 Case Report
Case Report
Nuclear Protein of the Testis Midline Carcinoma of the Frontal Sinus
Blanda Kamin1, Gunnar Gaffke2, Thabit Abo Dalla1 and Tino Just1*
1Department of Otorhinolaryngology, Head and Neck Surgery, KMG Klinikum, Güstrow, Germany
2Department of Diagnostic and Interventional Radiology, KMG Klinikum, Güstrow, Germany
Tino Just, M.D., Department of Otorhinolaryngology, Head and Neck Surgery Friedrich-Trendelenburg-Allee 1, D-18273 Güstrow, Germany.
Received Date: April 02, 2025; Published Date:April 11, 2025
Abstract
Introduction: This report presents a patient with an acute, 1cm severe swelling on the medial eyebrow/orbital rim, raising the question of
whether it is a preseptal orbital complication of acute rhinosinusitis, a frontoethmoidal mucocele, or a rare neoplastic entity.
Material: We report on a 40-year-old female patient who presented with an acute swelling of the left medial orbital rim, accompanied by
cephalgia and hypesthesia, which had progressively increased in size over the past three weeks.
Results: An initial native CT scan revealed a mass at the level of the nasal root, with osseous destruction of the anterior wall of the left frontal sinus. Following a combined endoscopic endonasal and external frontal sinus surgery, extent of the osseous destruction and tumor infiltration into the periorbital soft tissue were suspected. Histopathological examination identified an undifferentiated NUT (Nuclear Protein in Testis) carcinoma, confirming a NUTM1 gene translocation on chromosome 15. The patient underwent multimodal therapy, which included local therapy with radical surgery (the patient declined exenteration due to the small tumor size), radiotherapy, ifosfamide-based chemotherapy, and immunotherapy.
Discussion: NUT carcinomas are rare, highly aggressive, genetically defined carcinomas that typically occur along the midline of the body, with a predilection for the nasopharynx, sinonasal tract, neck and thoracic region. They can affect patients of any age. Due to their rapid progression andpoor prognosis, early diagnosis is essential for maximizing survival. Given the rarity of NUT carcinomas, this case aims to raise awareness of this tumor entity, which can be mistaken for a benign mucocele in differential diagnoses..
Introduction
NUT carcinomas, or midline carcinomas (NMC), are highly aggressive, rare genetically defined, poorly differentiated squamous cell carcinomas (SCC) characterized by the translocation of the NUTM1 gene on chromosome 15 [1]. They typically occur along the midline of the body, primarily in the head, neck, and thoracic regions, and can affect patients of any age. This case report describes a 40-year-old female patient with an osseous destructive mass in the left frontal sinus, with early infiltration into the left orbit. The clinical, morphological, and therapeutic data of this case are discussed.
Clinical Presentation and History
The patient -a 40-year-old female- presented in August 2022 with an acute and progressive swelling, cephalgia, and hypesthesia on the left medial orbital rim that had been present for approximately three weeks. There was no history of head trauma. She denied experiencing diplopia, visual impairment, or symptoms of sinusitis. Clinically, there was a noticeable swelling of the left medial orbital rim/eyebrow without external erythema, tenderness, or peripheral edema. Nasal endoscopy revealed a nonirritated nasal mucosa with serous secretion.
