 Research Article
Research Article
Comparative Phyto Extractive Potentials of Jatropha curcas, Saccharum spontaneum and Helianthus annuusin Heavy Metal Contaminated Soil Using Remediation Indices
Kever D . G1, Oluma H . O . A 1, Aguoru C . U1, Eneji I . S2 and Olasan J . O1
1Botany Department, Joseph Saawuan Tarka University Makurdi, Nigeria
2Chemistry Department, Joseph Saawuan Tarka University Makurdi, Nigeria
Kever D . G, Botany Department, Joseph Saawuan Tarka University Makurdi, Nigeria.
Received Date:September 04, 2023; Published Date:September 19, 2023
Abstract
Environmental pollution has become a global issue. The adoption of a cost effective and friendly remediation technique is thus encouraged. This study evaluated the potentials of Jatropha curcas L (Jatropha), Saccharum spontaneum L (Wild Sugarcane) and Helianthus annuus L (Sun flower) in the phytoremediation of Cr, Cd, Pb and Hg heavy metal contaminated soils. A potted experiment was used for the study. Data analysis was carried out using Minitab (17.0) for descriptive and inferential statistics. All pollution indices (PIs) were <1.0 with the highest pollution index found in sunflower soil (0.63) and the least in Jatropha soil (0.46). Only the augmented wild sugarcane had Overall Bio-concentration Factors (OBCFS) >1.0 in order of augmented wild sugarcane (1.1) > wild sugarcane (0.84) > augmented sunflower (0.81) > augmented Jathropha (0.78) > sunflower (0.6). All test plants had Overall Translocation Factors (OTFs) of >1.0 in order of augmented sunflower (1.98) > augmented and non-augmented wild sugarcane (1.94) > augmented Jatropha (1.93) > Jatropha (1.86) > sunflower (1.15). The study revealed that three test plants were good phytoremediants. However, only the augmented wild sugarcane was a hyperaccumulator. The test plants had high heavy metal uptake based on their translocation factors. This study recommends the use of the test plants in phytoremediation of heavy metal contaminated soils using cow dung.
Keywords:Heavy Metals; phytoextraction; Phytoremediation; Contaminated soil; Remediation indices
Introduction
Soil is one of the essential gifts of nature which support the biotic community. It often referred to as the cradle of life, plays an irreplaceable role in supporting ecosystems. Despite its significance, alarming rates of soil contamination are threatening this vital resource [1]. However, soil contamination is a major environmental issue that requires effective preventive measures [2]. In recent years, the contamination of soil by heavy metals has become a major environmental concern Pollutants such as heavy metals are non-biodegradable nor destroyed thus pose environmental risk. Heavy metal contamination in soil is a significant environmental issue that poses risks to human health, plants, and animals [3]. Heavy metals are toxic and can accumulate in the soil, leading to long-term environmental damage [4,5]. Therefore, remediation of heavy metal- contaminated soil is crucial to mitigate these risks and restore the ecological balance.
Heavy metal is defined as a chemical element with a specific gravity that is at least five times that of water (Soni and Jain, 2014). They have been reported to play positive and negative roles in human life. Some heavy metals like Cadmium (Cd), Chromium (Cr), Lead (Pb) and Mercury (Hg) even in trace amounts, are considered very harmful to the environment since they do not biodegrade while others like Iron (Fe), Zinc (Zn) and Copper (Cu) are essential for biochemical reactions in the body [6].
The most common heavy metals found in contaminated environments, in order of abundance are Fe, Pb, Cr, As, Zn, Cd, Cu, and Hg [7]. Heavy metals cannot be degraded and hence accumulate in the environment, tending to contaminate the food chain. Heavy metals are released into the environment by both natural and anthropogenic sources [8]. Uptake and accumulation of heavy metals in plants is influenced by attributes such as natural occurrences derived from parent material (rock), atmospheric deposition (depending on traffic density), concentration and bioavailability of heavy metals in soil (through addition of pesticides, herbicides, and fertilizers), the nature of soil where plants are grown (pH and organic matter concentration), individual plant performance (degree of maturity of the plant, time of harvest) [9]. Soil is recognized as a repository for pollutants due to the absorption processes that bind inorganic and organic substances to it [10]. Some soils may have higher levels of heavy metals due to volcanic activity or weathering of parent materials[11]. Anthropogenic activities such as smelting, mining, use of pesticides, fertilizers, sludges, and emissions from industries are also responsible for heavy metals accumulations in the soils [7].
