They continued by saying that there was no discernible difference between the tiny and medium-sized egg groups and that the big egg size group experienced the least amount of weight loss over the course of various incubation intervals, with losses ranging from 3.27% to 11.32%. The ideal holding time for quail eggs prior to incubation is neither documented nor advised [74]. According to Egbeyale et al. varied storage periods had an impact on the weight loss of laying hen eggs during incubation, which decreased as the storage period before incubation rose.
While a sizable body of literature has been devoted to studying the intrinsic and extrinsic characteristics of eggs, less study has concentrated on the common elements that can affect both aspects. The weight or size of the egg is probably one of the primary components, along with other aspects, that can alter or shape both features in poultry [50]. It is widely acknowledged that egg size impacts incubation success, embryonic mortality, and hatchability. Egg size also affects eggshell qualities and the exterior quality of the egg [62]. At the very beginning of life, thyroid hormones are crucial for the healthy development of nearly all bodily structures in the embryo. The most significant endocrine regulators of the development of embryonic muscle, the process of hatching, and post-hatch metabolism, for instance, are thyroid hormones [72]. As pulmonary breathing starts in an embryo at a late stage, plasma T3 levels rise noticeably [15], and fetal thyroid hormone levels rise along with an improvement in embryonic survival rates. To our knowledge, no research has been done on the connection between egg weight and fetal thyroid hormone levels. On day 6, thyroid hormone levels did not significantly differ across egg groups; however, on day 14 (p ≤ 0.05), there was a significant difference [29] (Figure 1).
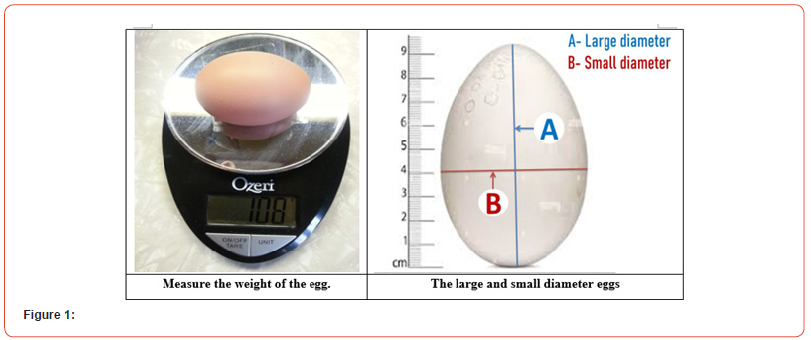
ii. Egg shape:
In addition to the specific gravity, mass, volume, surface area,
and percentage of the eggshell, its strength is influenced by its
microstructure, thickness, and loading rate. In recent years, some
academics have become interested in one of these elements: the
morphology of laying eggs [46]. An essential factor in identifying
the quality of the egg is the egg shape index, which is calculated as
the width-to-length ratio of the egg. Domestic chickens typically lay
oval-shaped eggs; therefore, eggs that are strangely shaped, such as
long and narrow, spherical, or flat-sided, cannot be categorized as
AA (nearly ideal) or A (slightly lower than AA) [72-76]. Eggs that
are round or exceptionally long do not look good and do not fit well
in egg cartons; as a result, they are significantly more likely to break
during transit than eggs that are of a typical shape [59]. A positive
link exists between the egg shape index and protein quality [16].
The study found definite relationships between the form index
and a variety of variables, including egg white length, yolk breadth,
height, and color [61]. Also, a lot of researchers have found a link
between egg length or width and form index [16]. Nevertheless, a
substantial inverse relationship between egg shape index and shell
thickness was discovered by Alkan et al. in.
iii. Egg shell:
The eggshell shields the embryo from physical harm and microbial
contamination, controls the exchange of water and gases
between the embryo and its surroundings, and serves as a calcium
source for the growing embryo [68]. Eggshell quality can be influenced
by a variety of parameters, but the most crucial ones are bird
genotype, usage type, rearing technique, environmental circumstances,
and mineral feed additions [73]. The main purposes of the
eggshell are to protect and provide calcium and other minerals to
the embryos as well as to ensure gas and water exchange between
the embryos and the outside environment, which is essential for
the success of the entire incubation and hatching process [51]. Particularly,
gas exchange takes place by diffusion through small holes
on the eggshell’s surface [54], and as a result, it is dependent on
the quantity of pores and the thickness of the eggshell [52]. Both
features alter the proportion of egg weight loss (EWL%) during incubation,
which has an impact on the eggshell’s ability to exchange
water. Gas exchange is hampered by an increase in eggshell thickness
and a decrease in the number of pores, which ultimately results
in embryonic death [67].
