 Research Article
Research Article
P53 And P16 Protein Expression and Survival in Patients with Squamous Cell Carcinoma of the Oral Cavity
Maria da Graça C Vidal1*, Onivaldo Cervantes2 and Alessandra S Bastianello3
1MSc, Chief of Head and Neck Surgery, Hospital Universitário de Santa Maria (HUSM), Av. Roraima, 1000, Camobi, 97105-900, Santa Maria, RS, Brazil
2PhD, Professor, Head and Neck and Ear, Nose and Throat Department, Universidade Federal de São Paulo (UNIFESP), R. Sena Madureira, 1500, 04021-001, São Paulo, SP, Brazil
3Medical Pathologist, Division of Pathology, HUSM, Av. Roraima, 1000, Camobi, 97105-900, Santa Maria, RS, Brazil
Maria da Graça Vidal, MSc, Chief of Head and Neck Surgery, Hospital Universitário de Santa Maria (HUSM), Av. Roraima, 1000, Camobi, 97105-900, Santa Maria, RS, Brazil.
Received Date:March 03, 2024; Published Date:March 20, 2024
Abstract
Objective: To examine p53 and p16 protein expression in patients with squamous cell carcinoma (SCC) of the oral cavity and to determine the influence of each protein and their association with survival.
Methods: We immunohistochemically assessed p53 and p16 expression on tumor sections from 69 patients with oral cavity SCC treated at our university hospital, between January 2014 and January 2018.
Results: Expression of p53 was observed in 69.6% of the patients, and of p16 in 30.4%. A positive p16 expression was less commonly found in the tongue and/or floor of the mouth (p = 0.0015). Patients with p53-positive tumors were 3.2 times more likely to die of the disease, whereas those with p16-positive tumors were 0.7 times more likely to die.
Conclusion: According to these results at primary sites, both p53 and p16 are relevant biomarkers, where p53 serves as a risk marker and p16 as a protective marker in oral cavity SCC.
Keywords:Mouth neoplasms; Squamous cell carcinoma of head and neck; Survival; Biomarkers; Prognosis
Introduction
Squamous cell carcinoma (SCC) of the oral cavity is a disease with high morbidity and mortality due to late-stage diagnosis, especially in Latin American countries such as Brazil [1, 2]. Traditional risk factors, such as smoking and alcohol consumption, are the main culprits due to the DNA damage that they cause in oral mucosal cells [3-5]. Specific biomarkers that have been investigated in the pathogenesis of oral cavity SCC may play a role in disease progression and, consequently, in treatment stratification, which could prevent major sequelae or treatment failure [6-8].
Tumor suppressor proteins p53 and p16 have been extensively studied in oral and oropharyngeal carcinogenesis [9-13]. The TP53 gene, mapped within chromosome band 17p13, encodes p53, which is involved in many cellular functions, including genomic stability, cell cycle progression, cell differentiation, DNA damage repair, and apoptosis [14-17]. Irreparable cellular damage due to continued cellular aggression can lead to a variety of p53 mutations, which result in its increased expression in cells [18-20]. One type of mutation is the transformation of this tumor suppressor into an oncogenic factor [21]. p53 mutations can result in greater resistance to radiotherapy, more infiltrative locoregional disease, greater recurrence, and more deaths [22, 23]. Nevertheless, favorable results with good treatment response have been reported in the presence of increased expression of p53 protein [24]. Increased p53 expression in SCC is closely related to smoking and alcohol consumption [25-27].
Cyclin-dependent kinase inhibitor 2A (CDKN2A), a tumor suppressor gene located on 9p21 that belongs to the family of cyclin-dependent kinase inhibitors, encodes p16, which is closely related to HPV16 when expressed above 70% in cell nucleus and cytoplasm [28-30]. Inhibition of the cyclin D1-CDK4/6 complex disrupts retinoblastoma (Rb) protein phosphorylation and releases E2F transcription factors, which inhibit the cell cycle in the G1-to-S phase transition [31-33]. Genetic alterations in p16 can contribute to cell growth, thus promoting tumorigenesis. Overexpression of p16 protein, corresponding to HPV16, has been most evident in oropharyngeal carcinoma and is associated with better prognosis [32-34]. In general, it is found in younger populations with different lifestyles, but it is not necessarily associated with smoking and/or alcohol consumption [33].
The present study aimed to examine the expression of p53 and p16 proteins in 69 patients with a diagnosis of oral cavity SCC treated at the hospital, between January 2014 and January 2018 and to determine the association between these proteins and survival.
Methods
This prospective longitudinal observational study is part of a line of research and doctoral dissertation that was approved by the ethics committee of the Universidade Federal de Santa Maria (Santa Maria, RS, Brazil) (CAAE 446429.156.0000.53.46). The study was conducted in accordance with the Helsinki Declaration.
We analyzed the expression of p53 and p16 proteins and their prognostic relationship in 69 patients with oral cavity SCC selected from a larger sample of 237 patients with carcinoma of the upper respiratory and digestive tracts (among the remaining 168 patients, 79 had laryngeal carcinoma and 89 had oropharyngeal and hypopharyngeal carcinoma). The tumors were staged according to the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging system [35]. Treatment was implemented according to tumor stage and surgical protocols from the medical literature, associated or not with radiotherapy and/or chemotherapy.
