 Research Article
Research Article
Cognitive Hearing Science: Clinical Implications in Audiology
Scott E Seeman* and Jennine Harvey Northrop
Department of Communications Sciences & Disorders, Illinois State University, Normal, Illinois, USA
Scott Seeman, Ph.D. Department of Communications Sciences & Disorders Illinois State University, Normal, IL, USA.
Received Date:March 07, 2024; Published Date:March 18, 2024
Abstract
The interdependent relationship of hearing and cognitive function has resulted in an accelerated growth of research across multiple fields.1 However, it is uncertain how modern clinical practice in audiology should evolve. This study is a systematic review of the cognitive hearing science literature with the purpose of identifying clinical application for audiologists. The resulted in a total of 118 articles, with 64 of those having direct clinical application. Results indicate audiologists should consider measures of cognitive health when diagnosing and treating their older patients with hearing loss. Further, cognitive screening tools should include measures of working memory capacity (WMC), attention, and processing speed. These tools should be sensitive to small changes in cognitive function and distinguish mild cognitive impairment from normal function. Specific recommendations for adaptable evidence-based treatment of hearing loss based on cognitive functioning are lacking. However, there were a few suggestions in the literature, including using speech-in-noise testing as part of the diagnostic test-battery, optimizing the signal-to-noise ratio (SNR) through stronger DNR settings and FM technology. Additionally, slower compression release times for those with poorer cognition as well as more automatic hearing aid functioning was recommended. While current literature provides preliminary, general guidelines for application to clinical practice, more research is needed that directly applies to assessment and intervention services for hearing loss in context of cognitive function.
Keywords:Cognition, Hearing, Diagnosis, Treatment, Review
Abbreviations:American Four Alternative Auditory Feature (AFAAF); At the Institute for Work, Learning and Aging Computer-based Cognitive Assessment (ALACog); Auditory Event Related Potential (AERP); Auditory Processing Disorder (APD); Auditory Trail Making Test (TMT-A); Awareness of Age-Related Change (AARC); California Verbal Learning Test (CVLT); Cambridge Neuropsychological Test Automated Battery (CANTAB); Clinical Global Impression of Change (CGIC); Cochlear Implant (CI); Computerized learning Exercises for Aural Rehabilitation (clEAR); Dichotic Sentence Index (DSI); Digital Noise Reduction (DNR); Distortion Product Otoacoustic Emissions (DPOAE); Ease of Language Understanding (ELU); Event Related Potential (ERP); Executive Function (EF); Frailty Index (FI); Frailty Profile (FP); Frequency Modulation (FM); Hearing Handicap Inventory for the Elderly (HHIE); Hearing Level (HL); hearing-related quality of life questionnaire for Auditory-Visual; Cognitive and Psychosocial functioning (hAVICOP); Hertz (Hz); Illness Behavior (IB); Inhibitory Control (IC); Integrated Care for Older People (ICOPE); Intelligence Quotient (IQ); Late Positive Potentials (LPP); Listening Effort (LE); Listening Span (LSPAN); Long Term Memory (LTM); Mild Cognitive Impairment (MCI); Mini-Mental-State-Exam (MMSE); Modified Telephone Interview for Cognitive Status (TICS-M); Montreal Cognitive Assessment (MoCA); Montreal Cognitive Assessment Hearing (MoCA-H); Montreal Cognitive Assessment Hearing Impaired (MoCA-HI); National Institutes of Health (NIH); Northwestern University Wordlist Six (NU-6);Personal Sound Amplification Product (PSAP); Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA); Pure Tone Average (PTA); Quality Of Life (QOL); Quick-Speech-In-Noise (QSIN); Reaction Time (RT); Reading Span (RSPAN); Repeatable Battery for the Assessment of Neuropsychological Status (RBANS); Signal-to-Noise-Ratio (SNR); Speech-In-Noise (SIN); Synthetic Sentence Index Ipsilateral Competing Message (SSI-ICM); Trail Making Test (TMT); Wechsler Memory Scale Version 3 (WMS-III); Wechsler Memory Scale Version 4 (WMS-IV); Word Auditory Recognition and Recall Measure (WAARM); Word Health Organization online Hearing test (hearWHO); Working memory (WM); Working Memory Capacity (WMC)
Introduction
There’s a longstanding appreciation of the role of cognition in communication, however, the field of cognitive hearing science has only formally emerged during the past two decades. Cognitive hearing science can be defined by Arlinger, et al. (2009) [1] as the “interactions between human hearing and cognition” (p.371) considering the role of both bottom-up (ear) and top-down (brain) processing in speech perception and communication. Research under the theoretical constraints of cognitive hearing science has shaped our understanding of human communication as evidence continues to emerge supporting the importance of the interplay between cognition and hearing. Recently, there have been several systematic reviews of the literature outlining this evidence in addition to new findings [2-4]. This evidence includes: the correlation between cognitive decline and hearing loss [5], hearing loss as a risk factor for dementia [6], neural plasticity and degeneration that occurs with hearing loss [7], reduced cognitive decline with hearing aid use [8], and better hearing aid outcomes for those with better working-memory capacity, verbal processing speed, etc. [9] The implications of cognitive hearing science on clinical audiology are significant, yet clinical practice protocols remain largely unchanged.
Cognitive hearing science research can be found as early as the 1950s, with the work of Broadbent [10, 11] and others [12, 13] explaining the ability to communicate in complex listening environments with use of a selective model of attention. However, much work in the second half of the 20th century considered hearing in isolation and focused primarily on sound processing in the cochlea. Many early models of the auditory system emphasized bottom-up processing for simplistic stimuli such as pure tones. Also, much of the cognitive science literature considered visual stimuli as it was easier to manipulate and could be examined in animals unlike language and music [1, 14].
Research investigating both cognition and hearing in the early 2000s grew sharply in several areas such as research examining speech perception in more realistic complex listening environments and studies considering age related differences in auditory perception [15]. Research including that by Humes and colleagues [16] has highlighted significant individual differences in successful communication despite similarities in auditory sensitivity or hearing loss and that cognitive capacity such as working memory may be a contributing factor [17]. There is also some emerging evidence that auditory rehabilitation and hearing aids my help counteract cognitive decline with aging [8].
However, despite the emergence of cognitive hearing science and the potential implications, there has been limited advancement in clinical application to audiology. Even if clinicians have a growing awareness of the significance of the interrelationship between cognition and hearing, it is not clear how clinical practice should evolve as the understanding of the relationship between cognition and hearing continues to grow. Specifically, clinical awareness of implications of hearing loss as an early warning sign or risk factor for dementia and cognitive decline, and that untreated hearing loss may be associated with more rapid cognitive decline with age, are known, but changes in clinical protocol remain sparse. Certainly, there have been calls for clinicians to account for the influence of cognition on communication, minimally supporting the use of cognitive screening tools as part of assessing hearing loss [18]. However, rigorous guidelines with specific recommendations for clinical protocol are underdeveloped. There are certain to be clinical opportunities and advancement in interdisciplinary practice to address cognition in during the diagnosis and treatment of hearing loss.
There is strong evidence linking hearing loss with cognition function, as well as an association between hearing loss and cognitive decline. How does knowledge of these relationships inform clinical practice in audiology? How should clinical practice be modified in regards to audiological diagnosis and treatment of older adults? The purpose of this paper is to review the cognitive hearing science literature for general and specific audiological recommendations for our older adult patients who may have both hearing loss and cognitive deficits or may be at risk for cognitive decline. We will examine findings in the cognitive hearing science literature with a scoping review following PRISMA guidelines [19]. This search will focus on research that has clinical recommendations or direct clinical application.
Methods
This review was developed using guidelines by Peters, et al.
(2015) [20], the Comprehensive Pearl Growing Method search
strategy outlined by Schlosser, et al. (2006) [21], and the Preferred
Reporting Items for Systematic reviews and Meta-Analyses
(PRISMA) checklist for data collection, documentation of inclusion
and exclusion criteria, as well as information bias risks [19]. The
objectives of the review include:
(1) What is the current state of Cognitive Hearing Science?
(2) How can Cognitive Hearing Science be applied to evidencebased
practice in audiology?
Data Collection Process
In accordance with guidelines set forth by Peters, et al. (2015)
[20] and Schlosser, et al. (2006) [21], the following steps to complete
the Scoping review were followed:
(1) Researchers compiled studies from any relevant reviews.
There were not any reviews on the Cognitive Hearing Science
area of specialty available.
(2) Researchers then identified Pubmed, ComDisDome, and
CINHL Complete as databases most relevant to the Cognitive
Hearing Science Literature.
(3) Key terms and quality filters were determined according
to this literature. The following key terms were identified:
Table 1:Key terms for the literature search.
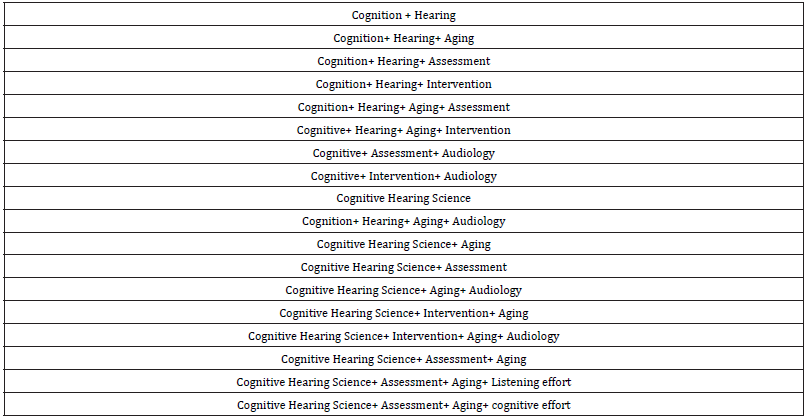
(4) Researchers searched all key terms across each of the
three identified databases. Key terms were used to narrow
the search results to 50 articles or less. Search results were
then reviewed for relevance to the topic of Cognitive Hearing
Science. Eleven key articles were identified.