Results
Initial Imaging Findings:
• CT Paranasal Sinuses (PNS):
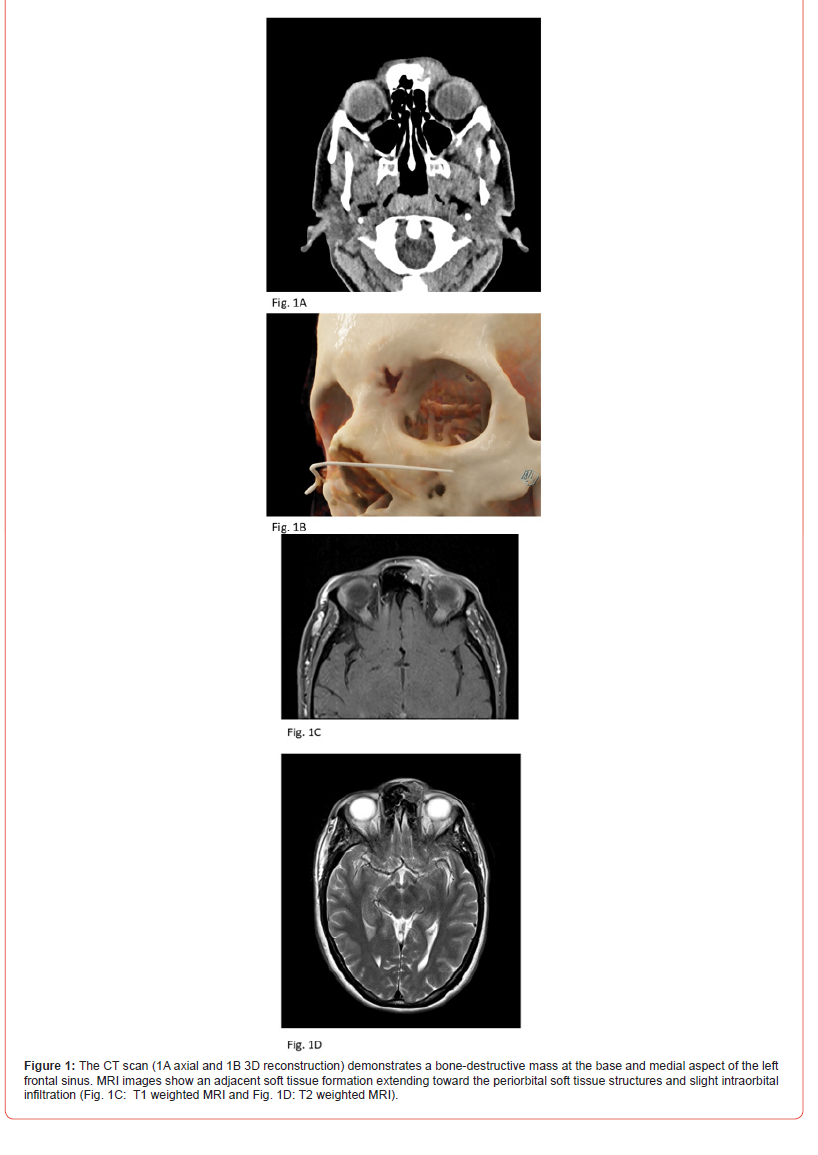
The CT scan (Figures 1A and 1B) demonstrated a bonedestructive
mass at the base and medial aspect of the left frontal
sinus, with adjacent soft tissue formation extending toward the
periorbital soft tissue structures and slight intra orbital infiltration.
• MRI Orbit:
MRI of the orbit showed a contrast-enhancing, largely
subcutaneous soft tissue formation (approximately 17 x 17 x
18mm) at the level of the nasal root, with osseous destruction
and infiltration of the anterior wall of the left frontal sinus and the
extraconal left orbit (Figures 1C and 1D). The tissue heterogeneously
enhanced the soft tissue lesion, predominantly subcutaneous, at the
level of the nasal root. The mass exhibited osteolysis of the anterior
wall of the left frontal sinus and infiltrated the extraconal space of
the left orbit, maintaining a close positional relationship with the
rectus medialis muscle, separated by a narrow fat lamella.
Given the locally destructive nature of the lesion, malignancy
was suspected. Differential diagnoses included:
• A chronic, osteodestructive inflammatory process, such as a
frontoethmoidal mucocele with secondary superinfection and
progressive bone resorption.
• An aggressive neoplastic process, including SCC of the nasal
and paranasal sinuses (SNSCC), sinonasal adenocarcinoma
(SNAC), primary sinonasal neuroendocrine carcinoma (SNEC),
primary sinonasal undifferentiated carcinoma (SNUC),
esthesioneuroblastoma, or NUT carcinoma.
Surgical Management:
The patient underwent a combined surgical approach, including
functional endoscopic sinus surgery (FESS) and an external
supraorbital eyebrow incision (Kilian’s approach) in August 2022.
• Endoscopic Findings: Anterior ethmoidectomy and frontal
sinusotomy were performed, revealing no significant mucosal
pathology within the ethmoid complex.
• External Approach: Exposure and excision of a destructive
subcutaneous soft tissue lesion were conducted. The anterior
wall of the frontal sinus was macroscopically eroded, while the
remaining sinus mucosa appeared intact.
Histopathological and Molecular Diagnosis:
Microscopy: Preliminary histopathological findings described a
solid, malignant, non-small cell tumor.
The samples were sent to the Reference Centre for
Histopathology:
NUT (midline) carcinoma with evidence of a BRD4-NUTM1
gene fusion was diagnosed.
• Strong nuclear positivity for NUT protein confirmed the
diagnosis of NUT carcinoma).
