 Research Article
Research Article
Creating A Protocol for the Management of Epistaxis in A Tertiary Care Centre-A Practical Application of the British Rhinological Society Multidisciplinary Consensus Recommendations on the Hospital Management of Epistaxis
Matt Lechner1, Oscar Emanuel1, Deepak Chandrasekharan1, Joshua Michaels1, Gulwish Moghul1, Nish Mehta2, Jacklyn Liu2, Claire Hopkins3, Valerie J Lund2, Hassan A Elhassan1, Santdeep Paun1 and Nicholas Eynon Lewis1*
1Department of Otolaryngology-Head and Neck Surgery, Royal London Hospital and Whipps Cross University Hospital, Barts Health NHS Trust, London, UK
2Royal National Throat, Nose and Ear Hospital/Head and Neck Centre, University College London Hospitals NHS Foundation Trust, London, UK
3Guy’s Hospital, Guy’s and St. Thomas’ Hospitals NHS Foundation Trust, London, UK
Mr. Nick Eynon-Lewis, FRCS (ORL-HNS), Department of Otolaryngology-Head and Neck Surgery Royal London Hospital, Barts Health NHS Trust, London, UK.
Received Date: January 03, 2022; Published Date: February 08, 2022
Abstract
Epistaxis is the most common acute presentation to ENT services in the UK. The recent British Rhinological Society multidisciplinary consensus recommendations [1] provide new guidance for its management. To better align with these, we reviewed current practice in our tertiary referral centre. We noted practice variation both within and between departments, particularly concerning the timing and utilisation of nasal packing, accounting for inpatient stay. The aim of this project was to reduce nasal packing for the initial management of epistaxis and increase attempted nasal cautery. We adapted the BRS guidelines for local use and worked with key stakeholders to establish a stepwise management algorithm for epistaxis, which would be suitable for all practitioners to initiate, regardless of specialty. This was discussed in an audit meeting with consultants, registrars, and junior doctors to assess clinical and practical acceptability. After refinement, we produced a step-by-step flowchart, which would serve as the local reference for management of epistaxis. After implementation, our prospective audit showed that inpatient admissions from epistaxis were reduced without adverse events or a significant increase in readmissions. Our QI process and protocol could be used as a safe way to disseminate and implement BRS guidelines at the local level.
Keywords: Epistaxis, Nose bleed, Cautery, Audit, Quality improvement, Acute Care, British Rhinological Society Multidisciplinary Consensus Recommendations on the Hospital Management of Epistaxis
Introduction
Epistaxis is the most common acute presentation to ENT services in the UK, accounting for 25000 acute presentations each year, which aligns with our institutional experience at Barts Health NHS Trust, which is the largest Trust in the UK. Recently, the British Rhinological Society (BRS) established multidisciplinary consensus recommendations for the management of epistaxis. At our institution, this condition is initially managed by the emergency department (ED) and by junior doctors in ENT (FY2- CT3 equivalent). Despite the publication of the BRS guidelines, we have observed low awareness of these in the ED and amongst ENT juniors. This may be one reason why nasal packing is used as the initial management in patients presenting with ongoing epistaxis, which may result in consequent inpatient stay. The aim of this quality improvement project was to disseminate and implement the BRS epistaxis guidelines at a local level. Specifically, we aimed to change initial management of epistaxis from nasal packing to attempted nasal cautery instead.
Methods
A presentation on the nationwide audit on epistaxis management options was given by the National ENT Trainee Research Network to the ENT department followed by further discussions with local stakeholders. At this meeting, it became clear that our ENT team did not have knowledge of the new BRS epistaxis guidelines. As a result, we created a new Protocol for Management of Epistaxis in Adults based on these new guidelines (Figure 1). With the proposed protocol, we prospectively audited our management of adult epistaxis patients over a 4-month period (November 2019 to February 2020) as part of a Barts Health NHS Trust Quality Improvement Project (project ID 11110). Data gathered included; type of epistaxis (anterior vs. posterior), location of patient in hospital, inpatient/outpatient status, whether nasal cautery was attempted and by whom, whether nasal packing was in situ and who had placed this, whether packing was removed and cautery was attempted, use of tranexamic acid, whether admission was needed and subsequent management (e.g. surgery or interventional radiology). Responses were anonymised at clinician and patient level.