(5) For each of the eleven articles, secondary hand-searching
reference lists from identified articles and citation tracking
of articles was completed. For each of the citations from the
eleven articles, abstracts were compiled.
(6) For all articles identified as relevant, the abstracts for
those articles were pulled.
(7) Abstracts were checked for relevance by two independent
investigators
(8) The independent investigators then reviewed their
findings, discussed discrepancies between the abstract review,
and identified articles to be included in the Scoping Review.
(9) Once the Articles to be included in the scoping review
were identified, each article was reviewed and documented
using the Preferred Reporting Items for Systematic reviews and
Meta-Analysis (PRISMA) checklist for data collection.
Measures
Extraction fields included: author, year of publication, aims/ purpose, study population and sample size, methodology, assessment type/ intervention type /concept, outcome measures, results, discussion, key findings, funding [19, 20].
Analysis
The extracted results will be discussed in two main conceptual categories of speech-in-noise perception and clinical implications and recommendations. The speech-in-noise perception category will include speech perception in noise, listening effort and related cognitive variables. The clinical implications and recommendations category will include studies that are relevant to diagnosis and treatment of older adults with hearing loss. In diagnosis, subjective assessments of hearing, overall health and well-being, and cognitive assessments are considered. Treatment includes any studies considering cognition in regards to hearing aid fittings, aural rehabilitation, cochlear implant fittings, outcome measures and auditory training.
Results
The final list of studies included 118 articles. There were 54 studies associated with auditory speech-in-noise perception, including measures of listening effort and relevant cognitive variables and 64 articles with specific clinical implications or recommendations regarding cognition and hearing. Clinical articles could be categorized as diagnosis of hearing and cognition, general health, quality of life and intrinsic capacity, treatment (e.g., cochlear implants and hearing aids) and auditory training.
Grouping Number 1: Speech in Noise Perception
This section included 54 papers regarding SIN performance, listening effort, as well as what cognitive factors are important in determining individual differences in speech-perception. Several of these 53 papers and one book chapter addressed the impacts of hearing loss on SIN performance and listening effort explicitly. Many of the listening effort studies also examined which cognitive factors are important in explaining individual differences in listening effort (Table 2).
Grouping Number 2: Clinical Implications and Recommendations
Although the scoping review resulted in 64 studies that had
suggestions for clinical consideration in diagnosing and treating
older adults with hearing loss, they did not necessarily have specific
data to back up these suggestions or the evidence available was not
of high quality. Researchers examined:
1) outcomes for people treated with hearing loss, including
cognition and mental health,
2) individual differences in cognition in relation to
performance and preferences with hearing aids,
3) testing for cognitive function and hearing loss in older
adults, and
4) general patient care regarding hearing and cognition in
older adults.
Discussion
Grouping Number 1: Speech in Noise Perception
Older adults often have more difficulties understanding speech in adverse listening conditions. This can be attributed to either decreased hearing sensitivity, deficits in cognitive function or both. Speech-perception-in noise can be quantified in terms of performance (percent-correct), SNR necessary to achieve a set level of performance (e.g., SNR for 50 percent-correct) or listening effort (dual-task, perceived effort, ERP, delayed recall, etc.). A number of studies have attempted to compensate for hearing loss by equating performance by adjusting SNR [22-24], through aided testing [25], or by spectrally shaping stimuli [15, 26]. This section had three categories of article methodology: SIN performance, SIN listening effort and SIN related cognitive variables. The studies explicitly examining cognitive variables were either cognition in context of SIN performance or listening effort. Subsequently, these three SIN subcategories of SIN articles will be discussed separately.
Speech-in-Noise (SIN) Performance
When correcting for hearing loss for SIN testing through adjustments to the SNR, aided testing, spectral shaping of stimuli to compensate for high-frequency hearing loss or through modeling [27], there remained significant differences between participants (young normal hearing versus older and hearing impaired) for SIN performance. Hearing loss was eliminated as a significant factor in a few studies [26, 28], while for some hearing loss remained significant [29]. Schneider, et al. (2000) [28] and Murphy, Daneman & Schneider (2006) [22] for the non-spatial separation condition were exceptions, with no significant differences between groups. Keep in mind the SIN test materials and the age and degree of hearing loss for the older participants in these studies varied considerably. Another study [30] analyzed QSIN psychometric functions in 139 young and older adults. The authors used modeling to characterize results and proposed two variables alpha (steep part of function) and lambda (shallow top of function), where alpha can explain the shape of psychometric functions in terms of “audibility and lowlevel central auditory processing” while lambda was associated with cognitive factors. Despite the lack of consensus across studies, hearing loss and age are certainly important factors in determining SIN performance.
Speech-in-Noise (SIN) Cognitive Variables
Several sources indicated that cognition is an important contributing factor determining SIN performance in older adults. Two systematic reviews of the literature [17, 31] found that working memory (WM) was significant (r=.28 for pooled data across studies) and IQ was not significant or less significant (r=.18 for pooled data across studies [31]. Also found inhibitory control (IC), processing speed and episodic memory were significant cognitive factors.31 Correlations for these four cognitive variables and SIN performance were around 0.3 [31].
Kim, et al. (2020) [27] utilized a listening span (LSPAN) test (a measure of WMC) that successfully predicted SIN performance in older adults. Humes (2005) [32] only found a small portion of variance for Auditory Processing Disorder (APD) test results (that included a dichotic listening task) associated with cognition (14% of total variance explained by IQ, age, and the auditory brainstem response). A total of 40% of variance in APD performance was explained by hearing loss; however, they did not include other cognitive measures such as working memory and processing speed. Nearly half (46%) of total variance was unexplained. Humes, Kidd & Lentz (2013) [15] could explain 60% of total SIN performance variance when including one global cognitive factor and 5 psychoacoustic hearing factors along with age, Environmental Sound Identification and Text Reception Thresholds. Schneider, Daneman & Pichora-Fuller (2002) [33] and Pichora-Fuller (2003) [23] attributed variation in speech intelligibility for older adults to cognitive processes such as memory, attention as well as slower cognitive processing. Another study examined QSIN performance in adults 60 years and older over an eight-year period [34]. Those with lower QSIN scores had similar cognitive function to those with better SIN performance but showed more rapid cognitive decline over the eight-year period. Chen, et al. (2022) [35] indicated significant cognitive function variance (general, WM, Executive Function (EF) and verbal learning) in older adults with hearing loss (with or without hearing aids) could be explained by SIN performance, while pure tones thresholds were not relevant to cognitive function. Lee and Lee (2023) [36] suggested using reaction time (RT) for SIN testing as a potential screener for MCI in older adults, with significant correlations between MMSE and SIN RT. Another study suggests auditory streaming task performance and reaction times are sensitive to both cognitive and auditory abilities [37]. Three articles [38-40] indicated WMC and IC (Inhibitory Control) may be causative factors for SIN performance and may be considered clinically. Lentz, Humes and Kidd (2022) [41] indicate that performance for psychoacoustic tasks, hearing (50% of tasks) and cognition (75% of tasks) are important factors. As listening task performance was impacted by either WM and/or hearing the authors stress both cognition and hearing as important determinants of higher-level auditory processing. In many studies WMC was found to be the significant cognitive factor. However, keep in mind that WMC was often the only cognitive factor measured. Other measures such as IC and processing speed were found to be important when included.
Another systematic review [42] indicated that for younger normal hearing adults, better working memory is not associated with better SIN performance. This lack of association between WMC and SIN performance in young adults with normal hearing and cognition may indicate that for even for individuals on the lower end of normal, WMC is still sufficient for most SIN tasks. Kocabay, et al. (2023) [43] found for younger adults (18-59) 72% of SIN performance variance was determined by hearing loss and age, while only 76.2% when considering specific cognitive and auditory factors. Fullgrabe & Rosen (2016) [42] suggest that older adults with hearing loss may require greater WMC to decipher phonological mismatches between the incoming message (which may be of low quality and/or distorted) and representations in LTM (see Ease of Language Understanding (ELU) model [14, 44, 45]). Such deficits in phonological representations (and processing) are seen for adults with severe hearing loss [46]. Listening for those with severe hearing loss may require more top-down processing and frontal lobe compensation that may be taxing on working memory [47]. Studies that were not necessarily interested in eliminating or reducing hearing loss as a factor often indicated both hearing and cognition are important SIN performance factors [15, 48].
Table 2:Summary of speech-in-noise research.