Recent advances in bioremediation techniques with the goal being to effectively restore polluted environments in an eco-friendly approach, and at a very low cost [12,13]. Environmentally friendly and cost-saving features are among the major advantages of bioremediation compared to both chemical and physical methods of remediation [14]. Plants are part of natural endowment as sources of food, shelter, medicine, aesthetics and other uses. By using plants in the cleanup of the environment, this method helps remediate the contaminated soil, reviving it to its natural state of being less harmful [15]. Plants have the potential to take up minerals from the soil, even in minute quantities. This is transported via the xylem to and stored in different parts of the plant’s tissues [16]. However, how well phytoremediation works depends on the specific plants chosen for the task, as the mechanism and plants’ ability to absorb different forms of minerals varies.
Phytoremediation is a set of ecological strategies that utilizes plants, in situ, to promote the breakdown, immobilization, and removal of pollutants from the environment [17]. This technique relies on the use of plant interactions (physical, biochemical, biological, chemical, and microbiological) in polluted sites to mitigate the toxic effects of pollutants [18]. Depending on the pollutant type (elemental or organic), there are several mechanisms involved in phytoremediation such as extraction, degradation, filtration, stabilization, and volatilization. [13].
Using plants helps remediate the contaminated soil or makes it less harmful, thereby restoring the soil’s health [15]. Plants can take up minerals from the soil, even in small amounts, and spread them through their systems [16]. However, how well phytoremediation works depends on the specific plants chosen for the task. By using plants, this method helps remediate the contaminated soil or makes them less harmful, thereby restoring the soil’s health [15,19]. Plants can take up minerals from the soil, even in small amounts, and spread them through their systems [16]. However, how well phytoremediation works depends on the specific plants chosen for the task.
The current state of research in the field of plant phytoextraction and heavy metal contamination has focused on the potential of various plant species for remediating metal-contaminated soils. However, there is a lack of consensus regarding the most effective plant species and the remediation indices that should be used to evaluate their Phyto extractive potentials. Some studies suggest that the chemical composition of foliage alone is not sufficient to assess the suitability of a plant species for phytoextraction. Other factors such as rooting zone depth, soil density, and harvestable biomass should also be considered. Jatropha, Wild sugarcane, and Sunflower are among the plant species that have shown promise in accumulating heavy metals from contaminated soil. However, a comprehensive comparative study evaluating their Phyto extractive potentials using remediation indices such as Plant Uptake Index (PI), Bioconcentration Factor (BCF), and Translocation Factor (TF) is lacking.
Therefore, there is need to examine the Phyto extractive abilities of different plants such as Jatropha, Wild Sugarcane, and Sunflower using remediation indices to identify the most effective plant for phytoremediation. Priya et al., [16] submit that, not all plants are capable of Phyto remediation, as only plants with high Phyto extractive potential can effectively remediate contaminants from the soil. The present study is designed to test the efficacy of Jatropha curcas (Jatropha), Saccharum spontaneum (wild sugarcane) and Helianthus annuus (sunflower) as potential heavy metal accumulators that could be used to clean up soils contaminated with toxic heavy metals such as Chromium (Cr), Cadmium (Cd), Lead (Pb) and Mercury (Hg) using remediation indices like Plant Uptake Index (PI), Bioconcentration Factor (BCF), and Translocation Factor (TF).
Materials and Methods
Study area
The study area is Makurdi, the State Capital of Benue State Makurdi. The experimental site is within the Joseph Saawuan Tarka University (the defunct Federal University of Agriculture) Makurdi (latitude 7o38’N-7o50’N, and longitude 8o24’E-8o38’E) [20]. The nursery barn of the Forestry Department, Joseph Saawuan Tarkaa University Makurdi, situated behind the water and works Unit of the University and enclosed by a fence, serves as a designated study area for soil preparation, treatment, and planting of test plants. This location provides a controlled environment for the conduct of the research work.