Eggs gain weight while generally losing thickness and strength during the manufacturing phase. The size and weight of the egg affect the eggshell’s quality. Strong correlations were found between the shell’s strength and thickness. The percentage of change in crushing strength is significantly influenced by the form index [10]. The handling of the egg after laying is particularly crucial since egg parameters like form index and shell thickness influence the likelihood of fractured eggs. Eggshell strength and integrity are crucial during storage and incubation to prevent water loss, microbial entry, and premature gas diffusion before incubation [18]. The viability of the embryo during egg storage is significantly impacted by the integrity of the eggshell, in addition to the durability of the interior components. Modifications to the egg’s physical properties, such as its protein pH, can induce nutrients to diffuse from the protein to the blastoderm and decrease the egg’s resistance to gaseous diffusion. This results in necrosis and regressive changes in the blastoderm, which hinder the embryo’s ability to develop [Egbeyale et al.] As a result, great care should be taken to ensure that eggs are of the best possible internal and external quality for storage and incubation (Figure 2).
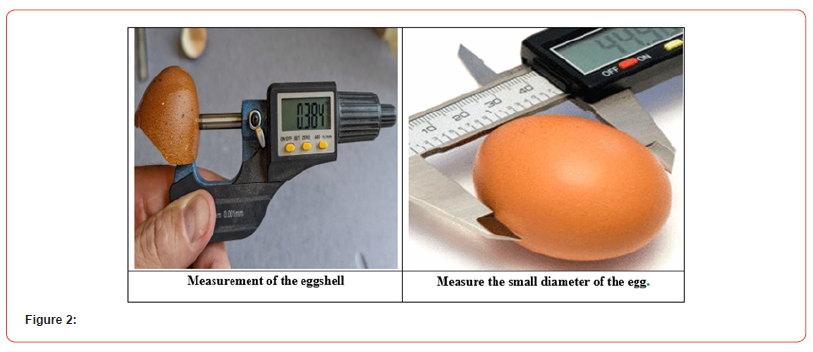
The total pore number, surface area, eggshell volume, and eggshell mass of large eggs were all considerably higher than those of small eggs in terms of eggshell quality metrics (p < 0.05), followed by a higher hatch rate and hatch mass. Egg weight and several exterior and internal measures of egg quality showed substantial relationships (p < 0.05) according to Pearson’s correlation coefficients [29]. The development and hatchability of chicken embryos can be impacted by the shell’s properties. The shell is a source of the building blocks required for the embryo’s healthy development because the egg is a closed system with regard to the minerals present [49]. A putative positive association between the processes of shell pigmentation and its calcification has been demonstrated to exist between the intensity of shell pigmentation, its thickness, and the hatchability of chickens [31]. Better hatchability may come from this, since it has been demonstrated that thicker-shelled eggs can produce hatch results that are up to 9% better than those of thinner- shelled eggs [13]. Japanese quail eggs’ “blue” or speckled shells do not seem to impact their quality if used for human food. However, the hatching success and live weight of the ensuing Japanese quail chicks might be altered by the color of the eggshell [20].
iv. Storage period of eggs:
Procedures for incubation are crucial for sustaining and enhancing
quail egg production. In the production of broilers, the
eggs are typically gathered for a period of 1 day to 3 weeks prior
to incubation in order to obtain enough eggs to fill the incubator
[38]. Eggs must be kept at low temperatures during storage to stop
embryonic development. Eggs should be kept at a room temperature
of 20 to 25 °C for storage times under four days, 16 to 17 °C
for storage times between four and seven days, and 10 to 12 °C for
storage times exceeding seven days [42]. The eggs evaporate water
during storage; the rate of evaporation is influenced by temperature
and relative humidity. According to studies [56, 11], the
quality of hatching eggs and table eggs is negatively impacted by
long-term storage. It is usual for eggs from broilers and turkeys to
lose 12 to 14% of their water during incubation [53]. The likelihood
of non-hatching increases as the number of storage days increases
because more embryos die while being stored and incubated [56].
After seven days of storage, some researchers have noted hatchability
declines of up to 5% per day [41]. In hens, it has been suggested
that a storage duration of 10 days is appropriate to retain higher
egg quality, a better Haugh unit, and lower the rate of unfavorable
physicochemical changes in the egg [81]. According to the findings,
the hatchability of eggs in meat and egg-type quails was approximately
84% up to 10 days of storage before it sharply declined [56].