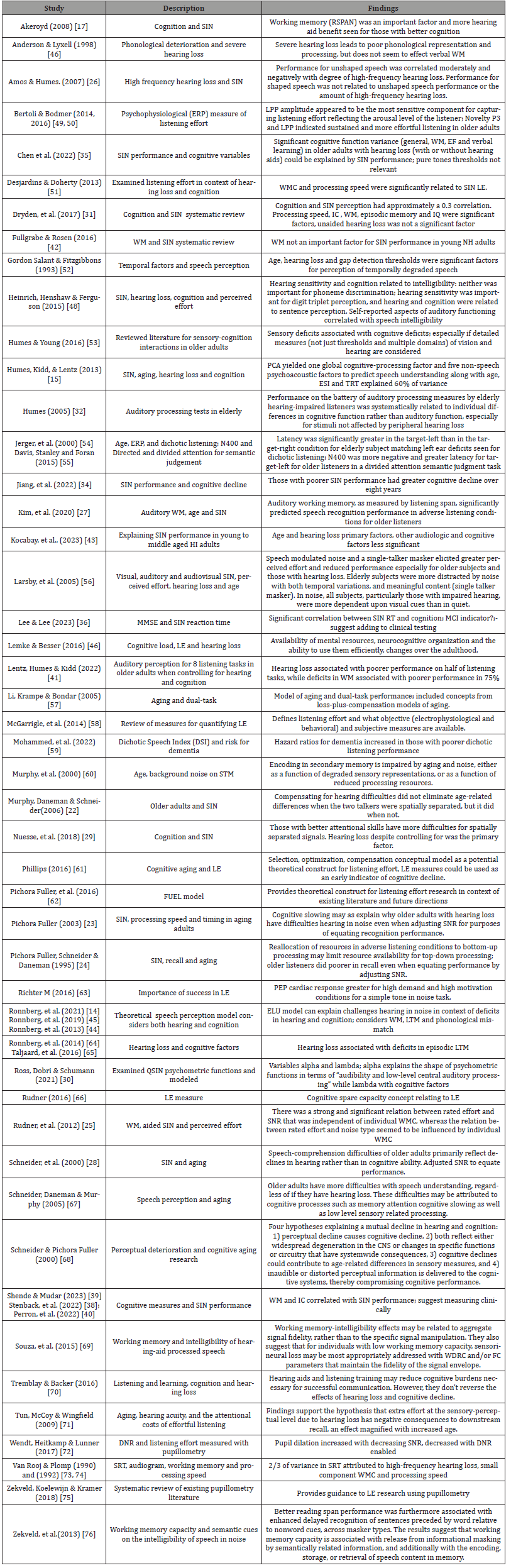
Table Abbreviations: Central Nervous System (CNS); Digital Noise Reduction (DNR); Frequency Compression (FC);Inhibitory Control (IC); Ease of Language Understanding (ELU); Environmental Sound Identification (ESI);Event Related Potential (ERP);Executive Function (EF); Late Positive Potential (LPP); Listening Effort (LE); Long Term Memory (LTM); Negative 400 (N400); Normal Hearing (NH); Quick-Speech-In-Noise (QSIN); Reaction Time (RT); Reading Span (RSPAN); Signal-to-Noise-Ratio (SNR); Speech-In-Noise (SIN); Text Reception threshold (TRT); Wide Dynamic Compression (WDRC); Working memory (WM); Working Memory Capacity (WMC)
Speech-in-Noise (SIN) Listening Effort
Although hearing loss can be compensated to equate intelligibility, listening to speech-in-noise can still require more listening effort for those with hearing loss and cognitive deficits. For degraded speech or speech embedded in noise, the additional cognitive resources necessary to understand what is spoken come at a cost. This cost related to bottom-up processing may leave fewer resources for top-down processing (storage and retrieval from memory, more in-depth listening, comprehension, etc.). There are several objective and subjective behavioral and physiological measures sensitive to the amount of effort required for listening [58]. Delayed recall [23, 76], dual-task procedures [56], perceived effort [25, 55], ERPs [27, 49, 50] and cardiac measures63 have been used as indicators of listening effort.
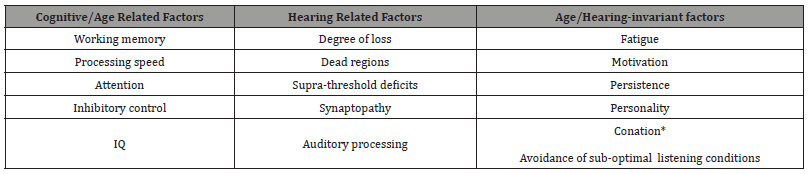
Research consensus suggests that although older individuals may have similarities in the type and degree of hearing loss, they often have differences in SIN performance or even when SIN performance is similar, they may achieve that level of performance with different amounts of listening effort (LE). Differences in LE can be attributed to age or cognitive factors, hearing factors or age/hearing invariant factors (Table 3) [61]. The cognitive factors include those that have been discussed so far (WMC, EF, processing speed and IC), with WM often quantified with use of a LSPAN or RSPAN task. The hearing factors include supra-threshold deficits or capacities, which include temporal processing [52] and psychoacoustic factors including auditory streaming and frequency selectivity [15]. The etiology of one’s hearing loss may also impact SIN performance and LE, such as cochlear dead regions, synaptopathy and auditory processing. These factors may further degrade the acoustic signal or make it more challenging to listen to speech in noisy backgrounds. This can be explained by the ELU model, where degraded speech inputs or hearing loss may result in mismatches between the speech signal (language) and semantic long-term memory, requiring greater effort through increased use of WM to resolve. Cognitive deficits also may limit the availability of resources necessary to resolve these mismatches [15, 45]. Subject factors such as motivation, conation and personality are also known to be important in listening effort performance (Table 3) [61]. Individuals that are more driven, motivated, and focused may apply greater effort to listening [46, 62, 63].
Table 3:Listening effort factors (adapted from Phillips 2016) [61].
There were nine articles in the search that pertained to listening effort. Three were theoretical or review papers, three were focused on perceived effort, one involved a simultaneous dual-task procedure, and two quantified LE with a delayed recall task, and three involved electrophysiologic measures.
The three theoretical or review papers on LE have a consensus that at a fixed or controlled level of SIN performance LE varies considerably among individuals. For listening in more challenging conditions there becomes mismatch between internal cognitive resources available and task demand. In order to maintain performance, the listener must allocate more resources to the processing load [46, 62]. Differences in LE may reflect cognitive differences hearing related differences and age invariant factors. These factors include episodic and working memory, attention and processing speed [61]. Normative age-related changes and significant deficits in cognition will impact communication especially when hearing loss is also present. These individual differences stress the importance in including cognitive assessments in research examining age related changes in communication as well as in the geriatric audiology clinic.
The two perceived effort studies indicate that older adults and those with hearing loss exhibit greater perceived effort [56]. Further, poorer SNRs elicited greater perceived effort and greater working memory was associated with lower perceived effort [25]. Results for measuring LE with a delayed recall task indicate that greater WM may enhance delayed recognition performance with more cognitive resources available for encoding, storage and retrieval of speech information [76]. Poorer delayed recall is often seen in older adults and those with hearing loss [71]. The dual-task study [51] showed a significant correlation between working memory, processing speed and performance on the secondary task when combined with a sentences-in-noise task, indicating these cognitive variables are two important LE factors. The review of pupillometry LE studies [75] shows a complex relationship with hearing loss and cognition with mixed results for both. Pupillometry results may be confounded by differences in age across participants, as pupil size decreases with age. Greater cognitive capacity for variables including scholastic aptitude, linguistic ability, WM, and vocabulary often correspond to larger and slower pupil responses in more challenging listening conditions, indicating more in-depth listening and greater effort. Greater hearing loss is often associated with smaller amplitude pupil responses in more challenging listening conditions, indicating reduced effort or less in-depth listening. However, larger and slower responses were found for hearing impaired listeners in more challenging (50 percent correct) compared to easier (95 percent correct) listening conditions [72]. Bertoli & Bodmer (2016) [50] found increased amplitudes for the late positive potential (LPP) in older adults and those with hearing loss compared to younger normal hearing adults, and all listeners had delayed and larger amplitude responses in more challenging compared to easier listening conditions, indicating greater effort.
Listening effort may be sensitive to individual differences in cognition and may at times explain why individuals with similar hearing loss may have dissimilar treatment outcomes. However, at this point it is unclear how this could be measured clinically in an efficient manner. Possibilities include subjective questionnaires and objective measures such as a delayed recall task58 or reaction time measures for word recognition testing [36].
Summary of Section 1
• When correcting for hearing loss (i.e., aided testing, SNR
adjustment) there remained significant age differences for SIN
performance [29].
• Researchers indicate cognition is an important factor in
determining SIN performance in older adults. Important
cognitive factors include WMC51, IC, processing speed [23] and
episodic memory [31].
• Studies with young normal hearing adults did not find WMC
as a relevant predictor of SIN performance [42].
• In two studies hearing loss was also associated with deficits in
episodic LTM [64, 65].
• Older adults with hearing loss place greater demand on
WMC for deciphering phonological mismatches and may also
have deficits in phonological representations. This increased
cognitive load and subsequent greater LE is realized in more
top-down and frontal lobe involvement in speech perception
[46].
• Listening effort appears more sensitive to individual
differences in cognition than SIN performance measures. LE
appears sensitive to cognitive related, hearing related and age/
hearing invariant factors. Cognitive factors that are significant
in LE studies include most often WMC, processing speed, IC and
selective attention.
Section 2: Clinical Implications and Recommendations
There were 64 articles that had direct clinical implications or
recommendations. These could be categorized under diagnosis or
treatment. Diagnosis articles examined:
(1) general health, quality of life and intrinsic capacity,
(2) subjective hearing assessments,
(3) special considerations for those with or at risk for
dementia, and
(4) cognitive assessments.
Treatment related articles investigated cognition in context of :
(1) general recommendations,
(2) hearing aids,
(3) cochlear implants, and
(4) auditory training.
Diagnosis: General Health, Quality of Life and Intrinsic Capacity: Cheong, et al. (2022) [77] suggest intrinsic capacity is predictive of mortality risk in older adults. Intrinsic capacity may be a good preindicator of frailty [78] and its assessment could provide a chance for early intervention. Measures of intrinsic capacity consider five domains of capacity: locomotion, cognitive, psychological, sensory and vitality [79]. Hearing loss often associated with frailty and may warrant measuring clinically. Beier, et al. (2022) [80] examined two different assessments of frailty. They determined that the Frailty Index (FI) is more sensitive to hearing loss while the Frailty Phenotype (FP) results are only associated with motor function. The FP model has five frailty criteria: unintentional weight loss, self-reported exhaustion, weak grip strength, slow gait speed and low physical activity level. Someone is classified as being frail if they have deficits in at least three of these criteria. The FI on the other hand has 40 variables covering physical, as well as psychological, social and cognitive aspects. This has similarities to the five domains of IC assessments such as the Integrated Care for Older People (ICOPE) tool [79]. The FI is calculated by dividing the number of deficits present by the total possible number of deficits (40). Scores exceeding 0.25 (10 deficits) are considered to indicate frailty [80]. The authors emphasize that hearing loss should be included in frailty assessments (using the FI) given their findings and considering previous described association between hearing loss and cognition, gait-speed, fall-risk, depression, hospitalization and mortality.
