 Research Article
Research Article
Efficiency of Plant Extracts and Fungicides in Controlling Root Rot Diseases in Peas
Sahar A ELSayed1*, Anwar M Aloufi2
1Department of Biology, Faculty of Science and Arts in Beljurashi, Al- Baha University, Al-Baha, Saudi Arabia
2Master’s in microbiology, Faculty of Science and Arts in Beljurashi, Al- Baha University, Al- Baha, Saudi Arabia
Sahar A. EL-Sayed, Department of Biology, Faculty of Science and Arts in Beljurashi, Al- Baha University Al-Baha, Saudi Arabia.
Received Date:January 17, 2024; Published Date:February 02, 2024
Abstract
Pea (Pisum sativum L) is an essential leguminous crop grown in many countries and Saudi Arabia. Peas are an important protein source for both humans and animals. Peas (Pisum sativum L) Pathogens attack the pea plant and cause severe damage to it, thus affecting its growth and production. In pathogenicity tests; twenty fastest-growing fungal isolates without regard to the isolation place including four isolates of F. oxysporum, F. solani, R. solani and Alternaria solani were examined for their pathogenicity on pea plants to select the most aggressive isolates to use in the further studies. In a study of the antifungal activity of plant extracts, i.e., Neem, Ginger, and Artemisia at concentrations of 0, 25, 50, 75, and 100%, all plant extracts at any concentration used occurred inhibition of the linear outgrowth of three examined pathogenic fungi. There is an opposite relation between increasing conc. of plant extracts from 0 to 100% and the linear outgrowth of three examined pathogenic fungi. In this respect, Neem and Ginger at 100% were the most efficacy. Neem extract at both 75 and 100% concentrations prevented the growth rate of F. solani.
Moreover, the high concentration (100%) also wholly suppressed the linear outgrowth of F. oxysporum. It caused more suppression in the linear outgrowth of F. solani in comparison with F. oxysporum in addition to R. solani. The reverse side, Ginger extract at high concentration wholly suppressed the linear outgrowth of F. oxysporum in addition to F. solani. Also, the concentration of 75% wholly suppressed the linear outgrowth of F. solani. In a study of chemical control, the Premium fungicide was more efficacious in lowering the linear spread of all examined fungi than the Tolex fungicide. Both high concentrations of Premium (75 and 100 ppm) prevented the outgrowth of F. oxysporum and R. solani. Tolex 500 wp fungicides at 100 and 75 ppm prevented the growth rate of R. solani. It is noteworthy that F. oxysporum was less effective with Tolex 500 while R. solani was more sensitive.
Keywords:Pathogenic fungi; chemical control; plant extracts; pea plants
Introduction
Pea (Pisum sativum L) is a leguminous plant with many advantages as it fixes nitrogen through the atmosphere [1]. Pea is a crop grown in several countries, such as the K.S.A. Studies found that it contains a high nutritional value of protein, iron, phosphorus, and calcium, as well as a group of vitamins such as Shula, vitamins A and B [2]. In furthermore, many of these characteristics, it is a useful and important food for both humans and animals, as it reduces cholesterol [3,4]. In numerous regions of the world, peas are cultivated and consumed as vegetables, as they are a significant source of protein and essential elements such as potassium and calcium. [5]. The pea plant is one of the most significant commercially plants due to its exposure to many diseases, especially root rot disease, and the harmful effect resulting from the use of fungicides, which are considered currently to be an effective treatment for the elimination of many diseases. However, scientists found that these pesticides threaten human and animal health, in addition to their ability to eliminate soil vitality and fertility. [6,7]. Showed that plants infected with root rot showed significant genetic variation.
This difference is evident in the decrease in the number of plants, which causes a constant threat to the formation of peas, particularly in temperate zones, where peas are sown in spring in cool and wet conditions. Seedling death can be a significant factor limiting yield and causing severe economic loss [8] reviewed that leguminous crops are susceptible to many diseases that limit their productivity and reduce quality. It was found that some varieties of peas may be infected with nearly a hundred fungal causes around the globe. From among fungal diseases, fusarium wilt is one of the worst diseases that affect legumes because it is a soil disease [9] found that root rot disease in peas is a complex disease caused by many fungi that are transmitted through the soil, and root rot disease is one of the significant aspects for determining crop yield. Sclerotinia sclerotium, Rhizoctonia solani, and Fusarium oxysporum were isolated from culture, recognized, and afterwards validated in a pathogenicity method as pathogens of root rot [10]. Peas are infected with many pathogens, whether fungal, bacterial, or viral, as well as nematodes, in addition to causing some physiological defects.
Fungal diseases, seedling death diseases, and root diseases are among the primary harmful conditions of the crop [11] found that R. solani is the highest significant destructive fungus caused a high % of both pre-emergence and post-emergence damping off 23.33% and 53.33% of viable plants. F. oxysporum showed (20, 26.66%) in pre- and post-damping off and 60% of survival plants. At the same time, F. oxysporum showed a high significant percentage in disease severity, followed by R. solani. Pythium spp. Gave 16.66%, 13.33% in both pre-damping and post-damping off, and 70 % of viable plants [12] showed that 14 isolates of F. oxysporum f. sp. pisi were evaluated in several areas of Manipur for their culture, morphogenetic and pathogenic activity. The color of the fungus varies from white to bright red, purple, and yellow. The development of pathogenic fungi from Fusarium isolates ranged from 5.4 cm to 8.9 cm at eight days after inoculation at twenty-six degree Celsius in 9 cm Petri plates. The isolates showed intermediate to abundant spores.
The size of the microconidia ranged from 11.6 × 3.1 to 25.2 × 6.2 μm and the size of the microconidia varied from 3.02 × 2.1 μm to 9.2 × 5.6 μm [13] found that plant extracts give a natural and protective effect against pathogenic fungi such as R. solani. He discovered that developing antifungals would not only offer an effective tool for controlling pea root rot but would also pledge successful and superior multifunction alternatives to traditional fungicides for managing those certain plant diseases [14] tested 13 leaf extracts of various plants in the laboratory for their capacity to keep Rhizoctonia solani under control. Garlic, eucalyptus, lemon, joker or van Tulsi greatly hindered fungal outgrowth in addition to sclerenchyma formation, except for Tulsi, onion, aka, jatropha, and bishram, which led to a decrease in the spread of Rhizoctonia solani [15] discovered this through experiments on about thirteen plant extracts from leaf extracts of Catharanthus roseus, Azadirachta indica, Lantana camara, Ocimum sanctum Ricinus communis, Saraca indica, or Thuja occidentalis latex-producing plants Calotropis procera, Nerium Indicum and Datura Ficus procera or Nerium Indicum tested.
Bulbs of Dinisosa, Allium cepa, or Allium siphon for pathogen eradication in vitro. These extracts’ antifungal activity was tested on fungi such as Rhizoctonia solani. It was discovered that when compared to the control, all-natural plant extracts reduced the pathogen [16] found that the methanol extract from (leaves, flowers, roots, fruit, and buds) of Trachystemon Orientalis, Smilax excelsa, Rhododendron ponticum, Phytolacca americana, or Prunus laurocerasus, these parts exhibited their activity against pathogenic fungi (Alternaria solani, Botrytis cinerea, and Rhizoctonia solani) [17] discovered that clerodendrum leaves (Clerodendrum infortunatum L), polyantha (Polyalthia longifolia Sonn), or ginger roots (Zingiber officinale Roscoe) were more efficacious towards Colletotrichum muse in addition to Rhizoctonia solani (Kühn) then resulted in growth suppression. Many fungi are pathogenic, whereas the highest potent plant extract is clerodendrum extract, which was found to be most potent towards the pathogen of rice sheath blight in either pot cultivation or field experiments [18] discovered extracts from Moringa oleifera Lam.
The plant parts (leaves, stem, fruit, and seeds) Strongly exhibited suppression in the spread of Macrophomina phaseolina, Rhizoctonia solani (Kühn), or Fusarium oxysporum, while the stem extract in addition to powder enhanced plant growth then demonstrated maximum suppression of root rot fungi on peas, so, this was clearly shown in bean crops under greenhouse conditions [19] that extracts of Moringa leaves and seeds contain antifungal properties that inhibit spread of R. solani in addition to F. solani. The antifungal effectiveness of the extracts was influenced by the concentrations of moringa extract [20] reported aqueous and ethanol-solvent extracts of four wild medicinal plants (Moringa Olivera, Osmium Basilica, Cinnamomum cavort, and Lantana Camara) were tested in vitro and under greenhouse for safeguarding of canola plants from root rot or wilt diseases. In vitro, all aqueous solvents in addition to ethanol extracts inhibited the linear growth of F. solani, R. solani, or F. oxysporum to various levels. Moringa oleifera was better than the fungal outgrowth of all examined fungi.