Moreover, changes in protein quality that are time-related and potentially impact the hatchability of eggs are strongly correlated with storage temperature [14]. According to Goodrum et al., when eggs were held at high temperatures, protein pH quickly increased. Early embryonic mortality is influenced most by albumin quality [14]. According to Van Schalkwik et al. storage temperature plays a significant role in influencing the viability of the embryo. Quail eggs must be stored at a lower temperature in order to increase hatchability. If adapted, a cold clay pot can serve as an alternative to buying a refrigerator and be the best option for places without electricity in the country [7].
The following formulas were used to determine the external
and qualitative characteristics of eggs:
Weight loss (g) = Initial weight – final weight
Weight loss (%) = Weight loss (g)
Initial weight (g)
Shape index (%) = [Egg width (mm) / Egg length (mm)] x 100
Shell ratio (%) = (Shell weight(g) / Egg weight(g)) x 100
Egg weight (g): The eggs were weighed separately.
Shell weight (g): After the eggs were broken, the eggshells were washed with water and dried to remove any remaining protein. Following this procedure, the weight of the shell (with membrane) was measured.
Shell thickness (mm): Shell thickness (with membrane) was measured at the sharp poles, blunt poles, and the equatorial portion of each egg. The average shell thickness was obtained from the averages of these three parts.
Surface area (S) was calculated using the formula S=4πr². The radius (r) was calculated as ¼ (length + width) of the egg.
The thickness of four shell pieces, one at both ends (wide end
and narrow end) and two from the body of the egg, was measured
to the nearest 0.01 mm using a helical grid and averaged.
Egg surface área (ESA, cm2) = 3.9782 x EW0.75056
Where EW is the egg weight (g)
Unit surface Shell weight (U, mg)=SW
Cm2 ESA
Where
SW= Shell weight (mg)
ESA= Egg surface área (cm2)
Hatchability %=Number of hatched chick x 100
Number of set eggs
For the subsequent determination of the indicator “weight and
volume ratio”, a comparative analysis of the distribution of eggs was
carried out according to the theoretically calculated indicator “egg
volume”.
N 1: V=((πLB²/6) - 0,022(πLB²/6))/1000;
N 2: V = (0.6057 − 0.0018* d) * D* d * d /1000 ;
N 3: V=0.523*D*d*d/1000;
The actual egg volume (Vf) was determined by the formula: Vf
= M1–M2,
where M1 is the mass of the egg in air and M2 is the mass of the egg in distilled water. The data on the calculation of the actual value of the density of eggs and the theoretically calculated values of the ratio of mass to volume are given.
v. Internal egg characteristics:
The egg’s functional qualities are correlated with its interior
quality. The internal quality of an egg has been measured using a
variety of techniques, but the impact of temperature has not been
completely investigated. Sometimes the temperature is not stated
or is stated as a range, such as room temperature [35,37]. This may
have an impact on the findings and make it challenging to compare
studies. The accepted method for assessing an egg’s interior quality
uses Haugh units (HU). HU, a non-linear function, measures the
rate of quality loss [28]. The reliability of Haugh’s approach and
its correction for egg weight have been contested by a number of
researchers. For instance, Eisen et al. observed a bias in the HU
regression when they compared direct measurements of albumin
height with HU calculations. According to their findings, protein
height was overestimated in smaller eggs and underestimated in
larger eggs when egg weight was taken into account. Wesley and
Stadelman discovered that HU correlates well with the appearance
of broken eggs and correlates strongly with other quality measures,
such as thin white diameter, yolk centering, thin white shape, thick
white shape, percentage of outer thin protein, percentage of thick
protein, and percentage of total liquid protein. However, the validity
of their findings has been questioned. According to Silversides et
al. the HU technique does not need to account for egg weight when
gauging the freshness of eggs at room temperature. Additionally,
they contend that it was incorrect to compare HUs from various
herds. They suggested gauging the protein’s height to assess the
eggs’ quality.
Wilgus and Van Wagenen contrasted the height of a fat squirrel with the height of a fat squirrel adjusted for egg weight while creating methods for assessing squirrel height. They discovered that the r2s were the same and that it was simpler to determine protein height without correction. The variety and age of the hen have been proven to affect albumin height in recent research by Silversides and Scott (Figure 3).