• PD-L1-TPS: 20%; PD-L1-CPS: 40.
• Diffuse p63 and cytokeratin expression were consistent with
squamous differentiation. The tumor was negative for S100,
synaptophysin, and chromogranin, excluding neuroendocrine
tumors.
• Molecular Analysis: Fluorescence in situ hybridization (FISH)
and next-generation sequencing (NGS) confirmed a BRD4-NUTM1
gene fusion, pathognomonic for NUT NMC.
Therapy and Clinical Course:
A) Initial Therapy
Due to the aggressiveness of the NUT carcinoma, multimodal
therapy was recommended by the interdisciplinary tumor board:
1. Surgical Resection:
Due to orbital invasion, radical excision was discussed. However,
the patient had refused an exenteracio orbitae in advance.
Radical frontal sinus surgery on the left was performed, including resection of the frontal sinus anterior wall, partial resection of the periorbita, plate osteosynthesis, and covering of the periorbita with PDS foil. pT4a cN0 cM0 V0 L1 Pn0 R1.
2. Adjuvant Radiotherapy:
Intensity-modulated radiation therapy (IMRT) was
administered to the tumor bed, nasal cavity, and paranasal sinuses
on both sides: Single dose of 2.0 Gy; total dose 50.0 Gy (AVG). Boost
tumor bed: Single dose 2.0 Gy; total dose 14.0 Gy.
3. Systemic Therapy:
Cisplatin 25 mg/m²
B) Five Months After Initial Diagnosis
Imaging Findings (1st Re-Staging with FDG PET-CT scan):
Local recurrence of intraorbital/extraconal tumor with
infiltration of intraorbital fatty tissue and additional osseous
metastasis in the sacrum.
Therapy:
In consultation with the Reference Center for Oncology
and inclusion in the International NMC Registry, Boston, USA, a
multimodal therapy was recommended:
1. Chemotherapy:
VIDE (Vincristine, Ifosfamide, Doxorubicin, Etoposide),
Filgrastim. Progressive cytopenia occurred during treatment.
2. Immunotherapy with Pembrolizumab.
3. Palliative Radiation Therapy of the metastasis at the T2
vertebra with a total dose of 30.0/40.0 Gy.
C) Thirteen Months After Initial Diagnosis
Imaging Findings (2nd Re-staging):
The second re-staging showed progression of an intraorbital,
extracranial mass on the left side, with infiltration of the levator
palpebrae muscle and expansion toward the frontal sinuses
bilaterally and the left ethmoidal sinus, without involvement of the
optic nerve. Additionally, there was osseous destruction of the left
frontal, ethmoidal, and maxillary sinuses, as well as progression
of the intraosseous metastasis at the T2 vertebra, but no vertebral
body collapse.
Therapy:
1. Radiotherapy:
Percutaneous conventional fractionated radiotherapy of the
left orbit, frontal, and ethmoidal sinuses bilaterally with a single
dose of 2.0 Gy and a total dose of 40.0 Gy.
2. Continuation of Systemic Therapy with Pembrolizumab.
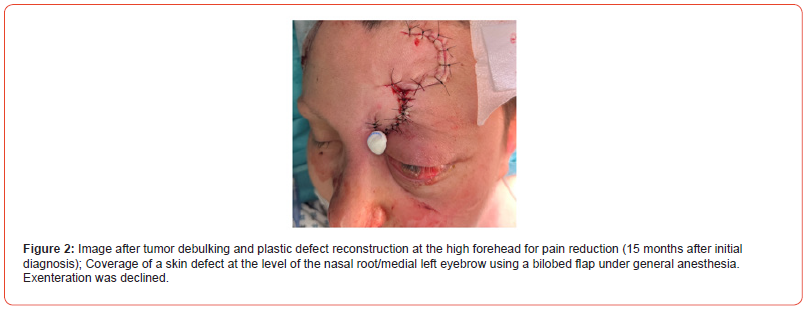
3. Tumor debulking and plastic defect reconstruction for
pain reduction:
Coverage of a skin defect at the level of the nasal root/medial
left eyebrow using a bilobed flap under general anesthesia (Figure
2).