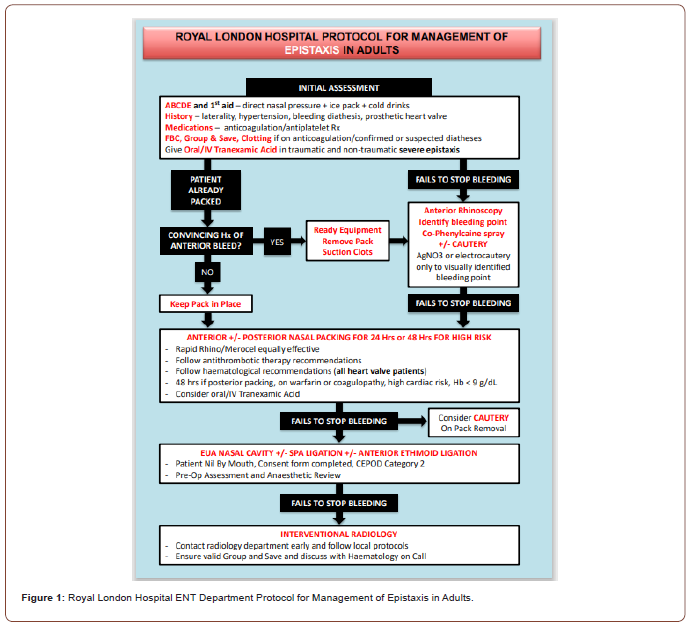
Data was collected through a proforma provided to ENT doctors. After assessing an eligible patient with epistaxis, the clinician filled in the proforma and returned it to the QI team for collation and data input. We included all patients presenting with epistaxis with no exclusion criteria.
Results
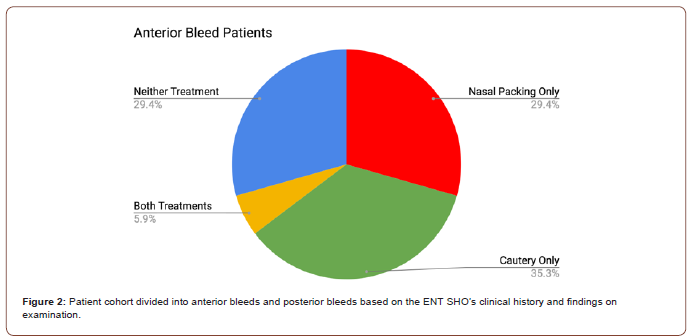
Our prospective audit cohort consisted of 25 consecutive patients presenting with epistaxis. The majority of patients were seen in the ED (n=22). 17 patients were diagnosed with an anterior bleed and, of these, 6 were cauterised as initial management and 10 required nasal packing and/or had IV/PO tranexamic acid. Of the 8 patients with a posterior bleed (defined as lack of an anterior bleeding point seen on rhinoscopy), 6 had nasal packing placed initially without cautery (Figure 2).
22 patients were reviewed in the ED, 2 in ambulatory care and 1 in a separate hospital without specifying the department. At the time of assessment, 3 were inpatients, 15 were outpatients and the remaining 7 were ED patients. Some interventions included in the audit had already been performed prior to the ENT SHO’s assessment; these instances will be alluded to in order to maintain data quality. For example, 2 patients had already undergone nasal packing in separate hospitals (1 by ED and 1 by ITU), while 1 patient had already undergone cautery again in a separate hospital.
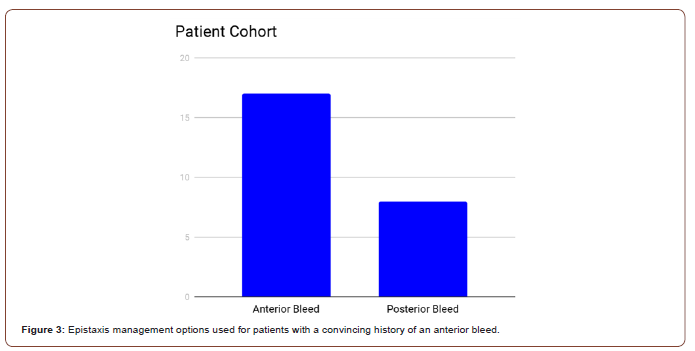
7/25 patients were cauterised (1 of which was performed before the audit participants reviewed the patient) and 12/25 patients were packed (2 before the audit participants reviewed the patient). 1 patient required both cauterisation and packing, while the remaining 5 patients received neither intervention. 11 patients were packed without cautery having been attempted (2 before the audit participants reviewed the patient), whereas only 6 were cauterised without nasal packing being used (1 of which was cauterised before the audit participants reviewed the patient) (Figure 3). At first glance, this seems to be out of keeping with the Integrate study guidelines, which state cautery should be attempted before nasal packing which only occurred in 39% of patients who received either of the two interventions.