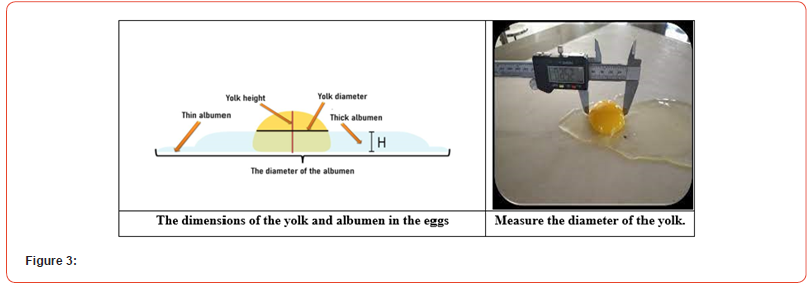
While egg weight has no bearing on the protein index, Heiman and Carver found that the albumen index—as computed in the Materials and Methods section—was superior to other quality indicators. Sauter et al. discovered that the albumen index was strongly linked with the quality loss indicated by candling. The albumen index’s quality loss rate is a non-linear function, much like HU, which is one of its main drawbacks.
For the sake of food safety, the robustness of the yolk membrane is becoming more and more crucial [44]. With rising egg age, the yolk membrane’s strength declines. This might enable any microbes in the white to access the nutrients in the yolk. Membrane strength levels are substantially correlated with the yolk index and HU, according to Kirunda and McKee’s research.
According to Wesley and Stadelman [1959], the yolk index was not as accurate even though it was connected with other internal quality indicators like the percentage of external thin protein, the percentage of thick protein, and the total percentage of thin protein. It provides a thorough picture of the overall quality of the eggs, much like HU. These findings concurred with those of Sharpe and Powell, who discovered that during storage, the height of the thick protein layer drops more quickly than the yolk index. The impact of elements like egg testing temperature and age on these quality indicators has received less study. According to Spencer et al. the interval between a breakthrough and a measurement can have an impact on quality metrics. HU and albumen height measurements of albumen quality revealed a linear deterioration with the logarithm of time since the breakthrough. Also, the age of the egg and the breed of laying hens both had an impact on the rate of loss. A linear drop of -1.15 HU with a 10°C increase in test temperature was noted by Stadelman et al. in (Figure 4).
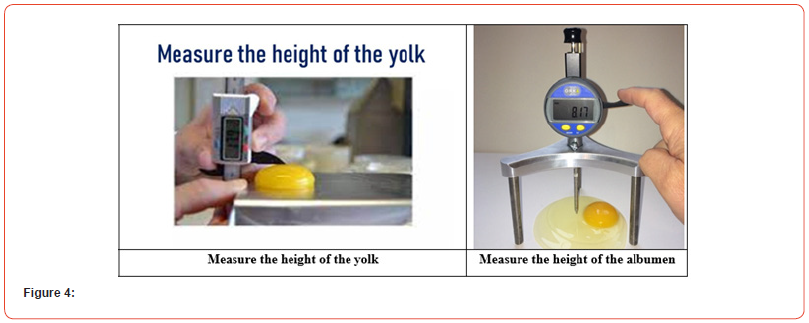
To determine the internal quality characteristics of eggs, the
following formulas were used:
Albumen weight (g) = Egg weight – (Shell weight + Yolk weight).
Yolk ratio (%) = (Yolk weight(g) / Egg weight(g)) x 100
Albumen ratio (%) = (Albumen weight(g) / Egg weight(g)) x
100
Yolk/albumen ratio (%): Yolk was weighed after being separated
from the broken eggs.
Albumen index (%) = [Albumen height(mm)/{(Albumen
length(mm) + Albumen width(mm)) /2}] x 100
Haugh unit = 100 log [Albumen height (mm) + 7.57 – 1.7 x Egg
weight(g)0.37]
Conclusion
Many factors can affect the external and interior egg quality characteristics, as was previously demonstrated. It is possible for the egg producer to regulate eggs and improve egg quality by being aware of this variety of elements. The quality of the finished product is influenced by good poultry management, best practices, and the careful gathering, handling, and processing of eggs.
Acknowledgment
None.
Conflict of Interest
None.
References
- Abdallah AG, Harms RH, El-Husseiny O (1993) Various methods of measuring shell quality in relation to percentage of cracked eggs. Poult Sci 72: 2038-2043.
- Ahn DU, Kim SK, Shu H (1997) Effect of egg size and strain and age of hen on the solids content of chicken eggs. Poult Sci 76(6): 914-919.
- Alabdallach A Z, (2020) "Histological and morphometric characteristics of chicken embryos with different genotypes." EurAsian Journal of BioSciences 14.1 (2020): 719-725.