Three articles [81-83] highlight the value of a more holistic approach in diagnosing and treating older adults with hearing loss given co-morbidities with cognitive and physical health. For example, hearing loss has been found to have a higher prevalence in those with diabetes and high blood pressure.83 Another issue is that hearing loss is inadequately screened in the primary care setting. Del Vecchio, et al. (2023) [84] suggest screening people for hearing loss and cognitive function earlier and consider high blood pressure and cholesterol to be risk factors for “common pathology linking the inner ear and brain damage” (p. 1). The broad scope of the co-morbidities experienced by older adults and their impact on quality of life and mortality risk necessitates interprofessional care. Minimally this may require appropriate and automatic referrals, such as a hearing assessment for those diabetic or those receiving memory services.
Self-efficacy and Illness Behavior (IB) may be important factors for Quality of Life (QOL) in context of hearing loss [85]. QOL is also known to be impacted by hearing loss and cognition [86]. Depression and reduced social engagement are associated with hearing loss, while less depression and more social engagement in those with higher cognition [5]. Hearing loss may indirectly impact cognition status through reduced social engagement [5, 87]. Emotional distress and exaggerated concerns of hearing loss were associated with lower self-efficacy and poorer QOL [87]. Poorer self-reported hearing associated with greater perceived agerelated losses (Awareness of Age-Related Change or AARC). This was especially true for interpersonal relationships in older adults and social cognitive and social emotional functioning in younger adults reporting hearing difficulties [88]. These studies indicate subjective assessments of hearing may be warranted as a screening tool, however perceived hearing loss often does not align with objective hearing assessments [89].
Three articles discussed more general patient care regarding
hearing and cognition in older adults. Heine & Browning (2002)
[90] discussed health care for those with sensory loss and the fact
that 70% of those with severe vision impairment have hearing loss.
Given the association between sensory loss with depression, anxiety,
lethargy and social dissatisfaction, the authors recommend a multidisciplinary
approach in dealing with such patients. Pichora Fuller,
et al. (2013) [91] and Pichora Fuller (2015) [92] emphasize the
importance of treating hearing loss in the older adult, particularly
in those with cognitive deficits. The author(s) stress:
1) identifying and treating hearing loss is especially
important given the potential causative effect of hearing loss
on cognition and the potential for hearing aids to slow down
cognitive decline,
2) the need for cognitive screening as part of audiological
assessments,
3) improving hearing health through amplification and
aural rehabilitation may be beneficial by improving cognitive
function and overall health and well-being.
One study considered gait and vestibular function (Danneels, et al. 2023) [93] in addition to cognition and hearing measures [92]. Slower gait speed in combination with moderate hearing loss was associated with a poorer MoCA score and higher fall incidence while hearing loss alone was not [93]. Bosmans, et al. (2022) [94] described an association between vestibular loss and cognitive impairment. Further research is needed on the causation as well as the benefits of treating vestibular loss on cognitive function.
Diagnosis: Subjective Hearing Assessments: Given the increased awareness of the relationship between cognition and hearing, it is not surprising that there were six studies that examined how we should screen/test for hearing loss. What should be the standard? Subjective questionnaires, pure tones and speech perception in noise assessments are three possibilities. However, one study found that subjective assessments have poor sensitivity and specificity, especially when considering older adults with MCI [95]. This may relate to another subjective hearing loss study by Sukurai, et al. (2023) [89] finding a large percentage of older adults fail to recognize they have hearing loss. These “over-estimators” of hearing sensitivity had associated lower cognition and poorer gait compared to those having an accurate gauge of their own hearing sensitivity. Those who self-identified as having hearing difficulty tended to be more depressed, as was seen in other studies.87 Another study introduced a self-report that considered hearing, vision, cognition and psychosocial functioning on quality of life (hAVICOP) [96]. Merten, et al. (2022) [97] found that psychosocial well-being was associated with better hearing, vision, neural and cognitive functioning. Goodwin, Hogervorst and Mairdment (2022) [98] looked at the hearWHO, digits in noise and the Modified Telephone Interview for Cognitive Status (TICS-M) as well as psychosocial well-being and cardiovascular health. The hearWHO is a free self-assessment hearing screener developed by the WHO with good sensitivity and specificity (0.962 and 0.903, respectively) [99]. Poorer hearing was associated with poorer cognitive function, social isolation and being sedentary. An objective self-assessment such as the hearWHO may be a good alternative to subjective measures of hearing sensitivity.
Diagnosis: Special Considerations for Dementia: Dawes, et al. (2022) [100] give recommendations for hearing assessments in individuals with dementia. They provided several recommendations in order to assess these often difficult-to-test individuals. This included testing 1000 and 4000 Hz by air conduction in both ears first, slowing down and having patients respond verbally with a “yes” instead of a button push, consideration of Distortion Product Otoacoustic Emissions (DPOAE) testing as a screening tool and functional hearing tests. One study has shown utility in using dichotic testing as a screening for dementia risk.59 Hazard ratios for dichotic auditory tests for dementia measured longitudinally were 1.82 and 4.19 for Dichotic Sentence Index (DSI) scores 50- 80% and below 50% respectively. Others suggest Auditory Event Related Potentials (AERP) may be useful indicators of pre-clinical AD [101].
Diagnosis: Cognitive Assessments: Twenty-one articles pertained to testing for cognitive function and hearing loss in older adults. Dupuis, et al. (2015) [102] indicated that more individuals pass general cognitive assessments (MoCA) that do not have sensory impairment compared to those that do. They stressed that hearing impairment may be both a confound for testing as well as a risk factor for cognitive decline. Gaeta, et al. (2019) [103] also found reduced MMSE scores in cognitively normal individuals without hearing loss, when the test materials presented were low pass filtered to simulate reduced audibility. Hill Briggs, et al. (2007) [104] outline which cognitive test procedures are appropriate for individuals that are deaf and use sign language and may also be appropriate for those with hearing loss. This included using the Wechsler Memory Scale (WMS-III) visual memory tests for immediate and delayed recall. These two subtests with normative data for 16-90-yearolds are the design memory and visual reproduction tests. The WMS-IV also includes the California Verbal Learning Test (CVLT) for auditory memory. Two visual WMC tests are also in the WMSIV (spatial addition and symbol span), but only have norms for 16-69-year-olds. Junkkila, et al. (2012) [105] provide support for using the Cambridge Neuropsychological Test Automated Battery (CANTAB) Paired Associate Learning subtest, which is a non-verbal, visual task sensitive to early stages of Alzheimer’s disease and MCI. However, clinicians are likely to use a screening tool such as the standard MoCA, that include an auditory only test for memory. The MoCA includes an immediate and delayed recall (5 minutes later) test for five words (such as face, velvet, church, daisy, red) read by the examiner. The MMSE asks the patient to recall the name of three objects the examiner reads until they get the names correct and then delayed recall after an attention task (count by 7’s up until 5 correct answers or spell world backwards). Shen, Anderson & Souza (2016) [18] provide a tutorial for assessing cognition in the audiology clinic. The recommendations were to look for signs of dementia and cognitive impairment in the case history, make sure to have a process for referring patients that are suspected of having a cognitive impairment and to include a cognitive screening tool sensitive to MCI such as the Montreal Cognitive Assessment (MoCA) and the Saint Louis University Mental State (SLUMS) test. Tests such as the MMSE may be more sensitive to Alzheimer’s but are as less sensitive to MCI. They also emphasized making sure hearing loss or difficulties hearing the test items is not the reason for a low score on the cognitive screening. Tests should be completed in a quiet room (such as a sound booth) and with amplification provided with a Personal Sound Amplification Product (PSAP) or demo hearing aid if the patient does not have their own hearing aids. Wong, et al. (2014) [106] examined performance on the MMSE in a group of older adults fitted with a monaural hearing aid. Results on the MMSE were poorer than results from the general population despite the use of amplification, consistent with the co-morbidity of hearing loss and cognitive decline.
Given that most cognitive assessments include an auditory memory test and other tests and instructions read by the examiner, there are concerns that audibility could result in lower assessment scores for those with hearing loss [107, 108]. Andries, et al. (2019) [109] argue that appropriate cognitive assessments for the hearing impaired should provide “visual support during administration”. Possibilities include the RBANS-H, HI-MoCA, CANTAB and ALACog. Others have confirmed that those with hearing loss do better on written vs. standard MoCA [95]. The development of the MoCA-H [100] and MoCA-HI [110] address this problem with the standard MoCA 8.1 test through a written format. A version of the MoCA-HI is available in paper format on the official MoCA website (https:// mocacognition.com). There are also cognitive screening tests for dementia that are also in written form [111]. Alternatively, hearing aids and PSAPs can offset the effects of hearing loss on audibility for cognitive assessments (i.e., MMSE) [103, 106]. A few cochlear implant studies and reviews examined cognitive assessments as outcome measures post-implantation [109, 112-114] or to determine candidacy [80, 115]. Assessments that are nonverbal (CANTAB and ALACog) or have visual support were preferred (HIMoCA).