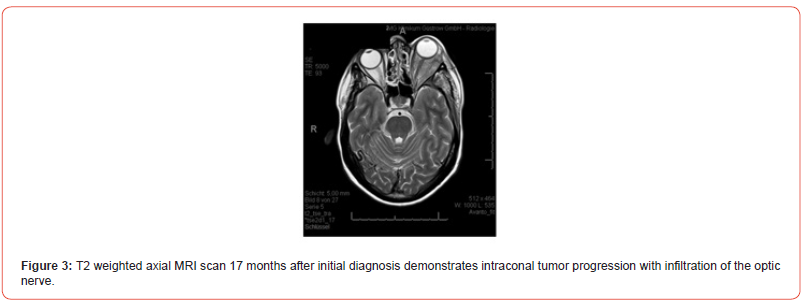
D) Seventeen Months After Initial Diagnosis
Imaging Findings (3rd Re-staging):
The CT scan and MRI imaging (Figure 3) showed further
progression of the primary tumor with infiltration of the eye
muscles, optic nerve, left temporal bone, left frontal sinus, left
temporal muscle, galea, and left frontal meninges, resulting
in exophthalmos. The metastasis at the T2 vertebra remained
unchanged, with no cervical lymphadenopathy. No thoracic or
intra-abdominal metastases were observed.
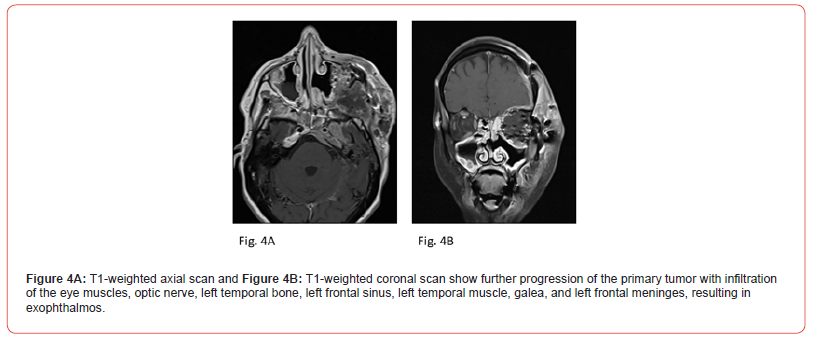
E) Twenty Months After Initial Diagnosis
Imaging Findings (4th Re-staging):
MRI imaging (Figure 4A: T1-weighted axial scan; Figure 4B: T1-
weighted coronal scan) demonstrated further progression of the
primary tumor with infiltration of the eye muscles, optic nerve, left
temporal bone, left frontal sinus, left temporal muscle, galea, and
left frontal meninges with exophthalmos.
Progression in the anterior cranial fossa on the left with meningeosis carcinomatosa, as well as progressive osseous metastases of the lumbar spine and pelvis, with a nucleus pulposus prolapse (NPP) at L1/S1 on the left, causing narrowing of the neuroforamen at L5/S1 and compression of the S1 nerve root.
Clinically, there was progression of pain due to the extensively locally advanced tumor growth.
Therapy:
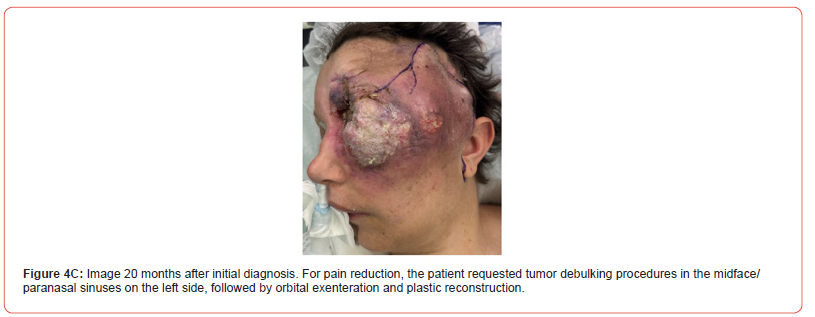
1. Two tumor debulking procedures were performed in
the midface/paranasal sinuses on the left, followed by orbital
exenteration and plastic reconstruction (Figure 4C).
2. Adjustment of pain management.
3. Initiation of antiproliferative therapy with Panobinostat.
4. Pelvic radiotherapy was planned.
5. Inpatient oncological palliative complex therapy was
administered in the last two weeks.
F) The Patient Died 22 Months After Initial Diagnosis.
Discussion
Theory of NUT Carcinoma
Definition:
NUT carcinoma, or NMC, is a rare, highly aggressive, genetically defined, poorly differentiated carcinoma. It was first characterized in the early 1990s [2, 3] and was included in the WHO tumor classification in 2015 [4]. It typically occurs along the midline of the body, primarily in the head, neck, and thorax regions [2, 3, 5, 6]. However, other rare lateral localizations (such as the larynx and salivary glands) have also been described [7, 8]. Since 2018, the terminology “midline carcinoma” has been replaced with “NUT carcinoma” in the WHO classification [9].