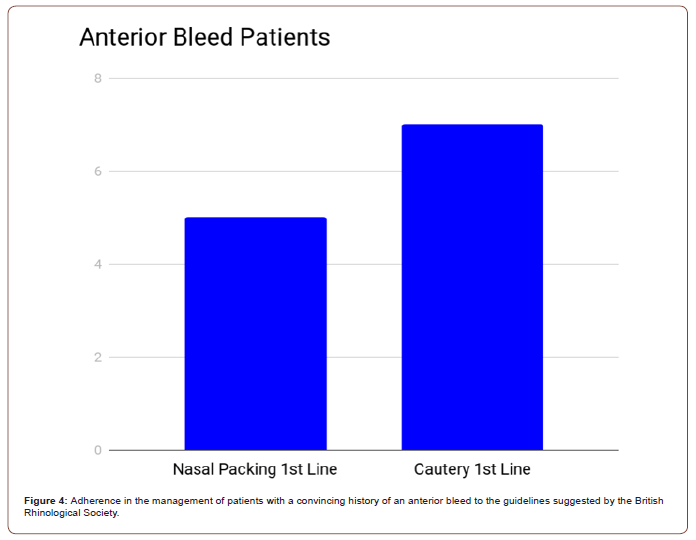
However, it must be considered that 17 of the 25 patients had a convincing history of an anterior bleed. Cautery was only performed on patients with a convincing history of an anterior bleed and corresponding findings on examination (Figure 4). Of the 8 patients without a convincing history of an anterior bleed, 6 were given nasal packing (1 before the audit participants reviewed the patient). 6 of the anterior bleed patients were cauterised without nasal packing being used (1 before the audit participants reviewed the patient), but 5 were packed without cautery having been attempted. 1 anterior bleed patient required both cauterisation and packing while 5 anterior bleed patients did not receive either intervention. Therefore, when anterior bleed patients are taken as a subset, 58% were treated according to the Integrate guidelines.
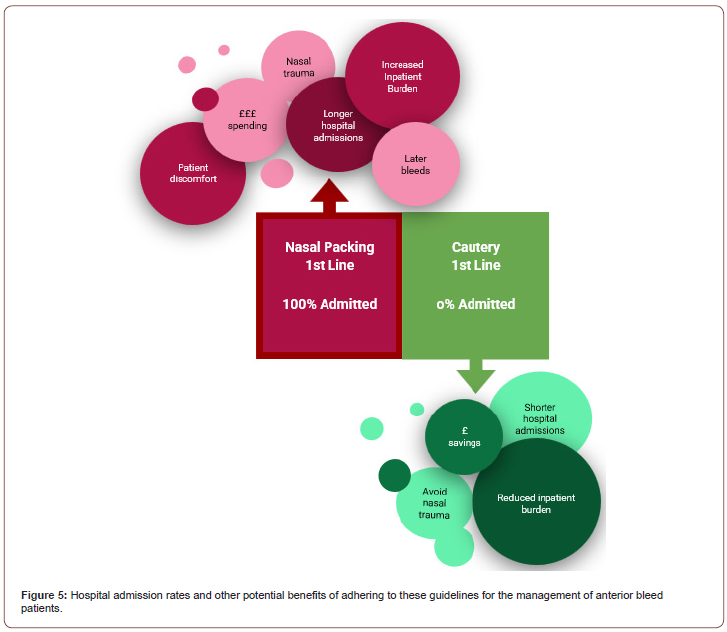
There is also an important point to be made regarding the inpatient burden of the anterior bleed patients. None of the 6 anterior bleed patients, who received cautery and not nasal packing, required an inpatient stay. This is in contrast to all of the 5 anterior bleed patients, who received nasal packing and not cautery, suggesting closer adherence to the Integrate guidelines would relieve some of this burden (Figure 5).
Tranexamic acid was given to 15 of the 25 patients. It was given intravenously in 13 cases and orally in 2. There was no statistically significant difference between the proportion of anterior bleed and presumed posterior bleed patients given tranexamic acid (5/8 patients without a convincing history of an anterior bleed and 9/17 patients with a convincing history of an anterior bleed).