Additionally, all of the evaluated plants’ aqueous extracts were less effective than the ethanol solvent [21] achieved the best production (66.55 q/ha) of peas when treating seeds with carbendazim and soil application of green manure + neem cake + antifungal. Piece [22] reported to identify the effect of indoor fungicide applications infected with root rot fungus of peas under greenhouse in addition to field conditions. Indoor fungicides generally lowered the severity of root rot, occasionally; the extent of seed treatment in the field is significantly exceeded. Even so, a difference between the level of control existed between hosts and pathogens in both greenhouses in addition to field experiments. Prothioconazole and fluopyram Penthiopyrad supplied the most regular outcomes across trials. According to the findings of these research, indoor application of fungicides offers growers a different method for controlling Fusarium root rot [23]. Found that fungicides of several classes like exogenous quinone inhibitors (QoIs), triazoles, Demethylation Inhibitors (DMIs) or Succinate Dehydrogenase Inhibitors (SDHIs) were employed for applications within limited locations.
QoI fungicides behave at the quinol-binding site. Exogenous cytochrome
bc1, which inhibits mitochondrial respiration then deactivates
membrane formation by preventing demethylation, is categorized
in the Fungicide Resistance Committee (FRAC) division 11.
DMI fungicides deactivate membrane formation by suppressing the
demethylation of sterol biosynthesis. The triazole fungicides are a
subdivision of the DMI FRAC division 3. The SDHI fungicides, FRAC
division 7, target the mitochondrial respiration chain; deactivate
the tricarboxylate cycle in addition to the electron transport chain
in mitochondria [24] found that the use of fungicides as an outcome
of substantial lowering in disease severity contrasted with the untreated
control. Some pesticides reduced infection by immersion;
in addition to Fosetyl Aluminum suppressed pathogens effectively
during seed treatment and spraying methods. Plant growth variables
were also evaluated, and then a considerable enhancement
was noticed in growth response of the treated plants contrasted
with the untreated control. The study exhibited that fungicides can
be used to effectively control peas. Wilt disease using the suitable
application method. Thesis’ objective, the purpose of this research
was to investigate the following items.
a) Groups of pea root rot inhibitors, fungi and their frequency
from different fields in Beljurashi and Bani Kabir governorates,
b) Isolation, purification, and identification of fungal root rot
causes.
c) Pathogenicity test for the fastest-growing pathogenic fungi.
d) Investigating the effects of plant extracts (Artemisia, neem, and
ginger) on growth of linear fungi.
e) The impact of Tolex 500 wpm and Premium fungicides on the
linear growth rate of fungi.
f) Evaluation of seed soaking in the best biological control treatments,
plant extracts, and fungicides against damping-off of
pea seedlings and survival of plants under artificial soil with
the most aggressive fungal pathogens in the greenhouse.
Materials and Methods
Source of Pea Seeds and Tested Materials
Pea seeds were obtained from the farm of Mr. Ahmed Ghoramallah in Beljurashi. While the used biological agents were isolated from the root of hygienic pea plants in the same sites in Beljurashi and Bani kabir provinces that were used to isolate fungal pathogens. Extracts from three plants have also been used as natural additives (neem, ginger, and Artemisia). Two fungicides, Tolex 500 and Premium, were used for comparison in this investigation.
Fungal Pathogens and their Frequency
Samples of pea plants appearing damping-off or root-rot symptoms were gathered from various fields at Beljurashi or Bani kabir Provinces and transferred into laboratories of the College of Arts and Sciences, Beljurashi, Al-Baha University to prepare for isolation and identification of root rot fungal pathogens of pea plants. Fifty isolates of root rot pathogens were isolated from the roots of pea plants under natural infection from diverse locations in Beljurashi and bani kabir Provinces, Saudi Arabia. As mentioned below, the frequency of isolated fungi species was recorded as a frequency percentage after isolated fungal genera.
Isolation and Purification of the Pathogens
Samples of pea plants appearing apparent symptoms of damping– off and root rot diseases were gathered from similar areas to infected fields at both Beljurashi and Bani kabir provinces, Saudi Arabia. All diseased root samples were carefully washed in tap water to eliminate the adjacent soil particles, dried, and then chopped into small pieces (approximately 1 cm). The samples were surface sterilized by soaking them in 0.5% sodium hypochlorite solution for 2 min.; after this, it was carefully rinsed in sterilized water, followed by drying between two sterilized filter papers. The surface sterilized samples were transported onto plates of Petri dishes loaded with Potato Dextrose Agar (PDA) medium that has been provided with 0.01% Streptomycin antibiotic (100 mg/ml) to prevent bacterial contamination. Plate’s Petri- dishes that contain infected root pieces were incubated at 25± degree Celsius for 5 days. The spreading of fungi was carefully moved to PDA slants and left at four degrees Celsius in a refrigerator for subsequent studies. The developing mycelium colons from pure cultures were got from each isolate were purified after five days by hyphal tip technique, in accordance with [25].
Identification Studies
The developing fungi after five days from incubation were obtained randomly from the ends of the growing colonies and transported onto poured PDA medium plates. The isolated fungi that were growing were recognized based on their morphological characteristics using a light microscope [26-28]. Sections of the fungal growth were placed on clean slides using lacto phenol cotton blue stain to observe and identify the morphological structures [29]. The size, texture, and color of the fungal colonies were checked under the light microscope; the previously prepared slides were examined using the objectives of 40x for vegetative mycelia i.e. septation, diameters conidiophores or sporangiophores and also reproductive structures i.e. conidia and sporangiospores. The 10x objective of the light microscope was used to examine fungal colonies. The frequency and frequency percentage of the isolated fungal species, whether it is defined or undefined, were calculated.
Pathogenicity Tests
The Pathogenicity tests were performed under greenhouse conditions of the College of Arts and Sciences, Beljurashi, at Al-Baha University. Twenty fastest-growing fungal isolates, including four isolates of F. oxysporum, six isolates of F. solani, five isolates of R. solani, and five isolates of Alternaria solani were examined for their pathogenicity on pea plants to select the most aggressive isolates to use in the further studies without regard to the isolation place.
Preparation of fungal inoculum
Pots with a diameter of twenty-five cm were sterilized by soaking them in a five percent formalin solution for fifteen min., and afterward left to air-dry in the open air. The soil was sterilized by extensively combining it with a five percent formalin solution. The treated soil was then lined with a plastic sheet for a week before removing it to allow the formalin to fully evaporate [30]. Fungi were cultured separately on sand-barley (SB) medium, which was made by combining twenty-five gram of clean sand, seventy-five gram of barley, and adequate water to lid the mix. Sterilized medium in flasks was inoculated with each individual fungus and incubated at a temperature of 25 degree Celsius for a period of two weeks. Each individual fungus was infested in the soil at four percent of the soil weight [31]. The soil in the pots was watered daily for a time of one week to promote the outgrowth of fungi. The soil in the control pots was combined with an equal quantity of sterilized Sand Barley (SB) medium that was free of fungus. Ten pea seeds were sterilized with a two percent sodium hypochlorite solution for 2 min, after that washed many times with sterilized water before being sown. For each treatment, three pots with a full of thirty seeds were utilized as replicates. The plants received a regular agricultural recommendation for irrigation and fertilization. Pots were organized under the greenhouse.
Sowing seeds
Healthy pea seeds. They were disinfected with one percent sodium hypochlorite solution for 5 min., after that washed in sterile water and sown at ten seeds per pot. For every treatment, three replicates were utilized. The plants received a regular agricultural recommendation for watering and fertilizing. The pots were set up in a totally randomized design under the conditions of a greenhouse.
Disease assessment
The disease assessments were recorded as ratios of pre- emergence and post-emergence damping-off in addition to the hygienic surviving plants in every treatment at 15, 30, and 45 days after sowing, respectively, using the formula of [32] as follows:
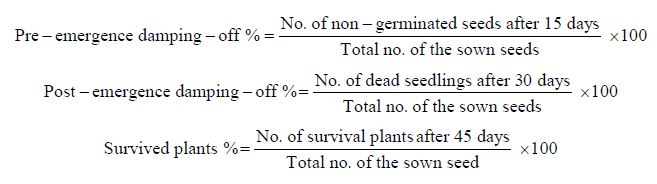
In Vitro Studies
Plant Extracts Preparation of Plant Extracts
A quantity of three plant species (Neem, Ginger, and Artemisia), chopped leaves and seeds of each plant then washed thoroughly in sterile distilled water. Each sample’s plant extract was made by mixing 20 grams of dried plant powder with 200 ml of distilled water in a glass flask (volume 500 ml). Large plankton is removed using two layers of gauze, and the filtrate is then separated from it using filter paper to provide a clear filtrate devoid of contaminants. Each plant sample’s initial solution is this. The extracts were then put in a dark, airtight flask and kept in the refrigerator at 10 degrees Celsius until they were used [33]. The extracts were considered 100% concentration. The solvent extract was diluted with sterile distilled water to prepare other dilutions i.e., 25, 50,75, and 100%.