NUT is characterized by the translocation of the NUTM1 gene to chromosome 15q14, usually as a fusion with the bromodomaincontaining protein 4 (BRD4) on chromosome 19q13 or BRD3 [3, 5, 6, 10]. BRD4 belongs to the family of BET proteins, consisting of two bromodomains (BD) and an extra terminal domain (ET). When BRD4 fuses with NUT, massive mega domains are formed that increase the transcription of various proto-oncogenes [11, 12].
Histopathologically, the carcinoma is characterized by proliferates of basaloid, medium-sized, undifferentiated cells with islands of abrupt squamoid differentiation (keratinization). Immunohistochemically, the NUT1 marker is used to define the diagnosis [3, 6].
Epidemiology:
Any age can be affected, although it was first described in children and young adults. The current reported mean age at diagnosis ranges between 21.9 and 30.5 years [11, 13, 14].
The incidence of NUT carcinomas is unknown. Data is only available from Western Australia, where the incidence in children (~0.41 per million children aged 0-16 years) was first identified in 2021 [15].
No significant differences in survival rates have been observed concerning gender, age, histology, geography, primary tumor location, or initial lymph node involvement (5, 10, 11). Risk groups were first defined in 2020 after analyzing data from the International NMC Registry. Three risk groups were identified based on the anatomical location of the primary tumor and the type of NUTM1 fusion. Patients with extra thoracic primary tumors with non-BRD4-NUT fusion (for example, fusion with BRD3) had the best prognosis, followed by patients with extra thoracic primary tumors with BRD4-NUT fusion. Patients with thoracic NUT carcinoma had the worst prognosis, regardless of the NUT fusion. Only in the first two groups were patients with long-term survival (≥ 3 years, 12 of 141 patients) identified [14].
Clinic:
The mean survival is less than one year, despite extensive
treatment. Since 2010, there has been an international NUT
carcinoma database (International NMC Registry) in Boston, USA
[11]. The mean survival time is reported to be between 6.5 and 9.7
months, with a 2-year survival rate of 19%-30% [10, 11, 14].
The primary tumor is localized in 41% of cases in the head and neck region, mostly in the sinonasal tract and nasopharynx, in 50% in the thorax, primarily in the lungs and mediastinum, and in 6% in bones and soft tissue [11, 14]. However, other areas along the median and paramedian lines of the body may also be affected. Lateral localization, such as in the salivary glands, is rare [8]. At diagnosis, 40%–50% of patients have metastases [13, 14].
Therapy:
Most patients receive intensive initial multimodal therapy. An optimal regimen has yet to be established, and no chemotherapeutic agents have been associated with improved survival [10, 11]. For the first time in this rare tumor entity, standard therapy recommendations were issued at the first International NUT Carcinoma Symposium in 2021 [12]:
For patients with localized NUT carcinoma, the most effective therapy is radical surgical resection combined with adjuvant radio chemotherapy [11-13].
For patients with unresectable NUT carcinoma, definitive radio chemotherapy or induction chemotherapy combined with surgery and adjuvant radio chemotherapy is recommended.
Commonly used chemotherapy regimens include Ifosfamide/ Etoposide, Ifosfamide/Etoposide/Vorinostat, Etoposide/Platinum, or Platinum/Paclitaxel. Ifosfamide-based chemotherapeutic agents appear to offer an advantage over platinum-based agents [12, 16].
Immunochemotherapy with checkpoint inhibitors may also be considered, particularly in tumors with high mutational burden and PD-L1 positivity [12, 17]. The combination of standard chemotherapeutic agents with bromodomain and extra terminal domain (BET) inhibitors is under investigation.
Summary and Conclusions
NUT carcinoma is a rare, highly aggressive, genetically defined SCC with a characteristic NUTM1 gene rearrangement [1]. It predominantly arises along the midline structures of the body and exhibits rapid locoregional progression with early metastases. Due to the rarity of NUT carcinomas, this case aims to raise awareness of this tumor entity, which often mimics benign inflammatory lesions (for example, mucoceles). This case highlights the critical importance of early recognition of NUT carcinoma in patients presenting with rapidly progressive, osteodestructive sinonasal and orbital lesions. Given the poor prognosis, early diagnosis is essential for maximizing survival. This case report describes an interdisciplinary treatment approach based on current treatment recommendations: a combination of local therapy with surgery and radiotherapy as well as ifosfamide-based systemic therapy and immunotherapy. With this therapy concept, survival was achieved far beyond the statistical mean, for an extended period with a good general condition.