Another option for audiologists is to use a delayed recall for speech stimuli they already use in the clinic such as the standardized monosyllabic word lists (NU-6) or a more formalized test of auditory memory such as the CVLT or the Word Auditory Recognition and Recall Measure (WAARM) [116]. The WAARM test has five sets of words (from the Words in Noise test) in blocks of 2,3,4, 5 and 6 words. Other options include using a list of 15 monosyllabic words presented visually or auditorily and ask them to recall as many as they can after reading or repeating back. This procedure also can be modified to be presented visually to offset audibility concerns [116]. However, such procedures only address auditory memory while formal cognitive assessments and screeners are more comprehensive.
Yueh, et al. (2003) [117] stressed the importance of screening for hearing loss in the elderly population. Screening recommendations included the Hearing Handicap Inventory for the Elderly (HHIE) and use of an Audioscope (handheld device that presents a 1000 and 2000 Hz tone at 40 dB HL) in the general practitioner’s office. Omar, et al. (2023) [118] indicate professionals (i.e., psychologists and audiologists) working with older adults with either memory or hearing problems often do not address the possibility of comorbidities despite acknowledgment from professionals that this comorbidity exists. In other words, audiologists rarely (4%) conduct or recommend cognitive assessments and psychologists do not often (4%) recommend or conduct hearing tests.
Quaranta, et al. (2014) [119] also provide evidence to support including more than just pure tone audiometric testing for older adult hearing assessments. The relationship between hearing loss when it includes SIN testing (SSI-ICM) in addition to pure tone testing has a stronger relationship with cognition in older adults. Xu and Cox (2021) [120] found that the American Four Alternative Auditory Feature (AFAAF) test was highly sensitive to individual differences in cognitive function (WMC). This test is available for free from the Hearing Aid Research Lab, University of Memphis website.
Treatment: Hearing aids: Seven articles examined outcomes for adults fit with hearing aids or PSAPS, including cognition and mental health. Acar, et al. (2011) [121] found that three-month post-hearing aid fitting, older adults showed improvements on cognitive function (MMSE) and psycho-social functioning. Allen, et al. (2003) [122] found in 42% of Alzheimer’s patients a significant improvement on the Clinical Global Impression of Change (CGIC) scale 24-week post hearing aid fitting, although cognition (MMSE) and problem behavior measures did not. Glick & Sharma (2020) [123] found evidence of neuroplasticity in individuals with hearing loss prior to being fit with hearing aids and three-months post-fit. Pre-frontal and frontal auditory areas were sensitive to visual inputs (cross-modal reorganization) prior to fitting and lost sensitivity to visual input post-fitting. Other studies indicated differences in outcomes and SIN performance attributed to individual differences in cognition. Sarant, et al. (2020) [8] examined longer term (18 months post-fitting) effects of treating hearing loss on cognition in older adults (60-84 years old). Almost all participants (97.3%) showed either no change or improvement in cognitive function overall (MMSE) while having a significant improvement for executive function (Groton Maze Learning Test). Digital Noise Reduction (DNR) may reduce processing effort as seen in cortical responses, although not behaviorally. DNR may be more beneficial to those with reduced noise tolerance [124]. Perron, Lau and Alain (2023) [125] determined that 60-70 percent of older adult participants with normal to mild hearing loss showed improved SIN performance and reduced effort with PSAP use. Age, hearing loss and cognition were predictors of PSAP benefit, with those with better cognition, older age and poorer hearing showing the most benefit. Visual aid use in vision impaired and hearing aid use in hearing impaired nursing home residents associated with slower cognitive decline (Kwan, et al., 2022) [126].
A large number of the remaining articles looked at individual differences in cognition in relation to performance and preferences with hearing aids. Gatehouse, Naylor and Elberling (2003) [127] showed that those with higher cognitive function benefited more from temporal variation in background noise and faster hearing aid time constants than those with poorer cognitive function. Lunner (2003) [128] found a significant negative correlation between SNR and WM (RSPAN), which was even stronger for aided performance. Ng, et al. (2015) [129] and Lunner, Rudner & Ronnberg (2009) [9] indicated that PTA, executive function and WM predicted variability and preference for directional microphones, digital noise reduction and speed of compression settings for their hearing aids. Souza & Sirow (2014) [130], Souza, Arehart & Neher (2015) [69], Gatehouse, Naylor and Eberling (2003) [127] Lunner & Sundewall Thoren (2007) [131] all found in association between fast versus slow compression release time and cognition (WM assessed with the RSPAN). Those with higher WMC did best and preferred fast, while those below the median WMC preferred and did better with slow. Xu and Cox (2021) [121] were the exception in not showing this relationship. Windle, Dillon and Heinrich (2023) [132] emphasize considering slow-acting compression for those with cognitive deficits and the negative impact of signal processing distortions of speech envelope on listening. They also emphasize the need for improving the SNR especially for older hearing aid users, as SIN performance decreases with age. This may require utilizing lower compression ratios, moderate digital noise reduction settings and increase use of directional microphones and closedear fittings to improve directionality. Lowering compression ratios may be especially necessary if utilizing fast-acting compression release times. They also warn against using frequency lowering/ compression and other advanced hearing aid features in those with cognitive impairment, as they may add to envelope distortions. Neher (2014) [133] found that those with higher cognitive function were better able to discern changes in hearing aid programming (fine tuning) and that those with poorer cognition may benefit from more automatic hearing aid functioning. Ronnberg, et al. [15, 44, 45] and Ng & Ronnberg (2019) [134] also indicate better SIN performance is associated with better WMC and hearing loss in general was associated with deficits in LTM, both auditory as well as visual. Further, Rudner, et al. (2012) [25] found an association between WMC and (aided) subjective listening effort.
Treatment: Cochlear implants: Six articles in this section looked at what are appropriate cognitive assessments to be used for older adults receiving CIs as outcome measures. These are summarized below: (1) MMSE improvement in particular was not associated with successful cochlear-implantation. The TMT and AlaCog showed improvement post-implantation [112].
(2) As mentioned elsewhere, the MMSE may be more appropriate for dementia screening than as a cognitive outcome measure or screener for MCI. For older adults receiving CIs there was a postoperative improvement in MMSE scores associated with better speech production only and not basic or advanced sound perception, self-esteem, activity limitations or social interactions as quantified with the Nijmegen Cochlear Implant Questionnaire (NCIQ) [114].
(3) Zucca, et al. (2022) [115] indicate CI outcomes (including word recognition and verbal fluency) were associated with age and the Auditory Trail Making Test (TMT-A) performance at baseline. The TMT-A is sensitive to cognitive shifting and cognitive processing speed.
(4 and 5) The NIH Toolbox Cognition Battery is an option for an outcome measure for older adult CI recipients [135]. However, processing speed was the only cognitive assessment associated with poorer SIN performance.
(6) Andries, et al. (2023) [109] found that individuals with MCI showed improvement in both cognitive functioning and SIN performance 12-months post-op cochlear implantation. Greater SIN improvement was associated with greater improvement on RBANS-H, which is a cognitive assessment that substitutes auditory tasks with auditory-visual ones.
Treatment: Auditory training: Henshaw, et al. (2022) [136] found working memory training did not generalize to other cognitive measures, SIN performance or self-reported hearing in older hearing aid users. The authors contend that more dynamic training protocols that may be more directly related to communication should be considered. Conversely, Sommers, et al. (2015) [137] found that for a commercial auditory training program (computerized learning Exercises for Aural Rehabilitation (clEAR)) greater word recall was retained at 3 months posttraining compared to pre-training results. Further, Lowe et al., (2023) [138] found at-home-training using conversation in noise and conversation in quiet resulted in improved self-reported hearing difficulty. The conversation in noise group also showed reduced listening effort as measured with a dual-task procedure. Kucuk, Dere and Mujdeci (2022) [139] found a significant decrease in MMN latencies as a result of auditory training. Additionally, Schumann and Ross (2022) [140] found that adaptive syllable training improves phoneme identification in older listeners with and without hearing loss. However, similar pre-test accuracy (80% correct) could only be achieved at lower SNRs in the listeners without hearing loss post-training.
Summary of Section 3
There were not frequent or consistent clinical
recommendations in the cognitive hearing science literature.
Researchers stressed the strong relationship between hearing
and sensory function with cognition in the elderly and that
it is important to screen for both hearing loss and cognitive
impairment. Also, many stressed the potential added benefit of
treating hearing loss in improving cognition or at least staving
off its decline [92].
• Audiologists should start screening for cognition utilizing a
screening tool that is sensitive to mild cognitive impairments
such as the MoCA. Authors also stress being careful to not
confound the inability to hear or see items on the cognitive
screening with cognitive impairment.18,100-101,106 Some
indication that speech perception tasks used in diagnosing
auditory processing disorders (dichotic listening) may be early
indicators of dementia [59].
•Consider individualized care for older adults in context of
quality of life with screenings for nor only hearing loss and
cognition but for comorbidities and risk factors including
dementia, cardiovascular disease, fall risk, vestibular function
and diabetes [84, 94, 96, 100, 106].
• Including additional audiological assessments for older adults
such as SIN testing and dichotic listening tests as these appear
sensitive to cognitive functioning [119].
• Preference for slower compression settings [69, 120] and
greater digital noise reduction9,128,133 in those with MCI.
Slower compression release and lower compression ratios
should result in easier listening through reducing speechenvelope
distortions [132].
• Deficits in cognitive functioning such as WMC and
processing speed may necessitate better SNRs for successful
communication technologies that improve SNRS such as
directional microphones and Frequency Modulation (FM)
systems. Also, more automatic functioning of hearing aids is
warranted in these individuals as well.
• Auditory training (Ferguson & Henshaw (2015) [141]; Lowe,
et al. (2023) [138]) may also be a beneficial treatment option.