Impact of Plant Extracts on Fungal Linear Outgrowth (mm)
The effects of plant extracts, i.e., Neem, Ginger, and Artemisia at concentrations of 0, 25, 50, 75, and 100% on fungal linear growth were tested in the laboratories of the College of Arts and Sciences, Beljurashi, Al-Baha University. All tested concentrations were combined separately with PDA medium prior solidification (1:9 v/v), after that emptied into sterilized Petri dishes. For each concentration, three plates were utilized as replicates. The plates were inoculated with fungal discs taken from the periphery of the 5-day-old culture of each tested fungi in the center of the plate. The check control was accomplished by growing one disc of the pathogen without any treatments. The plates were incubated at 27oC. The linear outgrowth of examined pathogenic fungi was estimated when the fungal cultivation covered the surface of any plate (each control). The most effective plant extracts were chosen for evaluation in a greenhouse experiment.
Chemical Fungicides
Effect of Tolex 500 wpm and Premium via Fungal Linear Outgrowth
The effect of both Tolex 500wp (Tolclofos- methyl) and Premium fungicides on three pathogenic fungal spread were examined at the concentration of 0,10, 25,50, and 100 ppm according to the active ingredient. The prepared conc. of fungicide was added to the PDA medium just prior solidification and emptied into Petri- dishes. Three dishes were made for every treatment. The percentages of fungal outgrowth lowering were estimated by comparing the growth in various concentrations to the control treatment.
Greenhouse Experiment
This experiment aimed to investigate the effectiveness of seed treatment in the best treatments of plant extracts, against the prevalence of damping off pea seedlings and survival plants under arti ficial soil with the most aggressive fungal pathogens. Pea seeds this study was done in sterilized pots (25cm), ten seeds for each one containing sterilized clay soil in the greenhouse. Both pots in addition to soil were sterilized with a 5% formalin solution. Plant extracts i.e., Neem and Ginger at the rate of 100%. as well as both fungicides (Tolex 500wp and Premium) at 100 ppm before sowing. A completely randomized design was used in this experiment. Soaked seeds were sown at rate of ten seeds per pot, six pots per replicate in each treatment. The inoculum of each of the three examined fungal pathogens was mixed with soil at a rate of 4% of soil weight. Disease incidences were recorded as percentages of pre-emergence and post-emergence damping-off in addition to the hygienic surviving plants in every treatment at 15, 30, and 45 days after sowing were assessed as mentioned before.
Statistical analysis
All collected information was assessed or statistically analyzed using the software package [34] for comparing means at 5% in one way completely randomized.
Results
Isolation and Identification of Fungal Pathogens and their Frequency
The outcomes shown in (Table 1) and represented in (Figure 1) show that fifty isolates of root rot pathogens were isolated from roots of pea plants under natural infection from diverse locations in both Beljurashi and Bani kabir Provinces, Saudi Arabia. Generally, 24 fungal isolates were isolated from Beljurashi in addition to 26 isolates from Bani kabir; after isolation and purification of the causal pathogens, these isolates represented four species of three genera. It was observed that the fungal species were identified as Fusarium, Rhizoctonia and Alternaria, in addition to eleven unidentified isolates (six isolates from Beljurashi and five isolates from Bani kabir). The genus Fusarium includes two species, i.e., F. oxysporum in addition to R. solani. At Beljurashi province, the unknown isolates fungi came in the first order with 6 isolates giving 25% frequency, followed by Rhizoctonia solani and Alternaria solani “five isolates for both,” giving 20.83% frequency.
Table 1:Isolated fungi and their frequency from pea plant roots appearing root rot disease symptoms at various fields in Beljurashi and Bani kabir Provinces..
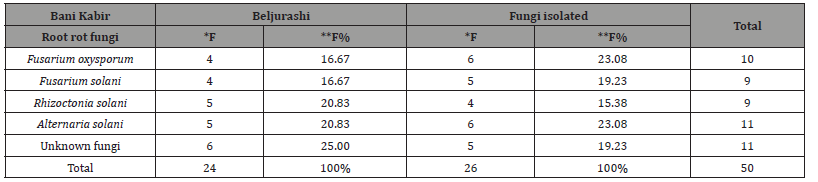
*F: Frequency, **F%: frequency%
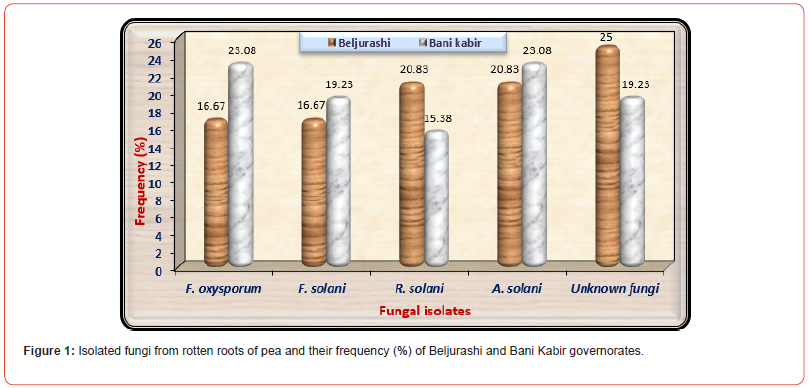
While Fusarium oxysporum and Fusarium solani came to an end with four isolates for both, which recorded 16.67% frequency. With respect to Bani kabir province, data in the same (Table) show that Fusarium oxysporum and Alternaria solani recorded the high isolates number “6 isolates for both” with high frequency “of 23.08%”. Both Fusarium solani and Unknown fungi came in the second order giving 19.23% frequency of both. However, Rhizoctonia solani came lately by four isolates recorded 15.38% frequency. Totally, Alternaria solani and Unknown fungi recorded the highest fungal isolates giving eleven isolates for both in both provinces together. In this respect, Fusarium oxysporum came the second order recording ten isolates, followed by Fusarium solani and Rhizoctonia solani, which gave nine isolates for both.
Pathogenicity Tests
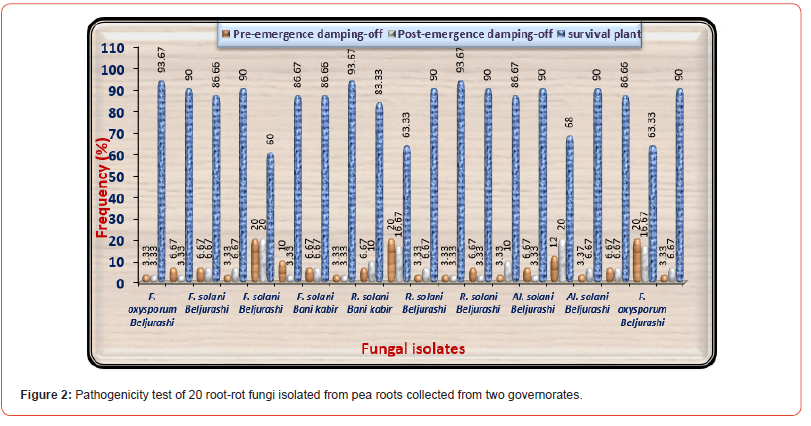
Table 2:Pathogenicity test of pre- and post-emergence damping-off in addition to survival of pea plants infected with isolated fungi (the fastest growing of twenty isolates).
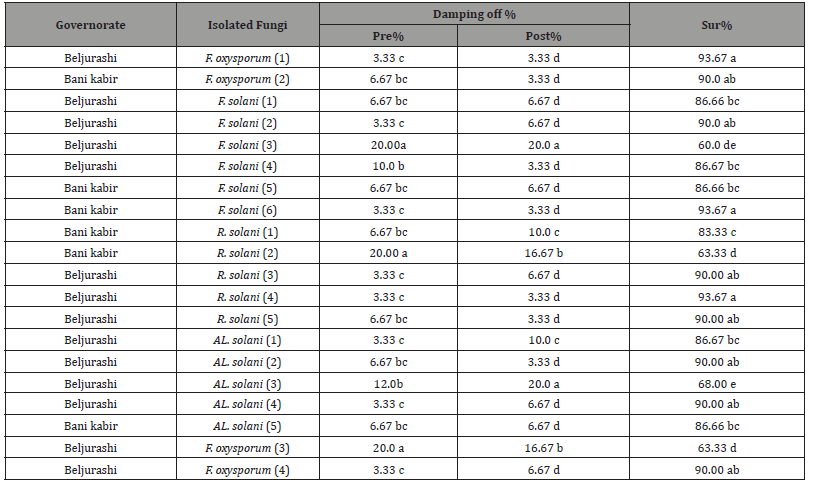
Pathogenicity tests were performed under greenhouse conditions at Saeed El- Khazim farm in Beljurashi. Twenty fastest-growing fungal isolates without regard to the isolation place, including four isolates of F. oxysporum, six isolates of . solani, five isolates of R. solani, and five isolates of Alternaria solani were examined for their pathogenicity on pea plants to select the most aggressive isolates to use in the further studies. Data in (Table 2) and illustrated in (Figures 2-2C) show that all examined fungi were pathogenic and induced pre-emergence and post-emergence damping-off in pea seedlings. A high percentage of pre-emergence damping-off (20.0%) take placed under infected soil with F. solani No3 isolated from Beljurashi province and R. solani No2, which was isolated from Bani kabir province as well as F. oxysporum No three isolated from Beljurashi province. While Alternaria solani No three came in second order (Figure 2A).