Experimental design
The Completely Randomized Experimental Design was used for this study. Randomization ensures that the treatment groups are comparable and that any observed differences in the response variable can be attributed to the treatments rather than other factors. This allows for proper statistical analysis, hypothesis testing, and estimation of treatment effects with confidence intervals. The Complete Randomized Design (CRD) experimental design using the General Full Factorial design (5x3x2) X 3 design structure, tool function of the Minitab 16.0 software was employed. A total of 90 experimental units of three factors were set.
Sources of heavy metals
The following heavy metal salts used in the work were procured from a standard commercial laboratory: CdCl2 (Cadmium Chloride), K2Cr207 (Potassium dichromate), PbCl2 (Lead II Chrolide) and Hg(NO3)2 (Mecury (II) Nitrate) for the preparation of Chromium (Cr), Lead (Pb), Mercury (Hg), and Cadmium (Cd) salts.
Soil sample collection (pre experiment)
Soil samples was collected from an undisturbed area behind the Botany Department of the University using the soil auger at the depth of 0-15cm for pre-soil analysis. Samples were collected in sterilized polythene bag and taken to the laboratory for pre-experiment of soil.
Preparation of heavy metal stock solutions for treatment Cadmium stock solution
In Cadmium, 18.33 g of CdCl2 (Cadmium Chloride), was dissolved in 200 cm3 of distilled water in a 1000 cm3 volumetric flask and diluted to mark to give a solution of 1000 mg/L stock solution of Cadmium and stored [21].
Chromium stock solution
In Chromium, 1084.14 g of K2Cr207 (Potassium dichromate), was dissolved with 200.00 cm3 of deionized water in a 1000 cm3 volumetric flask and diluted to mark with water to give a 1000 mgL- 1 stock solution of Chromium and stored [21].
Lead stock solution
In Lead, 27.81 g of PbCl2 (Lead II Chrolide) was dissolved in 50.00 cm3 of deionized water in a 1000 cm3 volumetric flask. The solution will be diluted to mark with distilled water to give a 1000 mgL-1 stock solution of Lead and stored [21].
Mercury stock solution
To prepare 1000 mL of a 0.1 mol/L solution of Mercury(II) nitrate we have to dissolve 32.46 g of Hg(NO3)2 (100 % purity) in deionized or distilled water. After the solid is completely dissolved, dilute the solution to a final volume with deionized (distilled) water. The solution was transferred to a clean container and stored [21].
Plant sample collection and authentication and analysis
Whole Plant samples and seeds of Jatropha plant (Jatropha curcas L.), wild sugarcane (Saccharum spontaneum L.) was collected at the Nursery and farm village of the forestry Department Joseph Saawuan Tarka University. The sunflower (Helianthus annuus L.) was commercially purchased. Both plants were authenticated in the Forestry Department, Joseph Saawuan Tarka University with further confirmation using the flora of West Africa album in comparism with description in literature. All plants were pre-established for eight weeks (two months) before the experiment proper. This was to allows for a pilot tests or preliminary experiments. The chosen test plants all exhibited morphological characteristics as described in literature.
Samples of the test plants pre-established for eight weeks for preliminary experiments were collected in a ziplock bag for pre-treatment and analysis of heavy metals. All plant samples were oven dried using GNLAB Mino economy oven of model MINO/75 at 1050C to a constant weight and crushed using wooden mortar and pestle [22]. 0.5 g of plant samples were weighed into a clean flat bottom flask of 250ml using a scale of model AR2130 Ohaus Corporation China. 5ml of concentrated Nitric per Chloric acid (HNO3 / HCLO4) in the ratio of 2:1 was added to the sample. Analysis of selected heavy metals (Cd, Pb, Cr, Hg) were done using Atomic Absorption Spectrophotometer AAS in mgl-1 of Model ICE 3000 Series, Thermo Scientific, USA at the Chemistry Advanced Research Centre (CARC), Sheda Science and Technology Complex (SHESTCO) Abuja.
Procedures of treatment application and planting of test plants
The soil in the pots were spiked with 50ml of the different heavy metals based on the experimental design and mixed thoroughly using a spatula, except in the control pot without treatments. For pots receiving manure treatments, the soil was mixed with 0.4kg of cow dung and allowed to stand for four weeks before plantation. This was to ensure that the organic matter in the cow dung undergoes decomposition and integrates with the soil, enhancing its overall fertility and quality. This integration period also allows for better nutrient release and transformation, creating a favorable environment for plant growth.