Conclusion
• Specific recommendations are lacking regarding tailoring
evidence-based treatment for hearing loss based on cognitive
functioning.
• Audiologists should consider measures of cognitive health
when diagnosing and treating hearing loss, including WMC,
attention and processing speed.
• In addition to speech testing in quiet, auditory processing or
SIN tests should be considered when evaluating older adults for
hearing loss.
• Considering ways to improve the Signal-to-Noise Ratio
(SNR) is vitally important in fitting hearing aids on those with
poorer cognition. This includes stronger DNR settings and
consideration of FM technology.
• May consider lower compression ratios and slower
compression release times for those with poorer cognition as
well as more automatic hearing aid functioning (i.e., disable
program button).
• Treating hearing loss is vitality important in maintaining
quality of life.
Acknowledgment
None.
Conflict of Interest
No Conflict of Interest.
References
- Arlinger S, Lunner T, Lyxell B, Pichora Fuller K (2009) The emergence of cognitive hearing science. Scand J Psychol 50: 371-384.
- Harrison B, Lister JJ, Lin FR, Betz J, Edwards JD (2015) Peripheral hearing and cognition: evidence from the staying keen in later life (SKILL) study. Ear Hear 36(4): 395-407.
- Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA (2018) Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg 144(2): 115-126.
- Yuan J, Sun Y, Sang S, Pham JH, Kong W J (2018) The risk of cognitive impairment associated with hearing function in older adults: a pooled analysis of data from eleven studies. Sci rep 8(1): 2137.
- Lin FR, Yaffe K, Xia J, Xue QL, Harris TB (2013) et al. (2013) Hearing loss and cognitive decline in older adults. JAMA Internal Med 173(4): 293-299.
- Thomson RS, Auduong P, Miller AT, Gurgel RK (2017) Hearing loss as a risk factor for dementia: A systematic review. Laryngoscope Investig Otolaryngo 2(2): 69-79.
- Kral A, Sharma A (2023) Crossmodal plasticity in hearing loss. Trends Neurosci 46(5): 377-393.
- Sarant J, Harris D, Busby P, Maruff P, Schembri A, et al. (2020) The Effect of hearing aid use on cognition in older adults: can we delay decline or even improve cognitive function? J Clin Med 9(1): 254.
- Lunner T, Rudner M, Rönnberg J (2009) Cognition and hearing aids. Scandinavian journal of psychology 50(5): 395-403.
- Broadbent D (1954) The role of auditory localization in attention and memory span. J Exp Psychol 47(3): 191-196.
- Broadbent DE (1958) Perception and communication. Amsterdam: Elsevier Science.
- Cherry E (1953) Some experiments on the recognition of speech, with one and with two ears. Journal of the Acoustical Society of America 25: 975-979.
- Moray N, Taylor A (1958) The effect of redundancy in shadowing one of two dichotic messages. Language and Speech 1: 102.
- Rönnberg J, Holmer E, Rudner M (2021) Cognitive Hearing Science: Three memory systems, two approaches, and the ease of language understanding model. J Speech Lang Hear Res 64(2): 359-370.
- Humes LE, Kidd GR, Lentz JJ (2013) Auditory and cognitive factors underlying individual differences in aided speech-understanding among older adults. Front Syst Neurosci 7: 55.
- Humes L E (2002) Factors underlying the speech-recognition performance of elderly hearing-aid wearers. J Acoust Soc Am 112: 1112-1132.
- Akeroyd M A (2008) Are individual differences in speech reception related to individual differences in cognitive ability? A survey of twenty experimental studies with normal and hearing-impaired adults. Int J Audiol 47: Suppl 2: S53-S71.
- Shen J, Anderson MC, Arehart KH, Souza PE (2016) Using Cognitive Screening Tests in Audiology. Am J Audiol 25(4): 319-331.
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7): e1000097.
- Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, et al. (2015) Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc 13(3): 141-146.
- Schlosser RW, Wendt O, Bhavnani S, Nail Chiwetalu B (2006) Use of information-seeking strategies for developing systematic reviews and engaging in evidence-based practice: the application of traditional and comprehensive Pearl Growing. A review. Int J Lang Commun Disord 41(5): 567-582.
- Murphy DR, Daneman M, Schneider BA (2006) Why do older adults have difficulty following conversations?. Psychol Aging 21(1): 49-61.
- Pichora Fuller MK (2003) Processing speed and timing in aging adults: psychoacoustics, speech perception, and comprehension. Int J Audiol 42 Suppl 1: S59-S67.
- Pichora Fuller MK, Schneider BA, Daneman M (1995) How young and old adults listen to and remember speech in noise. J Acoust Soc Am 97(1): 593-608.
- Rudner M, Lunner T, Behrens T, Thorén ES, Rönnberg J (2012) Working memory capacity may influence perceived effort during aided speech recognition in noise. J Am Acad Audiol 23(8): 577-589.
- Amos NE, Humes LE (2007) Contribution of high frequencies to speech recognition in quiet and noise in listeners with varying degrees of high-frequency sensorineural hearing loss. J Speech Lang Hear Res 50(4): 819-834.
- Kim S, Choi I, Schwalje AT, Kim K, Lee JH (2020) Auditory working memory explains variance in speech recognition in older listeners under adverse listening Conditions. Clin Interv Aging 15: 395-406.
- Schneider BA, Daneman M, Murphy DR, See SK (2000) Listening to discourse in distracting settings: the effects of aging. Psychol Aging 15(1): 110-125.
- Nuesse T, Steenken R, Neher T, Holube I (2018) Exploring the link between cognitive abilities and speech recognition in the elderly under different listening conditions. Front Psychol 9: 678.
- Ross B, Dobri S, Schumann A (2021) Psychometric function for speech-in-noise tests accounts for word-recognition deficits in older listeners. J Acoust Soc Am 149(4): 2337.
- Dryden A, Allen HA, Henshaw H, Heinrich A (2017) The association between cognitive performance and speech-in-noise perception for adult Listeners: a systematic literature review and meta-analysis. Trends Hear 21: 2331216517744675.
- Humes LE (2005) Do 'auditory processing' tests measure auditory processing in the elderly?. Ear Hear 26(2): 109-119.
- Schneider BA, Daneman M, Pichora Fuller MK (2002) Listening in aging adults: from discourse comprehension to psychoacoustics. Can J Exp Psychol 56(3): 139-152.
- Jiang K, Armstrong NM, Agrawal Y, Gross AL, Schrack JA, et al. (2022) Associations of audiometric hearing and speech-in-noise performance with cognitive decline among older adults: The Baltimore Longitudinal Study of Aging (BLSA). Front Neurol 13: 1029851.
- Chen Y, Wong LLN, Chan SS, Yu J (2022) Speech perception in noise is associated with different cognitive abilities in Chinese-speaking older adults with and without hearing aids. Front Psychol 12: 640300.
- Lee SJ, Lee S (2023) Clinical utility of response time in speech audiometry in elderly with mild cognitive impairment. Int J Audiol 62(5): 418-423.
- Johns MA, Calloway RC, Phillips I, Karuzis VP, Dutta K, et al. (2023) Performance on stochastic figure-ground perception varies with individual differences in speech-in-noise recognition and working memory capacity. J Acoust Soc Am 153(1): 286.
- Stenbäck V, Marsja E, Hällgren M, Lyxell B, Larsby B (2022) Informational masking and listening effort in speech recognition in noise: The role of working memory capacity and inhibitory control in older adults with and without hearing impairment. J Speech Lang Hear Res 65(11): 4417-4428.
- Shende S A, Mudar RA (2023) Cognitive control in age-related hearing loss: A narrative review. Ear Hear 436: 108814.
- Perron M, Dimitrijevic A, Alain C (2022) Objective and subjective hearing difficulties are associated with lower inhibitory control. Ear Hear 43(6): 1904-1916.
- Lentz JJ, Humes LE, Kidd GR (2022) Differences in auditory perception between young and older adults when controlling for differences in hearing loss and cognition. Trends Hear 26: 23312165211066180.
- Fullgrabe C, Rosen S (2016) On The (un)importance of working memory in speech-in-noise processing for listeners with normal hearing thresholds. Front Psychol 7: 1268.
- Kocabay AP, Aslan F, Yüce D, Turkyilmaz D (2022) Speech in noise: implications of age, hearing loss, and cognition. Folia Phoniatr Logop 74(5): 345-351.
- Rönnberg J, T Lunner, AA Zekveld, P Sörqvist, H Danielsson, Lyxell B (2013) The Ease of Language Understanding (ELU) model: theoretical, empirical, and clinical advances. Front Syst Neurosci 7: 31.
- Rönnberg J, Holmer E, Rudner M (2019) Cognitive hearing science and ease of language understanding. Int J Audiol 58(5): 247-261.
- Andersson U, Lyxell B (1998) Phonological deterioration in adults with an acquired severe hearing impairment. Scand Audiol Suppl49: 93-100.
- Lemke U, Besser J (2016) Cognitive Load and Listening Effort: Concepts and age-related considerations. Ear Hear 37 Suppl 1: 77S-84S.
- Heinrich A, Henshaw H, Ferguson M A (2015) The relationship of speech intelligibility with hearing sensitivity, cognition, and perceived hearing difficulties varies for different speech perception tests. Front Psychol 6: 782.
- Bertoli S, Bodmer D (2014) Novel sounds as a psychophysiological measure of listening effort in older listeners with and without hearing loss. Clin Neurophysiol 125(5): 1030-1041.
- Bertoli S, Bodmer D (2016) Effects of age and task difficulty on ERP responses to novel sounds presented during a speech-perception-in-noise test. Clin Neurophysiol 127(1): 360-368.
- Desjardins J L, Doherty K A (2013) Age-related changes in listening effort for various types of masker noises. Ear Hear 34(3): 261-272.