Written informed consent was obtained from each study participant. This document also ensured patient confidentiality and made it clear that the purpose of the study was to examine p53 and p16 protein expression in oral cancer and to determine its association with clinical presentation and survival. Follow-up was conducted clinically or by active search for those patients who were absent from subsequent consultations.
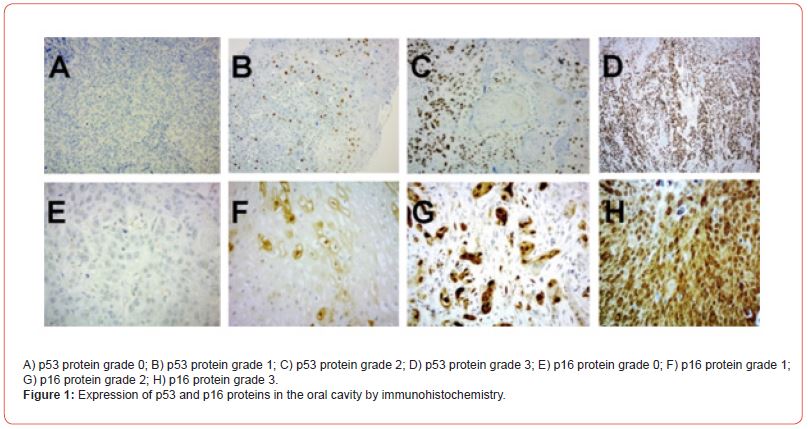
For all 69 participants with oral cavity SCC, we performed immunohistochemical analysis of p53 and p16 expression on primary site biopsy samples or surgical specimens using a Benchmark GX automated system (Roche, Basel, Switzerland). The nuclear staining of p53 and both nuclear and cytoplasmic staining of p16 were titrated and scored as follows: negative or grade 0 (up to 30% positivity for p53 and/or p16); grade 1 (31% to 50% positivity for either protein); grade 2 (51% to 70% positivity for either protein); and grade 3 (more than 70% positivity for either protein) (Figure 1) [36, 37].
We performed comparative analyses using the chi-square test. We analyzed factors associated with the occurrence of deaths by univariate Cox regression analysis to obtain the hazard ratio with its 95% confidence interval. We estimated survival using the Kaplan-Meier method and compared survival curves by the logrank test. We analyzed the data in SPSS 19 (IBM Corp., Armonk, NY, 2010) and R (R Core Team, Vienna, Austria, 2018), and set the level of statistical significance at 5%.
Patients were analyzed only if there were records from the first consultation to the last update or a death record, adequate immunohistochemistry, outpatient clinical follow-up, and written informed consent and authorization forms. Patients who were lost to follow-up, those with metastatic carcinoma with no known primary site, and those with inadequate immunohistochemistry with no possibility of repeating the test were excluded from the study.
Results
Mean patient age was 60.7 years (median, 60 years), and most patients were men (n = 51, 73.9%). A total of 57 patients (82.6%) were current/previous smokers and 41 (59.4%) were current/previous drinkers. Habitual consumption of maté (a hot tea-like beverage brewed from the dried and minced leaves of Ilex paraguariensis) was an added factor in 65 patients (94.2%).
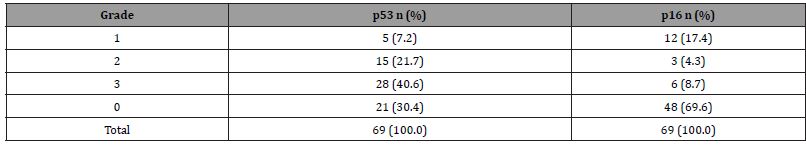
Forty-eight cases (69.6%) showed a positive p53 expression; grade 3 staining was observed in 28 (40.6%). Twenty-one cases (30.4%) showed a positive p16 expression, whereas 48 (69.6%) showed a negative expression (grade 0 staining) (Table 1).
Table 1:Expression of p53 and p16 proteins in patients with squamous cell carcinoma of the oral cavity (n = 69).