- Z Ahmad Alabdallah , A Norezzine , Y Anatolyevich Vatnikov , A Alekseevich Nikishov , E Vladimirovich Kulikov, et al. (2021) Influence of Different Genders of Japanese Quail on the Functional State of Kidneys. Archives of Razi Institute 76( 3): 667-680.
- Alabdallah Z A, Nikishov A A, Karamyan A (2021) S. Sex-related of some haematological and serum biochemical changes, fed high-protein diet in Japanese quail (Coturnix japonica). Iranian Journal of Ichthyology 8: 150-154.
- Alabdallah Z, Nikishov A, Vatnikov Y, Al-Ragawi A, Seleznev S (2021) The effect of high protein in the feed mixture on the morphological changes in the kidneys of quail birds. Journal of Chemical Health Risks 11(4): 383-392.
- Alaga A A, D M Ogah , I D Hassan (2015) Effects of Various Storage Temperature on the Hatchability of Quail Eggs in Hot Humid Environment of Lafia, Nasarawa State, Nigeria. PAT December 11 (2): 240-245.
- Alasahan S, Gulsen C.A, Sibel C ,Mikail B (2015) "Determination of Some External and Internal Quality Traits of Japanese Quail (Coturnix Coturnix Japonica) Eggs On the Basis of Eggshell Colour and Spot Colour" Journal of Veterinary Science 31(4): 235-324.
- Alkan S, K Karabag, A Galic, T. Karsli, M S Balcioglu (2010) 2010: Effects of selletion for body weight and egg production on egg quality traits in Japanese quails (Coturnix coturnix japonica) of different lines and relationships between these traits. Kafkas Univ Vet Fak Derg 16(2): 239-244.
- Anderson K E, J B Tharrington, Pa Curtis, Ft Jones (2004) 2004: Shell characteristics of eggs from historic strains of single comb white leghorn chickens and relationship of egg shape to shell strength. International Journal of Poultry Science 3: 17-19.
- Angel Daniel P U, Jhonn Lenon C J, Laura Candelaria C H, Alabdallah Z, Nikishov A A (2022). Alterations in the mass of quail eggs of different densities during storage. Caspian Journal of Environmental Sciences: 1-4.
- Arfenia K, Sachivkina N, Liseitse A, Alabdallah Z, Byakhova V (2022) Biochemical parameters of quail blood in experimental gastrointestinal tract candidiasis. In FEBS OPEN BIO 12: 292-292.
- Bennet C D (1992) The influence of shell thickness on hatchability in commercial broiler breeder flocks. J Poult Res 1(1): 61-65.
- Brake J, Walsh TJ , Vick S V (1993) Relationship of egg storage time, Storage conditions, flock age, eggshell and albumen characteristics, incubation conditions and machine capacity to broiler hatchability. Zootech Int 16(1): 30-41. FAO, 1980. Production year book. pp: 345-370.
- Christensen VL, Davis GS (2004) Maternal dietary iodide influences turkey embryo thyroid function. Poultry Science 3: 550-557.
- Cicek-Rathert T, F Uckardeş, D Narinc, T Aksoy (2011) 2011: Comparison of principal component regression with the least square method in prediction of internal egg quality characteristics in Japanese quails. Kafkas Univ Vet Fak Derg 17(5): 687-692.
- Cook F and Briggs GM (1997) Nutritive value of eggs, in Egg Science and Technology, ed. by Stadelman WJ and Cotterill OJ. AVI, Westport: 92-108.
- Cook MI, Beissinger SR, Toranzos GA, Arendt WJ (2005) Incubation reduces microbial growth on eggshells and the opportunity for trans-eggshell infection. Ecology Letters 8(5): 532-537.
- Crawford RD (1984) Domestic fowl, in Evolution of Domesticated Animals, ed. by. Mason IL, Longman, London: 298-311.
- Drabik K, Batkowska J, Vasiukov K, Pluta A (2020) The impact of eggshell colour on the quality of table and hatching eggs derived from Japanese quail. Animals 10(2): 264.
- Egbeyale LT, Bosa MK, Sogunle OM, Adeleye OO (2013) Effect of preincubation storage periods on weight loss, embryonic development, and hatchability of pullet eggs. The Pacific Journal of Science and Technology14: 416-424.
- Eisen E J, B B Bohren, and H E McKean (1962) The Haugh Unit as a measure of egg albumen quality. Poult Sci 41: 1461-1468.