- Gordon Salant S, Fitzgibbons P J (1993) Temporal factors and speech recognition performance in young and elderly listeners. J Speech Hear Res 36(6): 1276-1285.
- Humes L, Young L (2016) Sensory-cognitive interactions in older adults. Ear Hear 37: 52S-61S.
- Jerger J, Greenwald R, Wambacq I, Seipel A, Moncrieff D (2000) Toward a more ecologically valid measure of speech understanding in background noise. J Am Acad Audiol 11(5): 273-282.
- Davis T, Stanley N, Foran L (2015) Age-related effects of dichotic attentional mode in interaural asymmetry: An AERP study with independent component analysis. J Am Acad Audiol 26 (5): 461-477.
- Larsby B, Hällgren M, Lyxell B, Arlinger S (2005) Cognitive performance and perceived effort in speech processing tasks: effects of different noise backgrounds in normal-hearing and hearing-impaired subjects. Int J Audiol 44(3): 131-143.
- Li K, Krampe R, Bondar A (2005) An Ecological Approach to Studying Aging and Dual-Task Performance. In R Engle, G Sedek, U Von Hecker, D McIntosh (Eds.), Cognitive Limitations in Aging and Psychopathology (pp. 190-218). Cambridge: Cambridge University Press.
- McGarrigle R, Munro K J, Dawes P, Stewart AJ, Moore DR, et al. (2014) Listening effort and fatigue: what exactly are we measuring? A British Society of Audiology Cognition in Hearing Special Interest Group 'white paper'. Int J Audiol 53(7): 433-440.
- Mohammed A, Gibbons LE, Gates G, Anderson ML, McCurry SM, et al. (2022) Association of performance on dichotic auditory tests with risk for incident dementia and Alzheimer dementia. JAMA Otolaryngol Head Neck Surg 148(1): 20-27.
- Murphy DR, Craik FIM, Li KZH, Schneider BA (2000) Comparing the effects of aging and background noise on short-term memory performance. Psychol Aging 15(2): 323-334.
- Phillips NA (2016) The implications of cognitive aging for listening and the Framework for Understanding Effortful Listening (FUEL). Ear Hear 37 Suppl 1: 44S–51S.
- Pichora Fuller MK, Kramer SE, Eckert MA, Edwards B, Hornsby BWY, et al. (2016) Hearing impairment and cognitive energy: The framework for understanding effortful listening (FUEL). Ear and Hearing 37(1): 5S-27S.
- Richter M (2016) The moderating effect of success importance on the relationship between listening demand and listening effort. Ear Hear 37: Suppl 1: 111S-7S.
- Rönnberg J, Hygge S, Keidser G, Rudner M (2014) The effect of functional hearing loss and age on long- and short-term visuospatial memory: Evidence from the UK Biobank resource. Front Aging Neurosci 6: 326.
- Taljaard DS, Olaithe M, Brennan Jones CG, Eikelboom RH, Bucks RS (2016) The relationship between hearing impairment and cognitive function: a meta-analysis in adults. Clin Otolaryngol 41(6): 718-729.
- Rudner M (2016) Cognitive spare capacity as an index of listening effort. Ear Hear 37 Suppl 1: 69S-76S.
- Schneider BA, Daneman M, Murphy DR (2005) Speech comprehension difficulties in older adults: cognitive slowing or age-related changes in hearing?. Psychol Aging 20(2): 261-271.
- Schneider BA, Pichora Fuller MK (2000) Implications of perceptual deterioration for cognitive aging research. In FI M Craik, T A Salthouse (Eds.), The handbook of aging and cognition (2nd), pp. 155-219). Lawrence Erlbaum Associates Publishers.
- Souza P, Arehart K, Neher T (2015) Working memory and hearing aid processing: literature findings, future directions, and clinical applications. Front Psychol 6: 1894.
- Tremblay KL, Backer KC (2016) Listening and Learning: Cognitive Contributions to the Rehabilitation of Older Adults With and Without Audiometrically Defined Hearing Loss. Ear Hear 37 Suppl 1(Suppl 1): 155S-62S.
- Tun PA, McCoy S, Wingfield A (2009) Aging, hearing acuity, and the attentional costs of effortful listening. Psychology and aging 24(3): 761-766.
- Wendt D, Hietkamp RK, Lunner T (2017) Impact of noise and noise reduction on processing effort: A pupillometry study. Ear and hearing 38(6): 690-700.
- van Rooij JC, Plomp R (1990) Auditive and cognitive factors in speech perception by elderly listeners. Acta oto-laryngologica. Supplementum 476: 177-181.
- van Rooij J C, Plomp R (1992) Auditive and cognitive factors in speech perception by elderly listeners. III. Additional data and final discussion. The Journal of the Acoustical Society of America 91(2): 1028-1033.
- Zekveld AA, Koelewijn T, Kramer SE (2018) The pupil dilation response to auditory stimuli: current state of knowledge. Trends Hear 22: 2331216518777174.
- Zekveld AA, Rudner M, Johnsrude IS, Rönnberg J (2013) The effects of working memory capacity and semantic cues on the intelligibility of speech in noise. J Acoust Soc Am 134(3): 2225-2234.
- Cheong CY, Yap P, Nyunt MSZ, Qi G, Gwee X, et al. (2022) Functional health index of intrinsic capacity: multi-domain operationalisation and validation in the Singapore Longitudinal Ageing Study (SLAS2). Age Ageing 51(3): afac011.
- Tay L, Tay EL, Mah SM, Latib A, Koh C, et al. (2023) Association of intrinsic capacity with frailty, physical fitness and adverse health outcomes in community-dwelling older adults. J Frailty Aging 12(1): 7-15.
- Leung AYM, Su JJ, Lee ESH, Fung JTS, Molassiotis A (2022) Intrinsic capacity of older people in the community using WHO Integrated Care for Older People (ICOPE) framework: a cross-sectional study. BMC Geriatr 22(1): 304.
- Beier F, Löffler M, Nees F, Hausner L, Frölich, L, et al. (2022) Sensory and motor correlates of frailty: dissociation between frailty phenotype and frailty index. BMC Geriatr 22(1): 755.
- Maidment DW, Wallhagen MI, Dowd K, Mick P, Piker E, et al. (2023) New horizons in holistic, person-centred health promotion for hearing healthcare. Age Ageing 52(2): afad020.
- Lydon EA, Nguyen LT, Nie Q, Rogers WA, Mudar RA (2022) An Integrative framework to guide social engagement interventions and technology design for persons with mild cognitive impairment. Front Public Health 9: 750340.
- Shafiepour M, Bamdad Z, Radman M (2022) Prevalence of hearing loss among patients with type 2 diabetes. J Med Life 15(6): 772-777.
- Del Vecchio V, Tricarico L, Pisani A, Serra N, D'Errico D, et al. (2023) Vascular factors in patients with midlife sensorineural hearing loss and the progression to mild cognitive Impairment. Medicina (Kaunas,) 59(3): 481.
- Prior KN, Bond VE, Bond MJ (2022) Does illness behavior contribute to the understanding of self-efficacy and quality of life among people with hearing loss? A test of concept. Am J Audiol 31(1): 211-219.
- Atef RZ, Michalowsky B, Raedke A, Platen M, Mohr W, et al. (2023) Impact of hearing aids on progression of cognitive decline, depression, and quality of life among people with cognitive impairment and dementia. J Alzheimers Dis 92(2): 629-638.
- Zhao IY, Parial LL, Montayre J, Golub JS, Ng JH, et al. (2023) Social engagement and depressive symptoms mediate the relationship between age-related hearing loss and cognitive status. Int J Geriatr Psychiatry 38(8): e5982.
- Wettstein M, Kornadt A, Heyl V, Wahl HW (2023) Self-reported hearing and awareness of age-related change : A domain-specific perspective. Z Gerontol Geriatr 56(4): 269-275.
- Sakurai R, Kawai H, Suzuki H, Ogawa S, Yanai S, et al. (2023) Cognitive, physical, and mental profiles of older adults with misplaced self-evaluation of hearing loss. Arch Gerontol Geriatr 104: 104821.
- Heine C, Browning CJ (2002) Communication and psychosocial consequences of sensory loss in older adults: overview and rehabilitation directions. Disabil Rehabil 24(15): 763-773.
- Pichora Fuller K, Dupuis K, Reed M, Lemke U (2013) Helping older people with cognitive decline communicate: Hearing aids as part of a broader rehabilitation approach. Semin Hear 34(04): 308-330.
- Pichora Fuller MK (2015) Cognitive decline and hearing health care for older adults. Am J Audiol 24(2): 108-111.
- Danneels M, Van Hecke R, Leyssens L, van de Berg R, Dhooge I, et al. (2023) Association of Bilateral Vestibulopathy With and Without Hearing Loss With Cognitive-Motor Interference. JAMA Otolaryngol Head Neck Surg 149(8): 670-680.
- Bosmans J, Gommeren H, Mertens G, Cras P, Engelborghs S, et al. (2022) Associations of bilateral vestibulopathy with cognition in older adults matched with healthy controls for hearing status. JAMA Otolaryngol Head Neck Surg 148(8): 731-739.
- Kim MW, Jin MH, Choi JY, Kwak MY (2023) Potential overestimation of cognitive impairment because of hearing loss: impact of test modalities on cognitive test scores. J Laryngol Otol 137(8): 845-850.
- Ceuleers D, Baudonck N, Keppler H, Kestens K, Dhooge I, et al. (2023) Development of the hearing-related quality of life questionnaire for auditory-visual, cognitive and psychosocial functioning (hAVICOP). J Commun Disord 101: 106291.