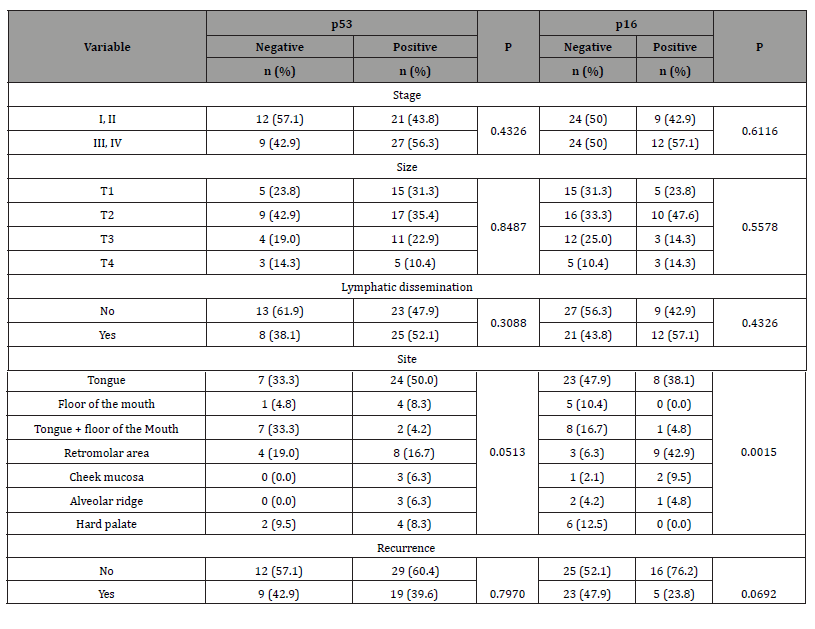
When analyzing the p53 protein, we observed a more frequent presence in the tongue and/or floor of the mouth (30 cases, 62.5%) than in other subsites (18 cases, 37.5%), although without statistical significance (p = 0.051). We did not find any statistical difference between positive p53 expression and tumor size (p = 0.848), lymphatic dissemination (p = 0.308), and recurrence (p = 0.797) (Table 2).
Table 2:Clinical presentation of squamous cell carcinoma of the oral cavity and association with the expression of p53 and p16 proteins.
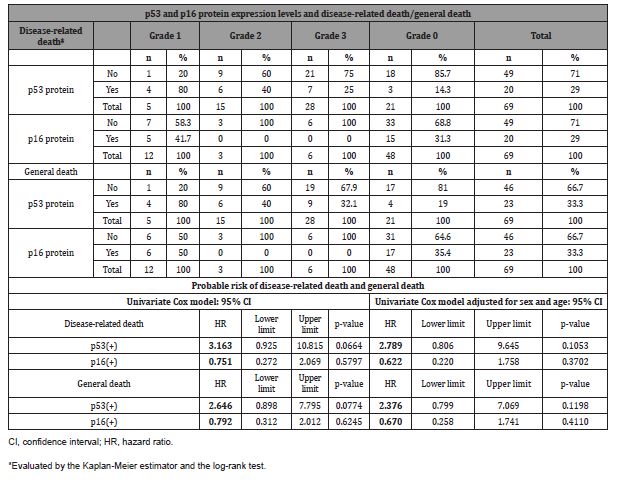
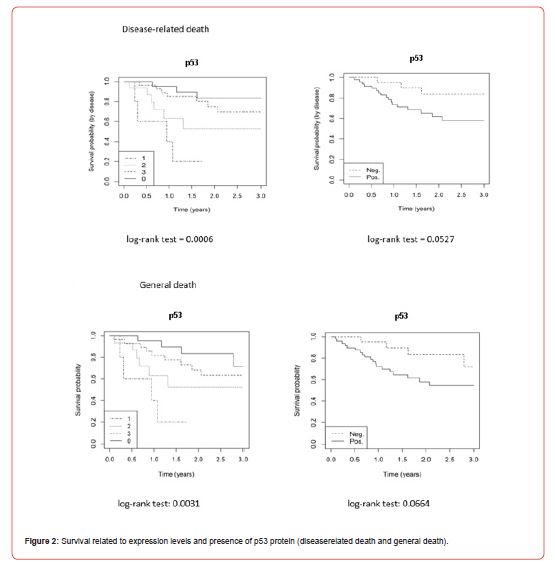
In the analysis of p53 protein and survival, statistical significance was not reached despite a higher frequency of deaths with p53 protein for both disease-related death (log-rank test = 0.0527) and general death (log-rank test = 0.0664). In the analysis of the expression levels of p53 protein, we obtained a statistical difference for disease-related death (log-rank test = 0.0006) and general death (log-rank test = 0.003), with p53-negative patients having the best survival. The probable risk of disease-related death with the p53 protein was 3.2 (p = 0.0664) and 2.8 (p = 0.1053) when adjusted for sex and age, while the risk of general death was 2.6 (p = 0.0774) and 2.4 (p = 0.1198) when adjusted for sex and age (Table 3, Figure 2).
Table 3:Number of deaths according to p53 and p16 protein expression levels and probable risk of death related to p53 and p16 protein expression.
The analysis of p16 protein showed that its low frequency in the tongue and/or floor of the mouth was statistically significant (p = 0.0015). The most frequent subsite was the retromolar area (n = 9, 42.9%). There was no statistical significance regarding tumor size (p = 0.557), lymphatic dissemination (p = 0.432), recurrence (p = 0.069), and staging (p = 0.611) with the p16 protein (Table 2).