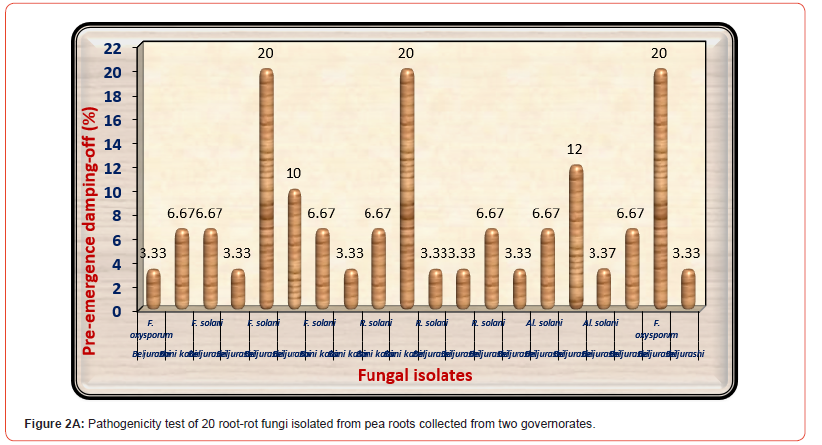
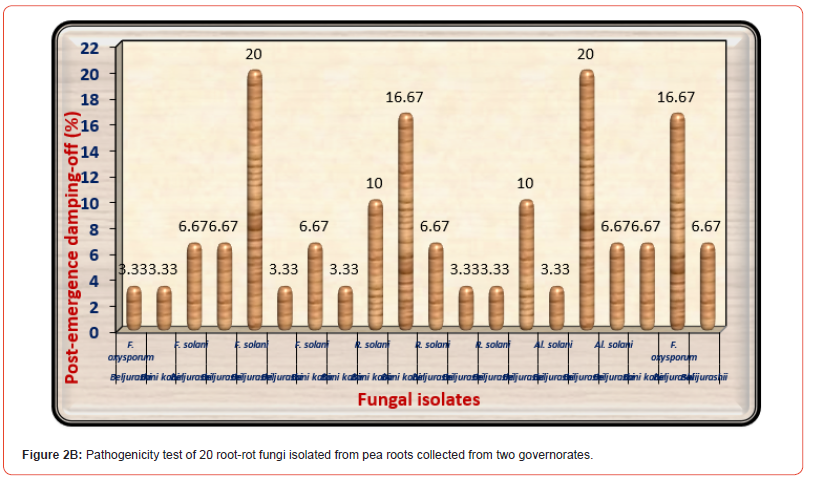
On the other side, as a consequence of the pathogenicity tests on 30 days old pea seedlings as shown in (Figure 2B) indicated that both of F. solani No3 and Alternaria solani No 3 isolated from Beljurashi province were found to be the most hostile fungi on the base of post-emergence damping-off giving 20% infection. Moreover, R. solani No2 and F. oxysporum No3 came the second order in this respect giving 16.67% infection of post-emergence damping-off. Regarding healthy survival plants, it is clear from the same Table and illustrated in (Figure 2C) the lowest values of healthy survival pea plants after 45 days from sowing occurred under infected with F. solani No3 (60%) accompanied by R. solani No2 equally with F. oxysporum No3 (63.33%). Moreover, Alternaria solani No3 came in the fourth order giving (68%). While other fungal isolates occurred a slight impact on this parameter. Based upon the seedling’s mortality after 30 days and survival plants after 45 days from sowing, F. oxysporum No3, F. solani No3, and R. solani No2 were the most hostile and selected for further studies. Figures in the exact column that are accompanied by the same letter or letters do not differ significantly (p 0.05).
Plant Extracts
The antifungal activity of plant extracts, i.e., Neem, Ginger, and Artemisia at concentrations of 0, 25, 50, 75, and 100% on fungal linear outgrowth of the examined pathogenic fungi are displayed in (Tables 3-3C) as well as illustrated in (Figures 3-3C). Data in (Table 3A) and (Figure 3A) showed an opposite correlation between raising the concentration of neem extract from 0 to 100% and the linear outgrowth rate of three examined pathogenic fungi. By other means, by raising the concentration of neem extract, the linear growth rate of examined pathogenic fungi decreased. Neem extract at both 75 and 100% concentrations prevented the development of F. solani. Moreover, the high concentration (100%) also wholly suppressed the linear growth of F. oxysporum. It induced more suppression in the linear outgrowth rate of F. solani in comparison with F. oxysporum in addition to R. solani.
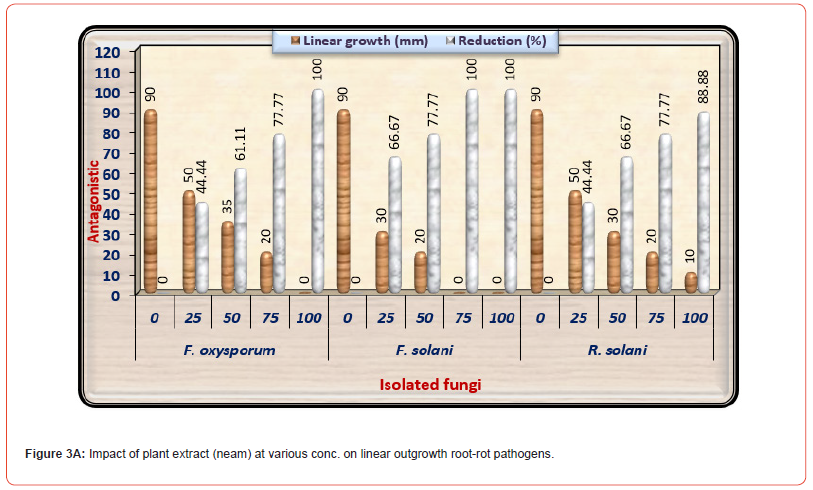
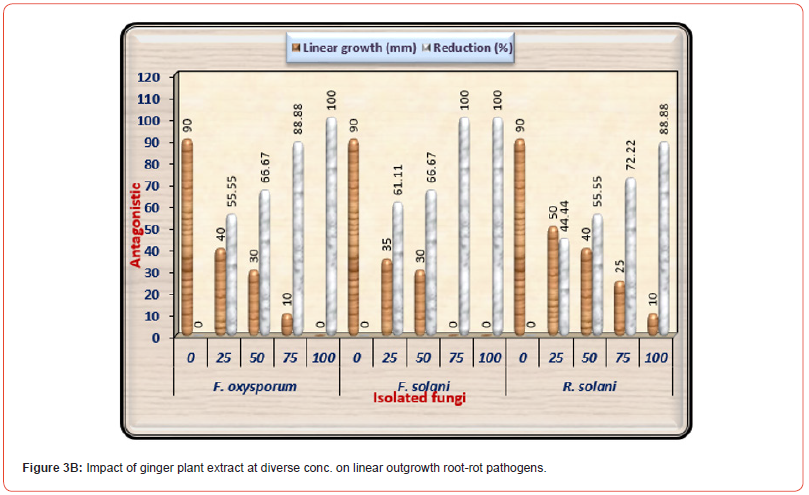
The results exhibit that the genus Rhizoctonia demonstrated greater tolerance for neem extract than the genus Fusarium. The impacts of various concentrations of Ginger plant extract on fungal linear outgrowth of the examined causal pathogens of pea root rot disease are displayed in (Table 3B) and illustrated in (Figure 3B). Results indicate that a gradual increase in the concentration of Ginger extract from 25% to 100% led to lowering in mycelium linear outgrowth of three examined pathogenic fungi. The minimum growth of three tested pathogenic fungi appeared under the high concentration of Ginger extract (100%) followed by the concentration of 75%. Ginger extract at high concentrations wholly suppressed the linear outgrowth of F. oxysporum in addition to F. solani. Furthermore, the 72-5% concentration wholly suppressed the linear outgrowth of F. solani. And it is worth noting that R. solani was more tolerant to Ginger plant extract than both species of Fusarium.
The antifungal activity of Artemisia plant extract at various conc. on linear outgrowth of pea root rot pathogens is presented in (Table 3C) and illustrated in (Figure 3C). There is a negative correlation between raising the concentration of this extract as well as the development of the examined pathogenic fungi. Also, there is a clear difference among pathogenic fungi in their impact on different concentrations of Artemisia plant extract. Artemisia extract at high concentration showed an increased effect as complete inhibition of mycelium linear growth of R. solani only. At the same time, the same concentration gives 88.88% and 77.77% reduction of F. solani and F. oxysporum linear growth, respectively. The results of plant extracts found that Artemisia extract was the least efficacious on the linear outgrowth of the examined pathogenic fungi, so it is neglected in the greenhouse experiment.