The test plants Jatropha plant (Jatropha curcas L.), wild sugarcane (Saccharum spontaneum L.) and sunflower (Helianthus annuus L.) were planted in the experimental pots filled with 5kg of pre-determined unpolluted soil. The method described by Paliza et al., [23] was employed with modifications.
Sample preparation
Collection of samples was done for weeks 5 and 10 after planting. The whole plant samples were carefully collected in a Ziplock bag, labelled, and conveyed to the laboratory. Plant samples were thoroughly washed with tap water and rinsed with distilled water to remove soil debris. The stems were separated from the roots and cut into smaller pieces.
All plant samples were oven dried using GNLAB Mino economy oven of model MINO/75 at 1050C to a constant weight and crushed using wooden mortar and pestle [22]. Porcelain mortar and pestle were also used to crush the soil samples to a homogenized state. The Porcelain mortar and the wooden mortar and pestles were rinsed with distilled water and dried after each sample ground to avoid cross contamination [24]. Each sample was passed through a sechi standard test sieve of 2mm, the fine powder of the samples was stored in airtight plastic containers with lid for analysis [25].
Heavy metal analysis in plants and soil
Digestion of samples was done in Advanced research laboratory, Joseph Saawuan Tarka University Makurdi. The method of AOAC [22] was adopted in digesting the heavy metals. 0.5 g of plant samples were weighed into a clean flat bottom flask of 250ml using a scale of model AR2130 Ohaus Corporation China. 5ml of concentrated Nitric per Chloric acid (HNO3 / HCLO4) in the ratio of 2:1 was added to the sample. The plant samples were allowed to stay for two minutes before been placed on the hotplate of model ES-3615, Everest China in a fume cupboard. This was heated gently until a clear solution was obtained which signified a complete digestion. The crushed plant material was allowed to cool to room temperature (25oc). Clean crucibles were used for soil samples digestion; 5ml of concentrated Nitric per Chloric acid (HNO3 / HClO4) in the ratio of 2:1 was added to each soil sample and shook for proper mixing. Soil samples in the crucibles were placed in the fume cupboard and allowed to stay for 24 hours before being filtered. Both plant and soil samples were filtered using Whatman no.1 Filter Paper. The filtrates were diluted with deionized water to 25ml mark and transferred into clean plastic bottles with lid and labelled accordingly for heavy metals analysis.
Analysis of selected heavy metals (Cd, Pb, Cr, Hg) were done using Atomic Absorption Spectrophotometer AAS in mgl-1 of Model ICE 3000 Series, Thermo Scientific, USA at the Chemistry Advanced Research Centre (CARC), Sheda Science and Technology Complex (SHESTCO) Abuja.
Results
Determination of pollution indices (PIs) of heavy metal contaminated soils
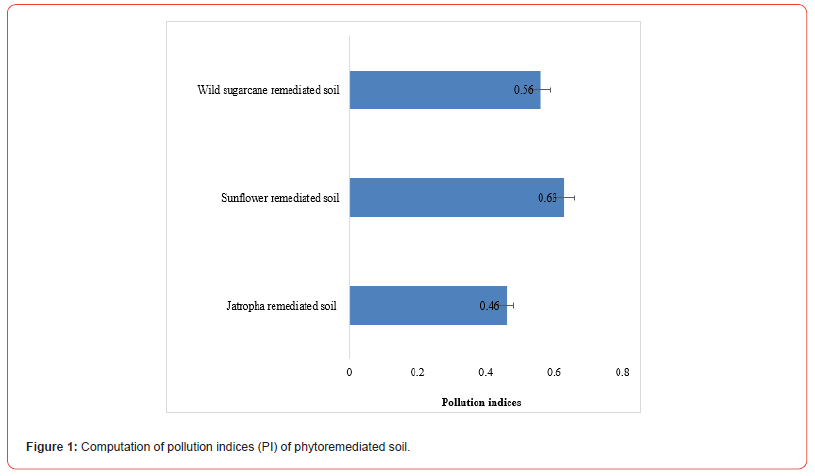
Table 1 -3 give the computed pollution indices of heavy metal contaminated soils under phytoremediation trials.