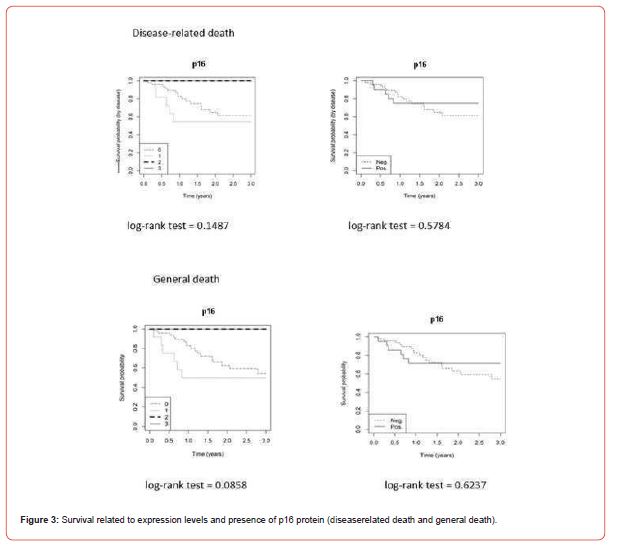
Regarding survival, the presence of p16 protein was not significant in disease-related death (log-rank test = 0.5784) and general death (log-rank test = 0.6237), and there was no significance between expression levels for disease-related death (log-rank test = 0.1487) and general death (log-rank test = 0.0858). The probable risk of disease-related death with the p16 protein was 0.7 (p = 0.5797) and 0.6 (p = 0.3702) when adjusted for sex and age, while the risk of general death was 0.8 (p = 0.6245) and 0.7 (p = 0.4110) when adjusted for sex and age (Table 3, Figure 3). None of the p16- positive patients classified as grade 2 or grade 3 died of the disease or general death.
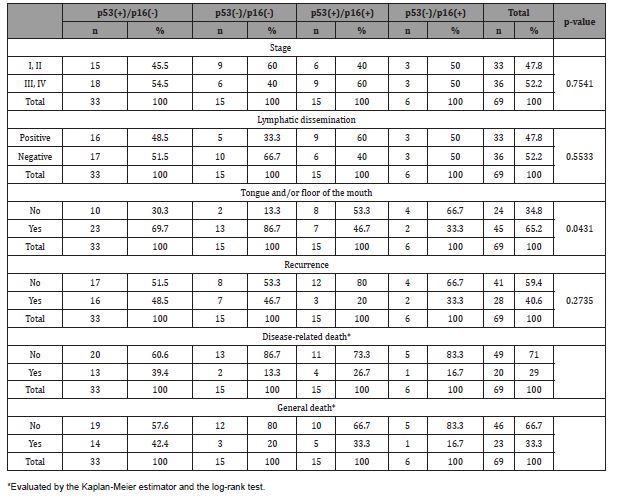
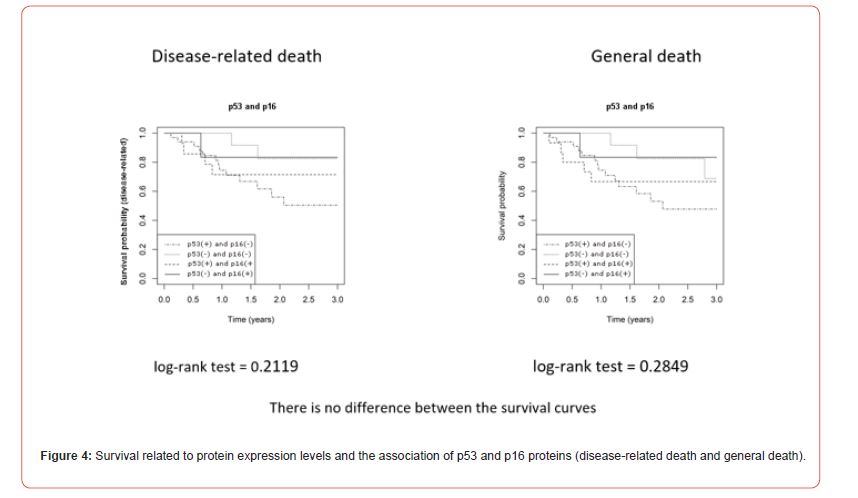
We identified different levels of concomitant expression of p53 and p16 in patients classified as grade 0 to 3 (Table 4). In the concomitant analysis of the expression of p53 and p16 proteins, we found 15 patients with positive and negative proteins for each association. We observed 33 patients with positive p53 protein alone and only 6 patients with positive p16 protein. We obtained statistical significance in the tongue and/or floor of the mouth (p = 0.0431) for negative proteins, but no correspondence for staging (p = 0.7541), lymphatic dissemination (p = 0.5533), recurrence (p = 0.2735), disease-related death (log-rank test = 0.2119), and general death (log-rank test = 0.2849) (Table 5, Figure 4).
Table 4:Comparison of p53 and p16 protein expression.
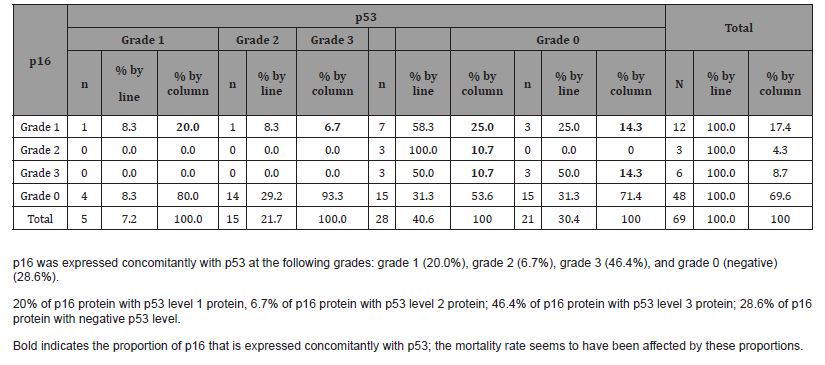
Table 5:Association of categorical variables with p16 and p53 proteins.