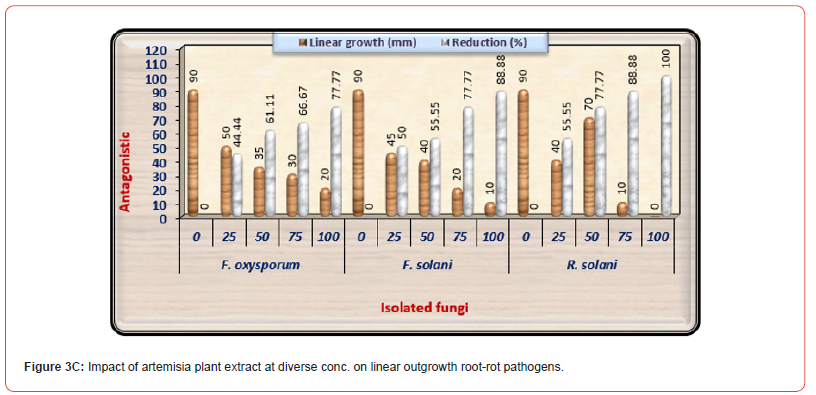
Table 3A:Impact of neem plant extract at various conc. on linear outgrowth of pea root- rot pathogens.
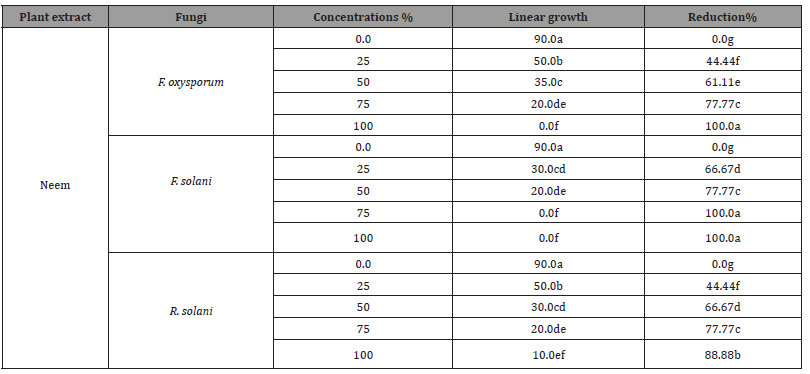
Table 3B:Impact of ginger plant extract at various concentrations on linear outgrowth of pea root- rot pathogens.
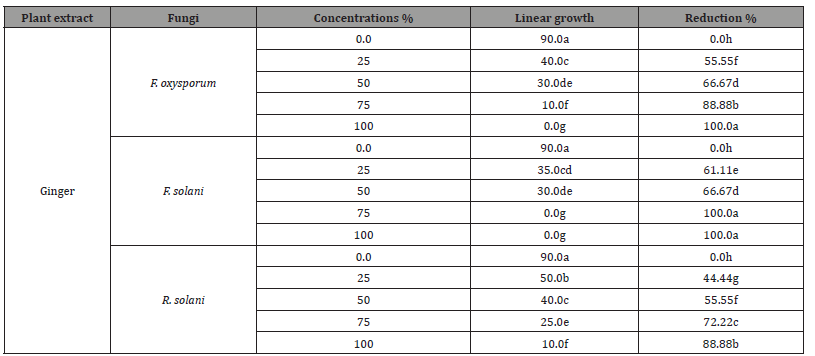
Table 3C:Impact of Artemisia plant extract diverse concentrations on linear outgrowth of pea root- rot pathogens.
Chemical Control
Effects of Tolex 500 wpm and Premium on the Linear Outgrowth of Pea Root Rot Pathogenic Fungi
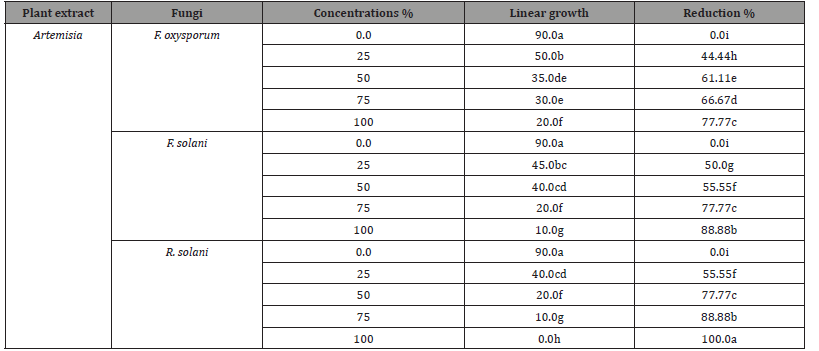
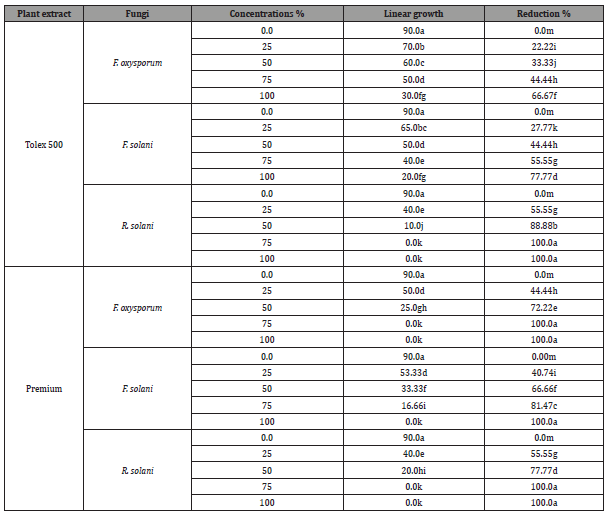
Results are displayed in (Table 4) and illustrated in (Figure 4) shows the effects of various concentrations of both chemical fungicides used on the linear outgrowth of the examined pathogenic fungi. It was observed that the impact of both chemical fungicides on the linear outgrowth rate of tested pathogenic fungi differed according to the type of fungicide, concentrations, and treated fungi. The reduction percentage rose as the fungicide concentration rises irrespective of fungal species. Generally, Premium fungicide was more efficacious in lowering the linear growth rate of all examined fungi than Tolex fungicide. Both high concentrations of Premium (75 and 100 ppm) prevented the outgrowth of F. oxysporum in addition to R. solani. While F. solani was less effective with the treatment of this fungicide, the high concentration only led to complete suppression of the linear growth rate of this fungus. On the other side, Tolex 500 wp fungicides at 100 and 75 ppm prevented the outgrowth of R. solani. And it is worth noting that F. oxysporum was less effective with Tolex 500 while R. solani was more sensitive. Based on these results, Tolex 500 and Premium at 100 ppm of both were used to evaluate under greenhouse conditions.
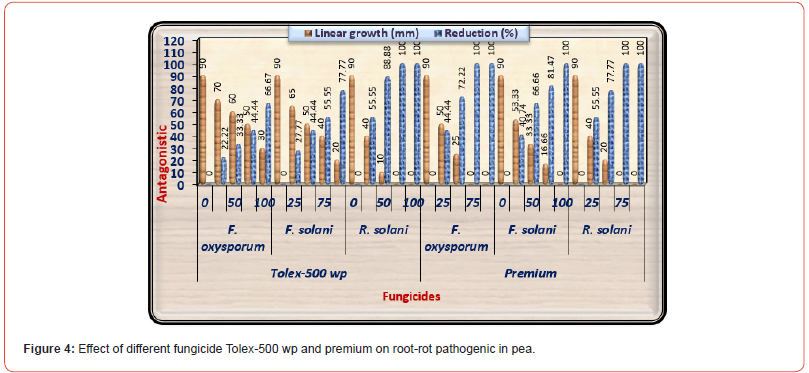
Table 4:Effect of fungicides (Tolex 500 wpm and premium) on linear outgrowth of pea root- rot pathogens.