- Farooq M, Mian MA, Ali M, Durrani FR, Asquar A and Muqarrab AK (2001) Egg traits of Fayomi bird under subtropical conditions. Sarad J Agric 17:141-145.
- Fowler CTS (1972) How management can affect egg size. Poult Sci 59: 2038-2046.
- Goodrum JW, Britton WM ,Davis JB (1989) Effect of storage conditions on albumen pH and subsequent hard-cooked eggs peelability and albumen shear strength. Poult Sci 68(9): 1226-1231.
- Hanafy, AM, Hegab IM (2019) Effects of egg weight and light sources during incubation period on embryonic development and post-hatch growth of Japanese quail (Coturnix japonica). European Poultry Science 2019: 83.
- Harms RH, Rossi AF, Sloan DR, Milles RD , Christmas RB (1990) A method for estimating shell weight and correcting specific gravity for egg weight in egg shell quality studies. Poult Sci 73:599-602.
- Haugh R R (1937) The Haugh unit for measuring egg quality. US Poult Mag 43: 552-573.
- Hegab IM, A M Hanafy (2019) "Effect of egg weight on external and internal qualities, physiological and hatching success of Japanese quail eggs (Coturnix coturnix japonica)." Brazilian Journal of Poultry Science: 21.
- Heiman V, J S Carver (1936) Albumen index as a physical measurement of observed egg quality. Poult Sci 15(2): 141-148.
- Ingram DR, Hatten LF, Homan KD (2008) A study on the relationship between eggshell color and eggshell quality in commercial broiler breeders. Int J Poult Sci 7: 700-703.
- Iposu SO, Onwuka CFI, Eruvbetine D (1994) The relationship between selected quality traits and egg size. Niger J Anim Prod 21:156-160.
- Iqbal J, Khan SH, Mukhtar N, Ahmed T, Pasha RA (2016) Effects of egg size (weight) and age on hatching performance and chick quality of broiler breeder. Journal of Applied Animal Research 44: 54-64.
- Kaminska BZ and Skraba B (1991) Analysis of hen types considering albumen:yolk ratio and its changes during the laying cycle, in Proceedings of the 4th European Symposium on the Quality of Poultry Products. II. Eggs and Egg Products, Doorwerth, Netherlands 43: 49.
- Keener KM, JD LaCrosse, PA Curtis, KE Anderson, B E Farkas (2000) The influence of rapid air cooling and carbon dioxide cooling and subsequent storage in air and carbon dioxide on shell egg quality. Poult Sci 79(7): 1067-1071.
- Khurshid A, Farooq M, Durrani FR, Sarbiland K, Chand N (2003) "Predicting Egg Weight, Shell Weight, Shell Thickness and Hatching Chick Weight of Japanese Quails Using Various Egg Traits as Regressors" International Journal of Poultry Science 2: 164- 167.
- Kirunda D F K, S R McKee (2000) Relating quality characteristics of aged eggs and fresh eggs to vitelline membrane strength as determined by a texture analyzer. Poult Sci 79(8): 1189-1193.
- Kuurman WW, Bailey BA, Koops WJ, Grossman M. (2002) Influence of storage days on the distribution for time of embryonic mortality during incubation. Poultry Science 81(1): 1-8.
- Lacin E, Yildiz A, Esenbuga N, Macit, M (2008) "Effects of Differences in The Initial Body Weight of Groups On Laying Performance and Egg Quality Parameters of Lohmann Laying Hens" Czech Journal of Animal Science 53: 466-471.
- Marion WW, Nordskog AW, Tolman HS , Forsythe RH (1964) Egg composition as influenced by breeding, egg size, age and season. Poult Sci 43(1): 255-264.
- Mayes FJ, Takeballi MA (1984) Storage of the eggs of the fowl (Gallus domesticus) before incubation. World’s Poultry Science Journal 40:131-140.
- Meijerhof R (1992) Pre-incubation holding of hatching eggs. World’s Poultry Science Journal 48: 57-68.
- Meir M, Nir A, Ar A (1984) Increasing hatchability of turkey eggs by matching incubator humidity to shell conductance of individual eggs. Poultry Science 63(8):1489-1496.
- Messens W, K Grijspeerdt, L Herman (2005) Eggshell penetration by Salmonella: A review. World Poult Sci 61:71-85.
- Narushin VG, Romanov MN (2002) "Egg-Physical Characteristics and Hatchability" World Poultry Science journal 58: 297-303,
- Nedomova S, L Severa, J Buchar (2009) Influence of hen egg shape on eggshell compressive strength. Int Agrophysics 23: 249-256.