- Merten N, Boenniger MM, Herholz SC, Breteler MMB (2022) The associations of hearing sensitivity and different cognitive functions with perception of speech-in-Noise. Ear Hear 43(3): 984-992.
- Goodwin MV, Hogervorst E, Maidment DW (2022) Test your health at home: comparing online screening tests of hearing, cognition, and cardiovascular health. Am J Audiol 31(3S): 950-960.
- Potgieter JM, Swanepoel W, Myburgh HC, Smits C (2018) The South African English Smartphone digits-in-noise hearing test: effect of age, hearing loss, and speaking competence. Ear Hear 39(4): 656-663.
- Dawes P, Littlejohn J, Bott A, Brennan S, Burrow S, et al. (2022) Hearing assessment and rehabilitation for people living with dementia. Ear Hear 43(4): 1089-1102.
- Tarawneh HY, Jayakody DMP, Verma S, Doré V, Xia Y, et al. (2023) Auditory event-related potentials in older adults with subjective memory complaints. J Alzheimers Dis 92(3): 1093-1109.
- Dupuis K, Pichora Fuller MK, Chasteen AL, Marchuk V, Singh G, et al. (2015) Effects of hearing and vision impairments on the Montreal Cognitive Assessment. Neuropsychology, development, and cognition. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 22(4): 413-437.
- Gaeta L, Azzarello J, Baldwin J, Ciro CA, Hudson MA, et al. (2019) Effect of reduced audibility on Mini-Mental State Examination scores. J Am Acad Audiol 30(10): 845-855.
- Hill Briggs F, Dial JG, Morere DA, Joyce A (2007) Neuropsychological assessment of persons with physical disability, visual impairment or blindness, and hearing impairment or deafness. Arch Clin Neuropsychol 22(3): 389-404.
- Junkkila J, Oja S, Laine M, Karrasch M (2012) Applicability of the CANTAB-PAL computerized memory test in identifying amnestic mild cognitive impairment and Alzheimer's disease. Dement Geriatr Cogn Disord 34(2): 83-89.
- Gaeta L, John A (2021) Hearing Loss and Cognitive Screening. Research in Gerontological Nursing 14(4): 170-172.
- Guerrero Arenas CI, Osornio García FU (2023) Cognition assessment technologies on deaf people. J Cogn 6(1): 18.
- Brown Quigley B, Gaeta L (2023) Considering individuals' hearing ability before administering cognitive assessments. Cogn Behav Neurol 36(1): 63-65.
- Andries E, Bosmans J, Engelborghs S, Cras P, Vanderveken OM, et al. (2023) Evaluation of cognitive functioning before and after cochlear implantation in adults aged 55 years and older at risk for mild cognitive impairment. JAMA Otolaryngol Head Neck Surg 149(4): 310-316.
- Völter C, Götze L, Falkenstein M, Dazert S, Thomas JP (2017) Application of a computer-based neurocognitive assessment battery in the elderly with and without hearing loss. Clin Interv Aging 12: 1681-1690.
- Ballasch I, De Kruif A, Hendel MK, Rohr C, Brünecke I, et al. (2023) O-DEM: a new cognitive screening in patients with hearing loss]. HNO 71(9): 599-606.
- Hamerschmidt R, Santos VM, Gonçalves FM, Delcenserie A, Champoux F, et al. (2023) Changes in cognitive performance after cochlear implantation in adults and older adults: a systematic review and meta-analysis. Int J Audiol 62(6): 521-532.
- Gundogdu O, Serbetcioglu MB, Kara E, Eser BN (2023) Effects of Cognitive functions on speech recognition in noise in cochlear implant recipients. ORL J Otorhinolaryngol Relat Spec 85(4): 208-214.
- Ohta Y, Imai T, Maekawa Y, Morihana T, Osaki Y, et al. (2022) The effect of cochlear implants on cognitive function in older adults: A prospective, longitudinal 2-year follow-up study. Auris nasus larynx 49(3): 360-367.
- Zucca M, Albera A, Albera R, Montuschi C, Della Gatta B, et al. (2022) Cochlear implant results in older adults with post-lingual deafness: The role of "top-down" neurocognitive mechanisms. Int J Environ Res Public Health 19(3): 1343.
- Smith SL, Pichora Fuller MK, Alexander G (2016) Development of the Word Auditory Recognition and Recall Measure: a working memory test for use in rehabilitative audiology. Ear Hear 37(6): e360-e376.
- Yueh B, Shapiro N, MacLean CH, Shekelle PG (2003) Screening and management of adult hearing loss in primary care: scientific review. JAMA 289(15): 1976-1985.
- Omar R, Kuo L, Costafreda SG, Hall A, Forbes M, et al. (2023) Managing comorbid cognitive impairment and hearing loss in older adults: a UK survey of audiology and memory services. Age Ageing 52(5): afad080.
- Quaranta N, Coppola F, Casulli M, Barulli O, Lanza F, et al. (2014) The prevalence of peripheral and central hearing impairment and its relation to cognition in older adults. Audiol Neurootol 19 Suppl 1: 10-14.
- Xu J, Cox RM (2021) Interactions between cognition and hearing aid compression release time: effects of linguistic context of speech test materials on speech-in-noise performance. Audiol Res 11(2): 129-149.
- Acar B, Yurekli MF, Babademez MA, Karabulut H, Karasen RM (2011) Effects of hearing aids on cognitive functions and depressive signs in elderly people. Arch Gerontol Geriatr 52(3): 250-252.
- Allen NH, Burns A, Newton V, Hickson F, Ramsden R (2003) The effects of improving hearing in dementia. Age Ageing 32(2): 189-193.
- Glick HA, Sharma A (2020) Cortical neuroplasticity and cognitive function in early-stage, mild-moderate hearing loss: evidence of neurocognitive benefit from hearing Aid Use. Front Neurosci 14: 93.
- Kim S, Wu YH, Bharadwaj HM, Choi I (2022) Effect of Noise Reduction on Cortical Speech-in-Noise Processing and Its Variance due to Individual Noise Tolerance. Ear Hear 43(3): 849-861.
- Perron M, Lau B, Alain C (2023) Interindividual variability in the benefits of personal sound amplification products on speech perception in noise: A randomized cross-over clinical trial. PloS one 18(7): e0288434.
- Kwan RYC, Kwan CW, Kor PPK, Chi I (2022) Cognitive decline, sensory impairment, and the use of audio-visual aids by long-term care facility residents. BMC Geriatr 22(1): 216.
- Gatehouse S, Naylor G, Elberling C (2003) Benefits from hearing aids in relation to the interaction between the user and the environment. Int J Audiol 42 Suppl 1: S77-S85.
- Lunner T (2003) Cognitive function in relation to hearing aid use. Int J Audiol 42 Suppl 1: S49-S58.
- Ng EH, Rudner M, Lunner T, Rönnberg J (2015) Noise reduction improves memory for target language speech in competing native but not foreign language speech. Ear Hear 36(1): 82-91.
- Souza P, Sirow L (2014) Relating working memory to compression parameters in clinically fit hearing aids. Am J Audiol 23: 394-401.
- Lunner T, Sundewall Thorén E (2007) Interactions between cognition, compression, and listening conditions: effects on speech-in-noise performance in a two-channel hearing aid. J Am Acad Audiol 18(7): 604-617.
- Windle R, Dillon H, Heinrich A (2023) A review of auditory processing and cognitive change during normal ageing, and the implications for setting hearing aids for older adults. Front Neurol 14: 1122420.
- Neher T (2014) Relating hearing loss and executive functions to hearing aid users' preference for, and speech recognition with, different combinations of binaural noise reduction and microphone directionality. Front Neurosci 8: 391.
- Ng EHN, Rönnberg J (2019) Hearing aid experience and background noise affect the robust relationship between working memory and speech recognition in noise. Int J Audiol 59(3): 208-218.
- Schvartz Leyzac KC, Giordani B, Pfingst BE (2023) Association of aging and cognition with complex speech understanding in cochlear-Implanted adults: use of a modified National Institutes of Health (NIH) toolbox cognitive assessment. JAMA Otolaryngol Head Neck Surg 149(3): 239-246.
- Henshaw H, Heinrich A, Tittle A, Ferguson M (2022) Cogmed training does not generalize to real-world benefits for adult hearing aid users: results of a blinded, active-controlled randomized trial. Ear Hear 43(3): 741-763.
- Sommers MS, Tye Murray N, Barcroft J, Spehar BP (2015) The effects of meaning-based auditory training on behavioral measures of perceptual effort in individuals with impaired hearing. Semin Hear 36(4): 263-272.
- Lowe SC, Henshaw H, Wild J, Ferguson MA (2023) Evaluation of home-delivered live-voice auditory training for adult hearing aid users involving their communication partners: a randomized controlled trial. Int J Audiol 62(1): 89-99.
- Kucuk Ceyhan A, Dere HH, Mujdeci B (2022) Evaluating the effectiveness of a new auditory training program on the speech recognition skills and auditory event-related potentials in elderly hearing aid users. Audiol Neurootol 27(5): 368-376.
- Schumann A, Ross B (2022) Adaptive Syllable Training Improves Phoneme Identification in Older Listeners with and without Hearing Loss. Audiol Res 12(6): 653-673.
- Ferguson MA, Henshaw H (2015) Auditory training can improve working memory, attention, and communication in adverse conditions for adults with hearing loss. Front Psychol 6: 556.
-
Scott E Seeman* and Jennine Harvey Northrop. Cognitive Hearing Science: Clinical Implications in Audiology. On J Otolaryngol & Rhinol. 6(5): 2024. OJOR.MS.ID.000647.
-
Hearing science; Audiology; Hearing loss; Ear; Cochlea; Speech perception; Noise; Hearing aid fittings; Mental health; Geriatric audiology
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