Greenhouse Experiment
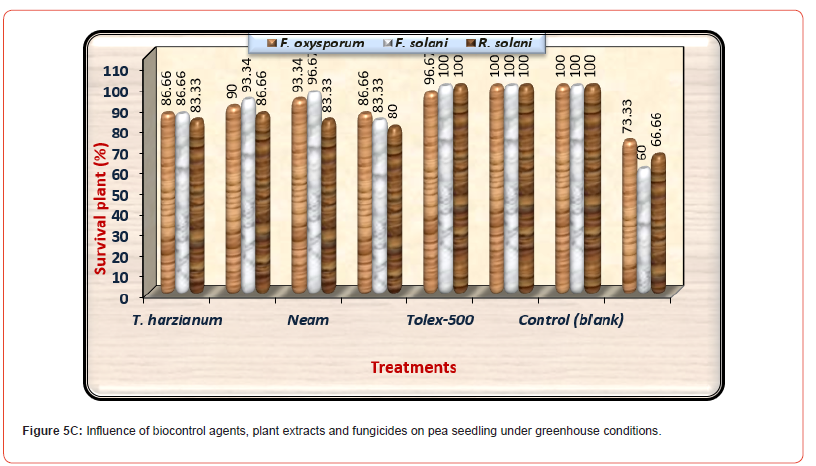
The purpose of this experiment was to examine the efficacy of seed the best treatments of plant extracts (Neem and Ginger at 100%), and fungicides (Tolex and Premium at 100 ppm) against the incidence of damping off on pea seedlings in addition to survival plants. The evaluation of both plant extracts, and chemical fungicide on disease prevalence (pre-emergence and post-emergence damping-off) of pea plants under infested with F. oxysporum, F. solani, and R. solani were presented in (Table 5) and illustrated in (Figures 5-5C). All tested treatments considerably lowered damping- off disease and significantly increased healthy survival plants compared with infected control under three tested pathogenic fungi. Generally, premium fungicide was more efficient in this regard, accompanied by Tolex 500 fungicide, then Neem extract, Ginger extract came at least in order without significant differences between them. Concerning healthy control (uninfected) grown in sterilized soil, it was observed that no recorded disease incidence.
Table 5:Influence of bio control agents, plant extracts and fungicides on pea seedling under greenhouse conditions.
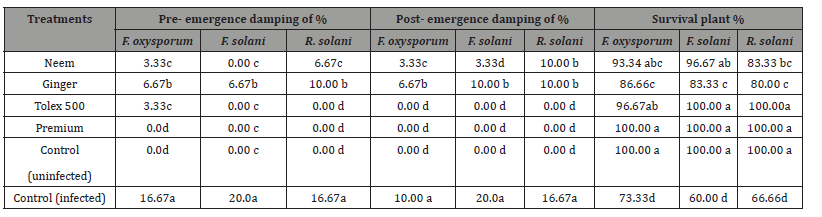
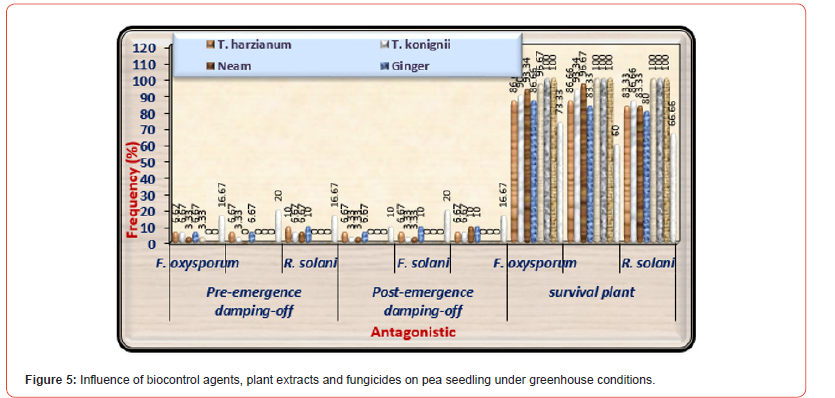
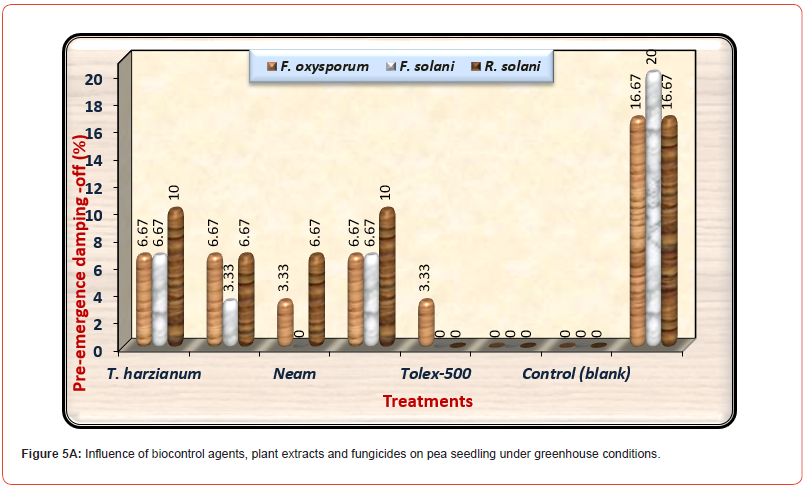
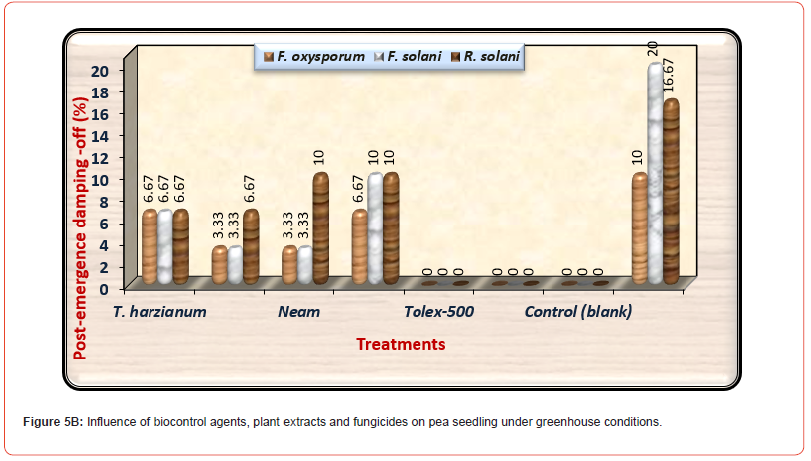
About survival plants, Premium fungicide nullified the effects of all pathogenic fungi on healthy survival plants leading to 100% healthy plants under-infested with the three pathogenic fungi. Moreover, Tolex 500 fungicide overcomes the harmful effects of F. solani and R. solani giving 100% healthy plants, but it is providing 96.67% healthy plants under infested with F. oxysporum. As shown in (Table 5) and (Figure 5A), pre-emergence damping-off of pea seedlings after fifteen days from sowing was decreased significantly due to the application of all treatments used at any dose under all infested soil by three tested pathogenic fungi as compared with infected control. Premium fungicide was more effective in this respect, where it completely inactivates the fungal pathogens, followed by Tolex 500. In relation to the effects of bio and natural additives on infected pea seedlings by pre-emergence damping-off, Neem extract was the most efficacious hence nullifying the injurious effect of F. solani. In contrast, both bio additions and Ginger extract come in the second order without significant differences. On the other side, both fungicides lead to the complete nullification of the tested pathogenic fungi in occurrence post-emergence damping- off, as shown in (Figure 5B).
Discussion
Pea (Pisum sativum L) is a crop high in protein and fixes atmospheric nitrogen. It is considered one of the plants that many countries cultivate, in addition to containing a high percentage of protein and carbohydrates and some vitamins. The pea plant is very important for its nutritional value for humans in addition to animals then plays a critical role in reducing cholesterol in the blood. Most countries in the world grow it for consumption in the form of vegetables because this plant is a significant source of protein and some fundamental elements. Through previous studies, it was found that several factors contribute to a decrease in the yield of the pea plant, like wilt diseases and root rot, which is considered the most effective disease because it is transmitted through the soil, which increases the damage resulting from many pathogens. Thus, these causes affect the growth of the crop and decrease Its production. The pea plant is considered one of the plants that are severely affected by root rot disease, which is transmitted through the soil [35].
Wilting and seedling death are diseases induced by many fungi present in the soil that cause diseases, such as Sclerotinia, Fusarium, Thielaviopsis, Aphanomyces, Rhizoctonia, Pythium and Phoma [36]. In this research, fifty isolates of root rot pathogens were isolated from the roots of pea plants exposed to natural infection from different sites in the Beljurashi and Bani Kabir governorates in the Kingdom of Saudi Arabia. 24 fungal isolates were isolated from Beljurashi in addition to 26 from the yard. It was observed that fungal species were identified from four species of three genera (Fusarium, Rhizoctonia, and Alternaria). In all, Alternaria solani recorded the highest number of fungal isolates, which gave 11 isolates in both counties together, while Fusarium oxysporum came in first place. The second order recorded ten isolates, accompanied by Fusarium solani then Rhizoctonia solani, which gave nine isolates for both. These outcomes matched those that were noted by [37,38].
The difference in the number, frequency, and species of fungal isolates according to the isolated province may outcome from the variation in soil texture, moisture, and composition of the soil, as well as the varying capability of fungal genera to thrive and adjust to diverse conditions. The present results of the pathogenicity tests study suggested that the twenty examined fungal isolates were pathogenic in addition to inducing pre- and post-emergence damping- off in pea seedlings. The largest proportion of pre-emergence damping-off (20.0%) happened under infected soil with F. solani No3 isolated from Beljurashi province and R. solani No2 isolated from Bani kabir Provence as well as F. oxysporum No3 isolated from Beljurashi province. While Alternaria solani No3 came in second order. On the contrary, findings obtained from the pathogenicity experiments conducted on thirty-day-old pea seedlings revealed that both F. solani No3 and Alternaria solani No3 isolated from Beljurashi province were found to be the most aggressive fungi because of post-emergence damping-off accompanied by R. solani No 2 then F. oxysporum No 3.