- Nikishov A A (2020) Morphometric Characteristic Of Kidneys In The Japanese Quail 157(2-3): 154-155.
- Nwachukwu EN, Ibe, SN Ejekwu K (2006) "Shortterm Egg Production and Egg Quality Characteristics of Main and Reciprocal Crossbred Normal Local, Neck and Frizzle Chicken X Exotic Broiler Breeder Stock in A Humid Tropical Environment" Journal of Animal and Veterinary Advance 5(7): 547-551.
- Nys Y, Gautron J, Garcia-Ruiz JM, Hincke MT (2004) Avian eggshell mineralization: Biochemical and functional characterization of matrix proteins. C. R. Palevol 3(6-7): 549-562.
- Onbasilar EE, Erdem E, Poyraz O, Yalcin S (2011) Effects of hen production cycle and egg weight on egg quality and composition, hatchability, duckling quality, and first-week body weight in Pekin ducks. Poultry Science 90(11): 2642-2647.
- Portugal SJ, Maurer G, Thomas GH, Hauber ME, Grim T, et al. (2014) Nesting behaviour influences species-specific gas exchange across avian eggshells. Journal of Experimental Biology 217(18): 3326-3332.
- Rahn H, Ar A (1974) The avian egg: Incubation time and water loss. Condor 76:147-152.
- Rahn H, Christensen VL,Edens FW (1981) Changes in shellconductance, pores,and physical dimensions ofegg and shell during the first breeding cycle of turkey hens; Poultry Science 60(11): 2536-2541
- Rahn H, Paganelli CV, Ar A (1987) Pores and gas exchange of avian eggs:a review. The Journal of Experimental Zoology 1: 165-172.
- Roberts JR (2004) "Factors Affecting Egg Internal Quality and Egg Shell Quality in Laying Hens" The Journal of Poultry Science 3: 161-177.
- Romao J M (2008) "Effect of egg storage length on hatchability and weight loss in incubation of egg and meat type Japanese quails." Brazilian Journal of Poultry Science 10: 143-147.
- S ekeroglu A, Kayaalp GT , Sarıca M (2000) The regression and correlation ˇanalysis an egg parameters in Denizli poultry. J Agric Fac Cukurova Univ 15: 69-74.
- Saatci M, Kırmızıbayrak T, Aksoy AR and Tilki M (2005) Egg weight, shape index and hatching weight and interrelationships among these traits in native Turkish geese with different colored feathers. Turk J Vet Anim Sci 29: 353-357.
- SARICA M, C ERENSAYIN (2009) Poultry Products. In: TURKOGLU M., M. SARICA: Poultry Science: 89-138.
- Sauter E A, J V Harns, W J Stadelman, B A McLaren (1953) Relationship of candled quality of eggs to other quality measurements. Poult Sci 32(5): 850-854.
- SEKEROGLU, A., G.T. KAYAALP, M. SARICA, (2000) The Regression and correlation analysis on egg parameters in Denizli poultry. Journal of Agricultural Faculty, Cukurova University 15: 69-74.
- Shafey TM (2002) Effects of egg size and eggshell conductance on hatchability traits of meat and layer breeder flocks. Asian-Austral J Anim 15: 1-6.
- Sharp P F, C K Powell (1930) Decrease in the internal quality of hen’s eggs during storage as indicated by the yolk. Ind Eng Chem 22:909.
- Silversides F G, T A Scott (2001) Effect of storage and layer age on quality of eggs from two lines of hens. Poult Sci 80: 1240-1245.
- Silversides F G, F Twizeyimana, P Villeneuve (1993) Research note: A study relating to the validity of the Haugh unit correction for egg weight in fresh eggs. Poult Sci 72(4): 760-764.
- Silversides, F.G., Korver, D.R. and Budgell, K.L. (2006). "Effects of Strain of Layer and Age at Photostimulation on Egg Production, Egg Quality Andbone Strength" Poultry Science 85(7): 1136-1144.
- Soliman FNK, Rizk RE, Brake J (1994) Relationship between eggshell porosity, eggshell thickness, egg weight loss, and embryonic development in japanese quail eggs. Poultry Science 73(10): 1607-1611.
- Solomon SE (2010) The eggshell: Strength, structure and function. Br Poult Sci 51: 52-59.
- Song KT, Choi SH , OH HR (2000) "A Comparison of Egg Quality of Pheasant, Chukar, Quail and Guinea Fowl" Asian -Australasian Journal of Animal Sciences 13: 986-990.