Discussion
The p53 protein has been widely studied for its tumor suppressive action in response to molecular damage to DNA by promoting DNA repair or apoptosis while inhibiting the G1-to-S phase transition, and for its oncogenic activity through the development of mutations in its codons [19-21, 23]. Mutated forms of p53 also have the ability to interact with the wildtype protein to prevent tumor suppression, a phenomenon known as “dominant negative effect,” where a mutation in one of the TP53 alleles produces what appears to be a dominant effect over the remaining normal allele [21-23, 27]. This occurs mainly through the action of carcinogenic agents from smoking and alcohol consumption [20, 25]. TP53 mutations are considered an early event in carcinogenesis of head and neck SCC [19-21, 25-27]. TP53 mutations have been identified in 70.4% of head and neck cancer tumors [21-23]. Therefore, the presence of p53 alterations may represent a risk in the process of oral carcinogenesis, with a guarded prognosis due to the loss of tumor suppressor function [18-23]. Using immunohistochemistry, we observed grade 3 of p53 protein in 28 (40.6%) cases, which shows its action from the beginning of carcinogenesis to more advanced stages, although there was no statistical significance between the variables staging (p = 0.432), tumor size I-IV (p = 0.848), lymphatic dissemination (p = 0.308), and recurrence (p = 0.797) [22, 27]. The most common sites were the tongue and/or floor of the mouth (n = 30, 62.5%), probably due to greater exposure to smoking, drinking, and/or mate [4, 11, 20, 25].
In terms of survival, we found a greater tendency for diseaserelated death (log-rank test = 0.0527) and general death (log-rank test = 0.066) in the presence of p53, although not relevant. Of note, among p53-positive patients, the best survival was achieved in those classified as grade 3 (7 deaths, 25%), and the worst survival was observed in those classified as grade 2 (6 deaths, 40%) and grade 1 (4 deaths, 80%), when we consider disease-related death [27]. There was a significant difference between them (p = 0.0006). The same was observed in the analysis of general death with p53 protein referring to grade 1 (4 deaths, 80%), 2 (6 deaths, 40%), and 3 (9 deaths, 32.1%) staining, with statistical significance (p = 0.0031) [26, 27, 38]. Survival was impressive among p53-positive patients because of the greater number of deaths that occurred in grade 1 staining. This impact may be due to the greater number of deaths in the lowest p53 protein grade, in which, proportionally, the number of patients is smaller. There is also the possibility of oncogenic mutations that compromise the suppressive activity in advanced disease [27, 39]. The majority of these patients were in advanced stage (80% in IV; 20% in III) [26, 27, 39]. Depending on the type of oncogenic mutation, there may be a greater expression or absence of p53 protein, especially in more undifferentiated neoplasms [21-23, 27].
The presence of the p16 protein, which belongs to the family of cyclin-dependent kinase inhibitors, has long been associated with a favorable prognosis due to its ability to inhibit the cell cycle in G1-S phase [28]. It is agreed that an expression greater than 70% is related to HPV16 [29-33]. That is, higher p16 protein intensity is associated with a better prognosis, especially in the oropharynx [31- 33]. An overexpression of p16 protein favors the early stages, with less recurrence and better survival [31, 40-45]. In the present study, most patients (n = 48, 69.6%) showed a negative p16 expression (grade 0 staining). When detected, the staining of p16 was graded as 1 in most cases (n = 12, 17.4%), followed by grade 3 (n = 6, 8.7%) and grade 2 (n = 3, 4.3%). We highlight the low frequency of HPV16- related grade 3 staining of p16 protein, found in 6 (8.7%) of our patients [29, 45]. Regarding primary site, a positive p16 expression was most commonly found in the retromolar area (n = 9, 42.9%), near the oropharynx, consistent with the results of Castellsagué, et al. [45], who reported a substantially lower p16 expression in the tongue and floor of the mouth (n = 1, 4.8%) (p = 0.0015) [34, 41, 42].
There was no significant association of p16 protein expression with tumor size (p = 0.557), lymphatic dissemination (p = 0.4326), staging (p = 0.6116), number of relapses (n = 5, 23.8%; p = 0.069), disease-related death (n = 5, 23.8%; log-rank test = 0.578), or general death (n = 6; 28.6%; log-rank test = 0.6237) The lowest rate of relapses and deaths was observed in the presence of the p16 protein. Most disease-related deaths occurred in patients with p16 expression graded as 0 (n = 15, 31.3%) and 1 (n = 5, 41.7%) [42- 44]. The same was observed for general death in patients with p16 expression graded as 0 (n = 17, 35.4%) and 1 (n = 6, 50.0%). There were no disease-related or general deaths in grade 2 or 3 staining. Our results favor a protective role of p16, similar to what has been reported by other authors [46-50].
We identified a greater association of grade 0 staining of proteins in the tongue and/or floor of the mouth (p = 0.0431), which may suggest less mutagenic potential in carcinogenesis, as there was a trend towards earlier stages, less lymphatic dissemination or relapses, and better survival (Table 5) [20, 38, 51].