The lowest values of healthy survival pea plants after 45 days from sowing occurred under infected with F. solani No 3 accompanied by R. solani No2 equally with F. oxysporum No3. Generally, F. oxysporum No 3, F. solani No3, and R. solani No2 were the most aggressive and selected for further studies. These results may be due to genetic variance by many fungal isolates. These outcomes are consistent with those achieved by [39-42]. In addition, the vari ation in pathogenic ability by many fungal isolates could be due to genetic variations of them. The negative consequences of the tested pathogenic fungi are caused by seed rot and damage, in addition to root system death, which reduces the absorption surface or consumption of water in addition to essential nutrients [43]. This is agreed with the present study where the three examined pathogenic fungi induce the destruction of pea root structure, in turn, causes damping-off. Moreover, [44] resulted that the negative consequences of fungal pathogens may be caused by enzyme formation, which results in rotten lesions on seed cotyledons, seed rot, and plumule soft rot, which causes damping off.
As for plant extracts, the outcomes revealed that all extracts that were used had the ability to inhibit pathogens at any concentration. where there is a negative relationship between plant extracts and pathogens from zero to 100% f. In this regard, 100% neem and ginger were the most efficient, so it was used for the greenhouse experiment. Neem extract at a concentration of 75 and 100% suppressed the outgrowth of F. solani. Moreover, the high concentration (100%) suppressed the linear outgrowth of F. oxysporum. It caused more significant inhibition of the linear outgrowth of F. solani compared to F. oxysporum and R. solani. So ginger extract has been shown to increase the inhibition rate of pathogens of F. oxysporum in addition to F. solani completely. Also, the 5-72% concentration suppressed the linear outgrowth of F. solani. Under greenhouse conditions, neem extract was the most effective and thus had the most substantial effect on F. solani. It is also most effective in reducing damping off after pea emergence 30 days after planting under soil infested with both types of Fusarium.
These results are like those of [45] they found that garlic oil has antimicrobial in addition to antiviral activities [46] tested some oils for inhibition of the growth of many pathogens and found that garlic juice suppressed linear growth rate of F. moniliforme, Helminthosporium Oryza, Sclerotium bataticola, Penicillium italicum, Alternaria citri, Aspergillus niger as well as Clove juice, suppressed the formation of Macrophomina phaseolina sclerotia. Plant extracts can produce secondary metabolites, and some of them, in addition to their derivatives, have antimicrobial properties, like phenolic compounds, which may sensitise phospholipids, preventing fungi movement [47-50] used thyme, cumin, clove, and rosemary essential oils which prevented the spread of Aspergillus parasiticus and aflatoxin formation. The effectiveness of different plant extracts towards fungal pathogens differs due to their solubility in water and/ or the existence of inhibitors to fungi toxic principle in the composition of plant extract.
In this connection, [51] stated that garlic oil possesses antifungal as well as antioxidant properties due to includes sulfur, phenolic compounds, terpenoids, allicin, and saponins [52] stated that there is an antifungal activity of many plant extracts i.e., Garlic, Artimisia, Capsicum, Caraway, Fleabane, Cumin, Camphor, Anise, Black piper, and Thyme against Aspergillus niger and F. oxysporum the causal pathogens of onion rot. Some plant oils i.e., carawy, cumin, coriander, and fennel completely suppressed the linear outgrowth of Rhizoctonia solani, Fusarium oxysporum, and Sclerotium rolficii [53]. Also, seed treatment with garlic and neem extracts were the most efficacious treatment for dominating the damping-off disease of bean due to activated soil microbes, decreasing the population of R. solani [54,55]. When fungicides are used to study their effect on pathogens according to the type of fungicide and its concentration lowered the growth of pathogens. The growth rate of pathogens lowered with a rise in the concentration of the pesticide. Overall, the premium fungicide was more effective in lowering the linear spread of all fungi examined than the Tolex fungicide.
Both high concentrations of Premium (75 and 100 ppm) suppressed the spread of F. oxysporum in addition to R. solani. Tolex 500 wp fungicides at 100 and 75 ppm inhibited the spread of R. solani. It is noteworthy that F. oxysporum was less effective with Tolex 500 treatment while R. solani was more sensitive. Also, under the greenhouse, this premium fungicide was most efficacious in reducing damping-off disease, thus significantly increasing the healthy survival of the plants. The effects of the differences in chemical fungicides may be due to a selective reaction between the fungicide and the fungicide. These outcomes are rather like those presented by [56-58] and suggested that the utilization of a fungicide as a seed treatment reduces the incidence of root rot due to the anticipated decomposition of this fungicide when introduced. soil and its exposure to environmental conditions. The decrease in harmful effects of fungal pathogens with the use of both fungicides may be due to the active ingredient in the fungicide formulation degrading in the fungal medium and causing toxicity.
Conclusions and Recommendation
From the foregoing results and discussions, it is possible to conclude that the use of neem extract from plant extractions could be utilized as substitute methods and antifungal agents towards fungal pathogens (F. oxysporum, F. solani or R. solani) which were discovered to be associated to symptoms of pea damping off and root rot diseases.
References
- Abi Ghanem R, Bodah ET, Wood M, Braunwart K (2013) Potential breeding for high nitrogen fixation in Pisum sativum L: germplasm phenotypic characterization and genetic investigation. Am J Plant Sci 4: 1597-1600.
- Hassan AA (1997) Vegetable fruits. Al-Dar Al-Arabia Publications and distribution, Cairo, Egypt pp. 241.
- Knopp RH, Superko HR, Davidson M, Insull W, Dujovne CA, et al. (1999) Long-term blood cholesterol lowering effects of a dietary fiber supplement. Am J Prev Med 17(1): 18-23.
- Ali I, Patil RV, Dharmatti PR, Sridevi O (2018) Study on genetic parameters and performance of garden pea (Pisum sativum L.) genotypes for yield and its components (under northern transitional belt of Karnataka, India). Int J Curr Microbiol Appl Sci 7: 986-999.
- Igbasan FA, Guenter W, Slominski AB (1997) Field Peas: chemical composition and energy and amino acid availability for poultry. Canadian J Ani Sci 77: 293-300.
- Ahmed GJ, Mao Q, Yan Y, Wu M, Wang Y, et al. (2020) Role of melatonin in arbuscular mycorrhizal fungi-induced resistance to Fusarium wilt in cucumber. Phytopathology 110(5): 999-1009.
- Lamichhane JR, Dsrr C, Schwanck AA, Robin MH, Sarthou JP, et al. (2017) Integrated management of damping-off diseases. A review. Agron Sustain Dev 37(10): 1-25.
- Sinha KK, Osiyevskyy O (2018) The impact of founding team’s human capital on mean and variability of new venture growth. In Academy of Management Proceedings 2018(1): 11516.
- Muhanna N, El-wan S, Dib N (2018) Biological Control of Root Rot Complex of Pea (Pisum sativum L). Egypt J Phytopathol 46 (1): 49-67.
- Ketta HA, El-Khateeb NM, Saleh MM, Kamel SM (2021) Efficiency assessment of combinations between Rhizobium leguminosarum and Trichoderma spp. for controlling of pea (Pisum sativum L.) damping-off disease. Egypt J Phytopathol 49(1): 1-14.
- Ibrahim (2022) Controlling of Fungal Root Diseases of Pea (Pisum sativum L.) by Safe Alternatives Fungicides in Egypt and Ethiopia Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy.
- Kripalini MKB, Sobita D (2018) Studies on Morphological, Cultural and Pathogenic Variability in Isolates of Fusarium oxysporumsp. pisi causing Wilt of Pea from different Districts of Manipur. In Int J Curr Microbiol App Sci 7(11): 2500-2506.
- Abdulaziz A Al-Askar, Younes M Rashad (2010) Efficacy of some plant extracts against Rhizoctonia solani on pea. Journal of Plant Protection Research 50(3).
- Bhumika K, D Prashantd, B Sanghmitra, RK Dantre, KP Verma (2014) Efficacy of plants leaf extracts on mycelia growth and sclerotial production of Rhizoctonia solani causing web blight of groundnut. International Journal of Plant Protection 7(1): 272-274.
- Nupur S, V Rashmi (2015) Bioefficacy of plant extracts in the control of root rot disease of sponge gourd. Journal Indian bot Soc 94(1&2): 126-130.
- Abdurrahman O, DS Hayriye (2016) Antifungal Activity of Some Plant Extracts against Different Plant Pathogenic Fungi. Journal of Advances in Agricultural & Environmental Eng 3(2): 1523-1531.