- Spencer, J. V., W. J. Stadelman, E. A. Sauter, and J. G. Darroch. 1956. Measured albumen quality as affected by time after break-out. Poult. Sci. 35:319-322.
- Stadelman W J, F Ziegler, J G Darroch 1954. The effect of egg temperature on its broken-out albumen quality evaluation. Poult Sci 33(2): 1082-1083.
- Too HC, Shibata M, Yayota M, Darras VM, Atsushi Iwasawa (2017) Iwasawa A. Expression of thyroid hormone regulator genes in the yolk sac membrane of the developing chicken embryo. The Journal of reproduction and development 63(5): 463-472.
- Tumova E, Zita L, Hubeny M, Skrivan M, Ledvinka Z (2007) The effect of oviposition time and genotype on egg quality characteristics in egg type hens. Czech J Anim Sci 52 26-30.
- Uddin M.S, Paul DC, Huque QME (1994) Effect of egg weight and pre-incubation holding periods on hatchability of the Japanese quail eggs in different seasons. Asian-Austral J Anim 7: 499-503.
- Van Schalkwyk SJ Brand Z, Cloete SWP, Brown CR (1999) Effects of time of egg collection and pre-incubation treatment on blastoderm development and embryonic mortality in ostrich embryos. S Afr J Anim Sci. 29: 154-163.
- Wesley R L, and W J Stadelman (1959) Measurement of interior egg quality. Poult Sci 38(2): 474-481.
- Wilgus H S, and A VanWagenen (1936) The height of the firm albumen as a measure of its condition. Poult. Sci. 15(4): 319-321.
- Wolanski NJ, Renema RA, Robinson FE, Carney VL, Fancher BI (2007) "Relationship Among Egg Characteristics, Chick Measurements, And Early Growth Traits in Ten Broiler Breeder Strains" Poultry Science 86(8): 1784-1792.
- Wolc A, Olori VE (2009) "Genetics of Hatchabilityegg Quality from the Perspective of a Chick" 6th European Poultry Genetics Symposium, World Poultry Science Association, Bedlewo, Poland.
- Yahaya HK, Oni OO, Akpa GN , Adejinka IA (2009) "Evaluation of Layer Type Chickens Under Reciprocal Recurrent Selection" Bayero Journal of Pure Applied Science 2: 77-82.
- Yilmaz AA, Bozkurt Z (2009) "Effects of Hen Age, Storage Period and Stretch Film Packaging on Internal and External Quality Traits of Table Eggs" Lucrari Stiintifice Zootehnie Si Biotehnologii 42: 462-9.
- Ziad A (2021) Changes in the morphological and anatomical structures of kidney in birds. Innovative approaches in the modern science: 134-139.
- Ziad A (2020) Histological structure differences of kidney in birds. Innovative approaches in the modern science: 72-76.
- Ziad Alabdallah (2022) Histological Comparison of Kidneys Between Female and Male Quail Birds at Different Age Stages. Open J Pathol Toxicol Res 1(3): 2022.
- Ziad Alabdallah (2022) The Presence of GFP in Embryos and its Effect on Cells. Open J Pathol Toxicol Res 1(2): 2022.
- Ziad, A (2018) "MORPHOMETRIC PARAMETERS OF CHICK EMBRYOS WITH DIFFERENT GENOTYPE." Innovative in Agriculture.
- Ziad A (2021) MORPHOLOGICAL CHANGES OF KIDNEYS IN RELATION TO AGE OF QUAIL. In Proceedings of the All-Russian Scientific Conference of Young Scientists and Specialists with International Participation 65(32): 131-133.
- Ziad, Alabdallah. (2021) "ARTIFICIAL CHANGE IN THE GENOTYPE AND THE EFFECT OF GFP ON CELLS, TISSUES AND ORGANS." Interscience 12-2: 95-97.
- Ziad, Alabdallah (2021) "DYNAMICS OF KIDNEY MORPHOLOGICAL CHANGES FEMALES AND MALES OF THE QUAIL BIRDS." Materials of the All-Russian scientific conference of young scientists and specialists with international participation, dedicated to the 155th anniversary of the birth of NN Khudyakov, Moscow: 7.
-
Ziad Ahmad Alabdallah*. External and Internal Conditions Affecting Fertilization and Hatching of Avian Eggs. Open J Pathol Toxicol Res. 1(4): 2023. OJPTR.MS.ID.000517.
-
Characteristics, Weight, genetic heterogeneity, shape, yolk, shell weakens, specific gravity, mass, embryo.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