We assessed the concomitant expression of p53 and p16 proteins at the different levels that can characterize risk and protection, respectively, in the analysis of patient survival (Table 4). In patients whose tumors were p53 grade 1 by immunohistochemistry, we observed the lowest number and grade of p16 protein (grade 1), accounting for 20% of cases. While patients whose tumors were grade 3 p53 protein by immunohistochemistry, there was a greater proportion of p16 protein in their different staining grades, about 46.4% of the cases (grade 1 in 25.0%, grade 2 in 10.7%, and grade 3 in 10.7%). This result seems to indicate a more aggressive oncogenic action in the lower expression of the p53 protein (grade 1), as well as a lower tumor suppressive action in the lower expression of the p16 protein (grade 1)—contrary to the higher expression of p16, especially grades 2 and 3, which favor patient survival [49, 50, 52]. In the analysis of the p53 protein, we must consider that there are more mutant forms than wild-type forms of p53, particularly in the absence of the p16 protein linked to HPV16 (p = 0.0015), which corroborates the molecular changes in oral carcinogenesis [38-44, 53]. In this study, p53 protein expression served as a risk factor, leading to a 3.2 times higher likelihood of progression to disease-related death. With p16 protein expression, patients were 0.7 times more likely to progress to disease-related death, which is characteristic of a protective profile [39-44, 50, 52, 53].
Despite some limitations such as absence of genetic sequencing of p53 and p16 proteins, non-identification of HPV16, and small sample size, both p53 and p16 proteins were shown to be potential biomarkers [49-52]. The p53 protein appeared to show a decrease in its suppressive activity, whereas the p16 protein, in grades 2 and 3, acquired a regulatory role in cell proliferation, improving the prognosis [49, 50, 52]. Possibly, future studies with a larger number of patients will determine their influence as prognostic markers [54, 55].
Conclusion
In patients with oral cavity SCC, both p53 and p16 proteins were relevant for survival. p53 was associated with risk of death even at lower levels of expression, probably due to mutations and loss of tumor suppressor function. For p16, higher expression was associated with greater protection, since it acts as a tumor suppressor. Also, p16-positive patients survived longer.
Data Availability
All relevant data are within the paper.
Declaration of Conflicting Interests
The Authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contributions
Conceptualization: MGCV, OC, ASB; Data curation: MGCV, OC, ASB; Formal analysis: MGCV, OC, ASB Investigation: MGCV, OC, ASB; Methodology: MGCV, OC, ASB; Project administration: MGCV, OC, ASB Resources: MGCV, OC, ASB; Supervision: MGCV, OC, ASB; Validation: MGCV, OC, ASB; Visualization: MGCV, OC, ASB; Roles/ Writing-original draft: MGCV, OC, ASB; Writing-review & editing: MGCV, OC, ASB
References
- S Perdomo, G Martin Roa, P Brennan, D Forman, MS Sierra (2016) Head and neck cancer burden and preventive measures in Central and South America. Cancer Epidemiol 44 Suppl 1: S43-S52.
- J Colombo, P Rahal (2009) Genetic changes in head and neck cancer. Rev Bras Cancerol 55: 165-174.
- MT Ruiz, E Pavarino Bertelli, JV Maniglia, MJ Ruback, EM Goloni Bertollo (2006) Head and Neck cancer epidemiology and biomarkers. Arq Cienc Saude 13: 34-38.
- D Goldenberg, A Golz, HZ Joachims (2003) The beverage mate: a risk factor for cancer of the head and neck. Head Neck 25: 595-601.
- PH Chen, B Huang, TY Shieh, YH Wang, YK Chen, et al. (2014) The influence of monoamine oxidase variants on the risk of betel quid associated oral and pharyngeal cancer. Scientific World Journal 2014 183548.
- L Ottria, V Candotto, F Cura, L Baggi, C Arcuri, et al. (2018) HPV acting on E-cadherin, p53 and p16: literature review. J Biol Regul Homeost Agents 32: 73-79.
- G Karpathiou, A Monaya, F Forest, M Froudarakis, F Casteillo, et al. (2016) p16 and p53 expression status in head and neck squamous cell carcinoma: a correlation with histological, histoprognostic and clinical parameters. Pathology 48: 341-348.
- SA Geisler, AF Olshan, MC Weissler, J Cai, WK Funkhouser, et al. (2002) p16 and p53 Protein expression as prognostic indicators of survival and disease recurrence from head and neck cancer. Clin Cancer Res. 8: 3445-3453.
- ER Cohen, IM Reis, C Gomez, L Pereira, ME Freiser, et al. (2017) Immunohistochemistry Analysis of CD44, EGFR, and p16 in Oral cavity and oropharyngeal squamous cell carcinoma. Otolaryngol Head Neck Surg 157: 239-251.
- P Galli, G Cadoni, M Volante, E De Feo, R Amore, et al. (2009) A case-control study on the combined effects of p53 and p73 polymorphisms on head and neck cancer risk in an Italian population. BMC Cancer 9: 137.