Acknowledgement
None.
Conflict of Interest
No Conflict of Interest.
References
- Solomon LW, Magliocca KR, Cohen C, Muller S (2015) Retrospective analysis of nuclear protein in testis (NUT) midline carcinoma in the upper aerodigestive tract and mediastinum. Oral Surg Oral Med Oral Pathol Oral Radiol 119(2): 213-220.
- Stelow EB, French CA (2009) Carcinomas of the upper aerodigestive tract with rearrangement of the nuclear protein of the testis (NUT) gene (NUT midline carcinomas). Adv Anat Pathol 16(2): 92-96.
- French CA (2012) Pathogenesis of NUT midline carcinoma. Annu Rev Pathol 7: 247-265.
- Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG (2015) Introduction to The 2015 World Health Organization Classification of Tumors of the Lung, Pleura, Thymus, and Heart. J Thorac Oncol 10(9): 1240-1242.
- French CA (2013) The importance of diagnosing NUT midline carcinoma. Head Neck Pathol 7(1): 11-16.
- Mikula M, Rooper L (2025) NUT carcinoma PathologyOutlines.com website.
- Hellquist H, French CA, Bishop JA, Coca-Pelaz A, Propst EJ, et al. (2017) NUT midline carcinoma of the larynx: an international series and review of the literature. Histopathology 70(6): 861-868.
- Agaimy A, Fonseca I, Martins C, Thway K, Barrette R, et al. (2018) NUT carcinoma of the salivary glands: clinicopathologic and molecular analysis of 3 cases and a survey of NUT expression in salivary gland carcinomas. Am J Surg Pathol 42(7): 877-884.
- Agaimy A, Haller F, Hartmann A (2018) Sinunasale Tumoren: Neues aus der WHO mit besonderem Fokus auf neue mesenchymale Entitä Pathologe 39(1):18-26.
- Bauer DE, Mitchell CM, Strait KM, Lathan CS, Stelow EB, et al. (2012) Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res 18(20): 5773-5779.
- Chau NG, Hurwitz S, Mitchell CM, Aserlind A, Grunfeld N, et al. (2016) Intensive treatment and survival outcomes in NUT midline carcinoma of the head and neck. Cancer 122(23): 3632-3640.
- French CA, Cheng ML, Hanna GJ, DuBois SG, Chau NG, et al. (2022) Report of the First International Symposium on NUT Carcinoma. Clin Cancer Res 28(12): 2493-2505.
- Saiki A, Sakamoto K, Bee Y, Izumo T (2022) Nuclear protein of the testis midline carcinoma of the thorax. Jpn J Clin Oncol 52(6): 531-538.
- Chau NG, Ma C, Danga K, Al-Sayegh H, Nardi V, et al. (2020) An anatomical site and genetic-based prognostic model for patients with nuclear protein in testis (NUT) midline carcinoma: analysis of 124 patients. JNCI Cancer Spectr 4(2): pkz094.
- Carter T, Crook M, Murch A, Beesley AH, de Klerk N, et al. (2021) Incidence of NUT carcinoma in Western Australia from 1989 to 2014: a review of pediatric and adolescent cases from Perth Children’s Hospital. BMC Cancer 21(1): 740.
- Luo J, Sanchez M, Lee E, Hertzler H, Luong N, et al. (2024) Initial chemotherapy for locally advanced and metastatic NUT carcinoma. J Thorac Oncol 19(5): 829-838.
- Davis A, Mahar A, Wong K, Barnet M, Kao S (2021) Prolonged disease control on nivolumab for primary pulmonary NUT carcinoma. Clin Lung Cancer 22(5): e665-e667.
-
Blanda Kamin, Gunnar Gaffke, Thabit Abo Dalla and Tino Just*. Nuclear Protein of the Testis Midline Carcinoma of the Frontal Sinus. On J Otolaryngol & Rhinol. 7(4): 2025. OJOR.MS.ID.000666.
-
Midline carcinoma, Frontal sinus, Rhinosinusitis, Nasopharynx, Sinonasal tract, Neck and thoracic, Left frontal sinus, Nasal root, Chemotherapy, NUT carcinomas
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