- Debjani C, Yumlembam RA, Susamoy K, Ranjan N, Ramen KK, et al. (2017) Effect of plant extracts against sheath blight of rice caused by Rhizoctonia Solani. Journal of Pharmacognosy and Phytochemistry 6(4): 399-404.
- A Ejaz, S Dawar, A Hanif (2017) Management of root rot fungi of crop plants by Moringa oleifera lam. Pakistan Journal of Botany 49(3): 1201-1209.
- M Goss, P Mafongoya, A Gubba (2017) Moringa oleifera Extracts Effect on Fusarium solani and Rhizoctonia solani Asian Research Journal of Agriculture 6(1): 1-10.
- Samar S, Abdel-Hafez, IN Ali, MA EL-Korashy, MI Ismaiel (2021) Effectiveness of some Plant Extracts as Safe Control Means against Damping-off and Root Rot Diseases in Canola Plants. Journal of Applied Plant Protection. Suez Canal University 10 (1): 21-27.
- Dutta S, Barman A Roy, Hembram S, Kuiry SP, Ghosh PP, et al. (2013) Integrated management of Rhizoctonia root rot of pea. Ann Plant Protect Sci 21: 118-20.
- Chryseis T Modderman, Samuel M (2018) Efficacy of In-Furrow Fungicides for Management of Field Pea Root Rot Caused by Fusarium avenaceum and F. solani Under Greenhouse and Field Condition. Plant Health Progress 19(3): 212-219.
- FRAC (2018) Fungicides sorted by mode of action (including FRAC code numbering).
- Saman Aslam, Muhammad U Ghazanfar, Nida Munir, Muhammad I Hamid (2019) Managing fusarium wilt of pea by utilizing different application methods of fungicides. Pakistan Journal of Phytopathology 31(1): 81-88.
- Dhingra OD, Sinclair JB (1985) Basic plant pathology methods. CRC Press, Inc. Boca Raton, Florida, USA.
- Gilman JC (1957) A manual of soil fungi. 2nd. Iowa Univ Press Ames, pp. 450.
- Booth C (1985) The genus Fusarium Commonwealth Mycological Institute Kew. Surrey, England, pp. 237.
- Barnett HL, Hunter BB (1972) Illustrated Genera of Imperfect Fungi, Burgen Publishing Co. Minnesota pp. 241.
- Robinson JM, SJ Britz (2000) Tolerance of a field grown soybean cultivar to elevated ozon level is concurrent with higher leaflet ascorbic acid level, higher ascorbate-dehydroascorbate redox status andlong-term photosynthetic productivity. Photosynth Res 64(1): 77-87.
- Whitenhead MD (1957) Sorghum is a medium suitable for the increase of inoculums for studies of soil-borne and certain other fungi. Phytopathology 47: 450.
- Metwelly MMM (2004) Resistance induction against diseases of faba bean crop. Ph.D. Thesis, Plant Pathol Fac Agric Suez Canal Univ, Egypt.
- El-Helaly AF, Elarosi HM, Assawah MW, Abd-Wafa MT (1970) Studies on damping-off and root-rots of bean in UAR (Egypt). Egypt J Phytopathology 2: 41-57.
- Pattnaik MM, Kar M, RK Sahu (2012) Bio-efficacy of some plant extract on growth parameters and control diseases in Lycoperscicum esculentum. Asian J of Plant Sci Research 2(2): 129s-s142.
- CoStat(2005) Cohort Software. 798 Lighthouse Ave. PMB 320 Monterey, USA.
- Grünwald NJ, Chen W, Larsen RC (2004) Pea diseases and their management. In: Disease Diagnosis and Management of Fruits and Vegetables. Naqvi SAMH, Mukerji KG, (Eds,.), Kluwer Academic Publishers, Dordrecht, the Netherlands, pp. 301-331.
- Kraft JM, Pfleger FL (2001) Compendium of Pea Diseases and Pests. 2nd ed, The APS Press, St Paul, MN, USA, pp. 110.
- Naglaa Muhanna AS, Safa Elwan E, Nsreen Dib D (2018) Biological Control of Root Rot Complex of Pea (Pisum sativum L.). Egypt J Phytopathol 46(1): 49-67.
- El-Nady IAI (2022) Controlling of Fungal Root Diseases of Pea (Pisum sativum L.) by Safe Alternatives Fungicides in Egypt and Ethiopia. Ph D. Theses, Department of Natural Resources, Faculty of African Postgraduate Studies, Cairo University, Egypt.
- Persson L, Bødker L, Larsson-Wikström M (1997) Prevalence and pathogenicity of foot and root rot pathogens of pea in Southern Scandinavia. Plant Disease 81(2): 171-174.
- Xue AG (2003) Biological Control of pathogens causing root rot complex in field pea using Clonostachys rosea strain ACM941. Phytopathology 93(3): 329-335.
- Riad SR, Mahmoud M Hamed (2008) Effect of Seed Treatment on Control of Root Rot Disease and Improvement of Growth and Yield of Pea Plants. Middle Eastern and Russian Journal of Plant Science and Biotechnology 2(2): 84-90.
- Nazir N, ZA Badri, NA Bhat, FA Bhat (2022) Effect of the combination of biological, chemical control and agronomic technique in integrated management pea root rot and its productivity. Scientific Reports 12(1): 11348.
- Porter DM, DH Smith, R edsrodriguez-Kabana (1990) Compendium of peanut diseases. American Phyto pathological Society, St. Paul, MN. USA.
- Mahmoud SYM, MH Hosseny, KAA Shaikh, AHA Obiaalla, YA Mohamed (2013) Seed borne fungal pathogens associated with common bean (Phaseols vulgaris L.) seeds and their impact on germination. J Enviro Studies 11(1): 19-26.
- Seow YX, CR Yeo, HL Chung, HG Yuk (2014) Plant essential oils as active antimicrobial agents. Crit Rev Food Sci Nutr 54(5): 625-644.
- Radwan IA (1980) Studies on storage diseases of garlic in the ARE. Ph. D. Theses, Fac of Agric, Zagazig Univ, Egypt.
- El-Mougy NS, Abdel-Kader MM (2007) Antifungal effect of powdered spices and their extracts on growth and activity of some fungi in relation to damping off disease control. J Plant Prot Res 47(3): 266-278.
- Qari SH (2008) In vitro evaluation of the antimutagenic effect of Origanum majorana extract on the meristemetic root cells of Vicia faba. J Taibah Univ Sci 1: 6-10.
- Sarhan Ehab, El-Far EMM, Ebrahiem AMY (2018) Systemic resistance in snap bean (Phaseolus vulgaris L.) elicited by some chemicals and biotic inducers against white mold disease caused by Sclerotinia sclerotiorum. Egypt J Phytopathology 46(2): 61-84.
- Farag RS, ZY Dawz, FM Hewedi, GS El-Barotyl (1989) Antimicrobial activity of some Egyptian spice essential oils. J Food Prot 52(9): 665-667.
- Rivlin RS (2001) Historical perspective on the use of garlic. J Nut 131(3s): 591s-594s.
- El-Saeed AAA (2004) Studies on post-harvest rot of onion (Allium cepa L.) in Egypt. Ph D Thesis, Faculty of Agric, Mansoura University, Egypt.
- Fahmy MS (1994) Biochemical studies on some medicinal plants cultivated in Egypt. M. Sc. Thesis, Faculty of Agric, Mansoura University, Egypt.
- Morsy SM, Drgham EA, Mohamed GM (2009) Effect of garlic and onion extracts or their intercropping on suppressing damping-off and powdery mildew diseases and growth characteristics of cucumber. Egypt. J. Phytopathology 37(1): 35-46.
- Ahmed MFA (2013) Studies on non-chemical methods to control some soil borne fungal diseases of bean plants (Phaseolus vulgaris L.). Ph.D. Thesis Fac of Agric, Cairo Univ, Egypt, pp. 141.
- Ismail AEA (1994) Effect of intercropping of soybean and maize plants on incidence of some soil fungal diseases. Ph.D. Thesis Fac Agric, Zagazig Univ.
- Abd El-Hai KM, Ali AA (2017) Down-regulation of damping-off and root rot diseases in lentil using kinetin and Trichoderma. J Plant Protection and Pathology 8(2): 45-54.
- Abd El-Hai KM, Ali AA (2019) Formulation of Trichoderma, Saccharomyces and Rhizobium metabolites against damping-off and root rot pathogens in peanut plants. Asian J Biol Sci 12(2): 114-112.
-
Sahar A ELSayed*, Anwar M Aloufi. Efficiency of Plant Extracts and Fungicides in Controlling Root Rot Diseases in Peas. Open J Pathol Toxicol Res. 1(5): 2024. OJPTR.MS.ID.000522.
-
Pathogenic fungi; chemical control; plant extracts; pea plants; iris publishers; iris publisher’s group
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