- MM Bloching, H Soulsby, A Naumann, W Aust, D Merkel, et al. (2008) Tumor risk assessment by means of immunocytochemical detection of early pre-malignant changes in buccal smears. Oncol Rep 19: 1373-1379.
- CG Ferreira, JC Rocha (2010) Oncologia Molecular, Atheneu, São Paulo, Brazil.
- AC Abrahao, BV Bonelli, FD Nunes, EP Dias, MG Cabral (2011) Immunohistochemical expression of p53, p16 and hTERT in oral squamous cell carcinoma and potentially malignant disorders. Braz Oral Res 25: 34-41.
- DP Lane, LV Crawford (1979) T antigen is bound to a host protein in SV40-transformed cells. Nature 278: 261-263.
- AJ Levine (1997) p53, the cellular gatekeeper for growth and division. Cell 88: 323-331.
- CY Gao, PS Zelenka (1997) Cyclins, cyclin-dependent kinases and differentiation. Bioessays 19: 307-315.
- T Weinert (1998) DNA damage and checkpoint pathways: molecular anatomy and interactions with repair. Cell 94: 555-558.
- AC Fett Conte, ABCF Salles (2002) The importance of the p53 gene in human carcinogenesis. Rev Bras Hematol Hemoter 24: 85-88.
- KR Patel, BN Vajaria, RD Singh, R Begum, PS Patel (2018) Clinical implications of p53 alterations in oral cancer progression: a review from India. Exp Oncol 40: 10-18.
- S Singla, G Singla, S Zaheer, DS Rawat, AK Mandal (2018) Expression of p53, epidermal growth factor receptor, c-erbB2 in oral leukoplakias and oral squamous cell carcinomas. J Cancer Res Ther 14: 388-393.
- A Diab, M Kao, K Kehrli, HY Kim, J Sidorova, et al. (2019) Multiple Defects Sensitize p53-Deficient Head and Neck Cancer Cells to the WEE1 Kinase Inhibition. Mol Cancer Res 17: 1115-1128.
- C Rodriguez Ramirez, JE Nor (2018) p53 and Cell fate: sensitizing head and neck cancer stem cells to chemotherapy. Crit Rev Oncog 23: 173-187.
- G Zhou, Z Liu, JN Myers (2016) TP53 mutations in head and neck squamous cell carcinoma and their impact on disease progression and treatment response. J Cell Biochem 117: 2682-2692.
- A Sathyamurthy, AS Koushik, M Gowri, MG Janaki, N Kilara, et al. (2016) Impact of molecular predictors on the response rates in head and neck cancer patients-an observational study. Indian J Surg Oncol 7: 380-385.
- MA Broglie, A Soltermann, D Rohrbach, SR Haile, M Pawlita, et al. (2013) Impact of p16, p53, smoking, and alcohol on survival in patients with oropharyngeal squamous cell carcinoma treated with primary intensity16 modulated chemoradiation. Head Neck 35: 1698-1706.
- B Joseph, RV Kumar, G Champaka, A Shenoy, KS Sabitha, et al. (2019) Biological tailoring of adjuvant radiotherapy in head and neck and oral malignancies - The potential role of p53 and eIF4E as predictive parameters. Indian J Cancer 56: 330-334.
- G Wichmann, M Rosolowski, K Krohn, M Kreuz, A Boehm (2015) The role of HPV RNA transcription, immune response-related gene expression and disruptive TP53 mutations in diagnostic and prognostic profiling of head and neck cancer. Int J Cancer 137: 2846-2857.
- K Syrjanen, S Syrjanen, M Lamberg, S Pyrhonen, J Nuutinen (1983) Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg 12: 418-424.
- KK Ang, J Harris, R Wheeler, R Weber, DI Rosenthal, et al. (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363: 24-35.
- L Satgunaseelan, SA Virk, T Lum, K Gao, JR Clark, R Gupta (2016) p16 expression independent of human papillomavirus is associated with lower stage and longer disease-free survival in oral cavity squamous cell carcinoma. Pathology 48 (2016): 441-448.
- Z Deng, M Hasegawa, K Aoki, S Matayoshi, A Kiyuna, et al. (2014) A comprehensive evaluation of human papillomavirus positive status and p16INK4a overexpression as a prognostic biomarker in head and neck squamous cell carcinoma. Int J Oncol 45: 67-76.
- C Fakhry, WH Westra, S Li, A Cmelak, JA Ridge, et al. (2008) Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 100: 261- 269.
- C Fakhry, WH Westra, SJ Wang, A van Zante, Y Zhang, et al. (2017) The prognostic role of sex, race, and human papillomavirus in oropharyngeal and non oropharyngeal head and neck squamous cell cancer. Cancer 123: 1566-1575.
- H Yang, Y Cao, ZM Li, YJ Li, WQ Jiang, et al. (2018) The role of protein p16(INK4a) in non-oropharyngeal head and neck squamous cell carcinoma in Southern China. Oncol Lett 16: 6147-6155.
- SB Edge, CC Compton (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17: 1471- 1474.
- LD Wang, JY Hong, SL Qiu, H Gao, CS Yang (1993) Accumulation of p53 protein in human esophageal precancerous lesions: a possible early biomarker for carcinogenesis. Cancer Res 53: 1783-1787.