Discussion
Epistaxis carries a lifelong incidence of 60% across the general population [2]. Although most cases are self-limiting and benign, those that become profuse and unrelenting carry significant clinical urgency and potential mortality. In fact, epistaxis in isolation is the most common acute emergency managed by ENT departments across the UK and accounts for over 25,000 presentations to secondary care each year. Therefore, the recently published BRS guidelines for the management of this condition, one that accounts for over £1.5 million in hospital beds alone, was much needed [3].
In a recent study of 111 junior doctors, it was found that 75% of participants subjectively lacked confidence in the management of acute epistaxis when presenting as an emergency admission and it was concluded that a lack of training in otorhinolaryngology during medical school (8.1 days of teaching) largely accounted for this [4]. It is routinely assumed that the initial investigation of acute epistaxis should include a patient history and clinical assessment and it is integral to these processes that particular aspects of a patient’s history are noted. These include but are not limited to the onset, duration and frequency of the epistaxis, as well as its laterality, family history and predisposing factors, such as anticoagulation, nasal trauma and recent surgery. Clinical examination should also be coupled with an appreciation of the previous attempts to resolve the epistaxis, be that through nasal compression, packing tamponade or attempted cautery. It is only through careful consideration of these details that an appropriate management plan can be formulated. When these strategies were assessed as part of a collaborative ENT prospective study on epistaxis, significant variation in clinical practice was found between hospital trusts [5].
Our data provides useful evidence that the British Rhinological Society guidelines can be implemented on a local level to improve patient outcomes. There are a few limitations to the project. Firstly, the sample size was small as we were limited by the number of epistaxis admissions that required ENT SHO input over the project period. Additionally, since ENT SHOs were aware of the ongoing audit, some may have adhered more closely to the newly created protocol compared to their normal practice, which may bias the self-reported data. Therefore, future studies should seek to increase sample size and evaluate patient outcomes and guideline compliance over a longer timescale.
Conclusion
In conclusion, the described use of the epistaxis management guideline form will allow relatively inexperienced doctors to better manage epistaxis through adhering to the most widely recognised and accepted up-to-date evidence. It will give them the confidence to remove packing in emergency departments and potentially expedite resolution of the bleed and subsequent discharge through cautery. We have seen that following the British Rhinological Society guidelines can reduce unnecessary hospital stays for patients with anterior bleeds. In fact, all patients in our cohort of anterior bleeds who initially received cautery instead of nasal packing did not require an inpatient admission, whereas all of those who were packed first did require admission, suggesting that adhering to the guidelines can reduce the resource burden of epistaxis inpatients.
Acknowledgement
We would like to thank the entire team of the ENT Department at the Royal London Hospital for their support with this quality improvement project and Mr. Edward Spirling from the Clinical Effectiveness Unit (Barts Health NHS Trust).
Author Contribution
ML, OE and DC led on audit design, data collection and analysis. All authors contributed to the manuscript.
Conflict of Declaration
None to declare.
References
- National ENT Trainee Research Network (2017) British Rhinological Society Multidisciplinary Consensus Recommendations on the Hospital Management of Epistaxis. J Laryngol Otol 131(12): 1142-1156.
- Cummings CW, Flint PW, Harker LA, Haughey BH (2004) Cummings Otolaryngology, Mosby Inc., USA.
- NICE (2015) Costing Statement: Implementing the NICE Guideline on Transition Between Inpatient Hospital Settings and Community or Care Home Settings for Adults with Social Care Needs (NG27). Putting NICE Guidance into Practice. NICE.
- Fox R, Nash R, Liu ZW, Singh A (2016) Epistaxis management: current understanding amongst junior doctors. J Laryngol Otol 130(3): 252-255.
- Mehta (2017) Can trainees design and deliver a national audit of epistaxis management? A pilot of a secure web-based audit tool and research trainee collaboratives. J Laryngol Otol 131(6): 518-522.
-
Matt Lechner, Oscar Emanuel, Deepak Chandrasekharan, Joshua Michaels, Gulwish Moghul, Nish Mehta, Jacklyn Liu, Claire Hopkins, Valerie J Lund, Hassan A Elhassan, Santdeep Paun, Nicholas Eynon Lewis. Creating A Protocol for the Management of Epistaxis in A Tertiary Care Centre-A Practical Application of the British Rhinological Society Multidisciplinary Consensus Recommendations on the Hospital Management of Epistaxis. On J Otolaryngol & Rhinol. 5(3): 2022. OJOR.MS.ID.000611.
-
ENT, Nasal packing, Nasal compression, Otorhinolaryngology, Anterior bleed, ENT SHO’s, ED patients, Radiology, Rhinological society, Acute Care.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