- MT Galgano, PE Castle, KA Atkins, WK Brix, SR Nassau, et al. (2010) Using biomarkers as objective standards in the diagnosis of cervical biopsies. Am J Surg Pathol 34: 1077-1087.
- V Singh, N Husain, N Akhtar, MY Khan, AA Sonkar, et al. (2017) p16 and p53 in HPV-positive versus HPV-negative oral squamous cell carcinoma: do pathways differ? J Oral Pathol Med 46: 744-751.
- GB Rasmussen, KE Hakansson, IR Vogelius, JH Rasmussen, JT Friborg, et al. (2017) Immunohistochemical and molecular imaging biomarker signature for the prediction of failure site after chemoradiation for head and neck squamous cell carcinoma. Acta Oncol 56: 1562-1570.
- JK Stephen, G Divine, KM Chen, D Chitale, S Havard, et al. (2013) Significance of p16 in site-specific HPV positive and HPV negative head and neck squamous cell carcinoma. Cancer Clin Oncol 2: 51-61.
- NMS De Britto, HSC Paula, VA. Saddi (2014) The role of p16 and Ki 67 in squamous cell carcinomas of the oral cavity and oropharynx. Rev Bras Cir Cabeça Pescoço 43: 193-199.
- RJ Bova, DI Quinn, JS Nankervis, IE Cole, BF Sheridan, et al. (1999) Cyclin D1 and p16INK4A expression predict reduced survival in carcinoma of the anterior tongue. Clin Cancer Res 5: 2810-2819.
- PW Yuen, M Man, KY Lam, YL Kwong (2002) Clinicopathological significance of p16 gene expression in the surgical treatment of head and neck squamous cell carcinomas. J Clin Pathol 55: 58-60.
- AR Kreimer, GM Clifford, P Boyle, S Franceschi (2005) Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review, Cancer Epidemiol. Biomarkers Prev 14: 467-475.
- X Castellsague, L Alemany, M Quer, G Halec, B Quiros, et al. (2016) HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst 108: djv403.
- J Laco, J Nekvindova, V Novakova, P Celakovsky, H Dolezalova, et al. (2012) Biologic importance and prognostic significance of selected clinicopathological parameters in patients with oral and oropharyngeal squamous cell carcinoma, with emphasis on smoking, protein p16(INK4a) expression, and HPV status. Neoplasma 59: 398- 408.
- P Lassen, JG Eriksen, S Hamilton Dutoit, T Tramm, J Alsner, et al. (2009) Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol 27: 1992-1998.
- PM Weinberger, Z Yu, BG Haffty, D Kowalski, M Harigopal, et al. (2006) Molecular classification identifies a subset of human papillomavirus--associated oropharyngeal cancers with favorable prognosis. J Clin Oncol 24: 736-747.
- T Isayeva, Y Li, D Maswahu, M Brandwein Gensler (2012) Human papillomavirus in non oropharyngeal head and neck cancers: a systematic literature review. Head Neck Pathol 6 Suppl 1: S104-120.
- A Namazie, S Alavi, OI Olopade, G Pauletti, N Aghamohammadi, et al. (2002) Cyclin D1 amplification and p16(MTS1/CDK4I) deletion correlate with poor prognosis in head and neck tumors. Laryngoscope 112: 472-481.
- AI Stukan, OY Chukhray, VA Porkhanov, VN Bodnya (2019) [Association of the expression of p53 and p16INK4A with the clinical and morphological characteristics of patients with head and neck squamous cell cancer]. Arkh Patol 81: 12-18.
- EM Smith, LM Rubenstein, H Hoffman, TH Haugen, LP Turek (2010) Human papillomavirus, p16 and p53 expression associated with survival of head and neck cancer. Infect Agent Cancer 5: 4.
- PS Ramesh, D Devegowda, A Singh, RK Thimmulappa (2020) NRF2, p53, and p16: Predictive biomarkers to stratify human papillomavirus associated head and neck cancer patients for de-escalation of cancer therapy. Crit Rev Oncol Hematol 148: 102885.
- A Almangush, I Heikkinen, AA Makitie, RD Coletta, E Laara, , et al. (2017) Prognostic biomarkers for oral tongue squamous cell carcinoma: a systematic review and meta-analysis. Br J Cancer 117: 856-866.
- S Tandon, C Tudur Smith, RD Riley, MT Boyd, TM Jones (2010) A systematic review of p53 as a prognostic factor of survival in squamous cell carcinoma of the four main anatomical subsites of the head and neck. Cancer Epidemiol Biomarkers Prev 19: 574-587.
-
Maria da Graça C Vidal*, Onivaldo Cervantes and Alessandra S Bastianello. P53 And P16 Protein Expression and Survival in Patients with Squamous Cell Carcinoma of the Oral Cavity. On J Otolaryngol & Rhinol. 6(5): 2024. OJOR.MS.ID.000648.
-
Squamous cell carcinoma; Oropharyngeal carcinogenesis; Tumor suppressor; Hypopharyngeal carcinoma; Oral cancer; Oropharynx; Oncogenic mutations; Immunohistochemistry; DNA repair
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