In Jatropha trials (Table 1), Concentration Factors (CFs) of heavy metal contaminated soils were 0.46 in Cr, 0.56 in Cd, 0.00 in Pb and 0.82 in Hg. Consequently, the computed pollution index of Jatropha soil after remediation was 0.46. In wild sugarcane trials (Table 2), Concentration Factors (CFs) of heavy metal contaminated soils were 0.63 in Cr, 0.49 in Cd, 0.45 in Pb and 0.65 in Hg. Consequently the computed pollution index of wild sugarcane soil after remediation was 0.56. In sunflower trials (Table 3), Concentration Factors (CFs) of heavy metal contaminated soils were 0.64 in Cr, 0.55 in Cd, 0.51 in Pb and 0.82 in Hg. Consequently, the computed pollution index of sunflower soil after remediation was 0.63. As shown figure 2, all pollution indices (PIs) were <1.0. The highest pollution index was found in sunflower soil (0.63) and the least in Jatropha soil (0.46).
Determination of BCFs of heavy metals in test plants
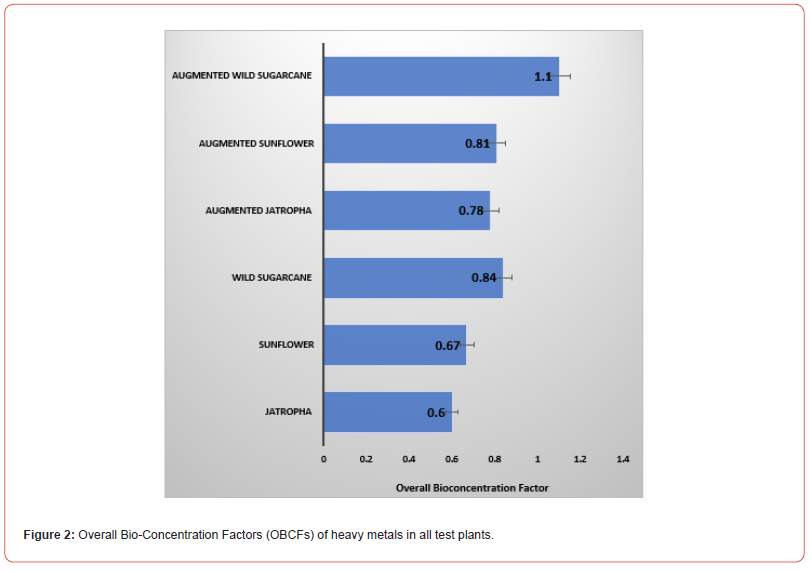
Bio-centration Factors (BCFs) are presented in Table 4. In the non-augmented experiments, the highest BCFs of heavy metal were found in Jatropha (BCFs of Cr was 0.29 and Cd was 0.24) and wild sugarcane (BCFs of Pb was 0.29; Hg was 0.1). In the augmented experiments, the highest BCFs of heavy metal were found in Jatropha (BCFs of Cr was 0.38), wild sugarcane (BCFs of Cd was 0.40; Pb was 0.36) and sunflower (BCF of Hg was 0.14). As a result, the augmented wild sugarcane had the highest overall Bio-Concentration Factors (OBCFs) of 1.1 as the only experimental trial that recorded >1.0 OBCF (Figure 3). This was followed by non-augmented wild sugarcane (OBCF=0.84). Augmentation of pots containing the test plants increased OBCFs. In descending order, OBCFS are given as follows: Augmented wild sugarcane (1.1) > wild sugarcane (0.84) > augmented sunflower (0.81) > augmented Jatropha (0.78) > sunflower (0.67) > Jatropha (0.6).
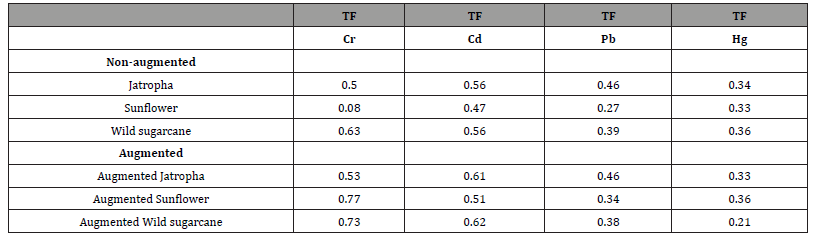
Determination of TFs of heavy metals in test plants
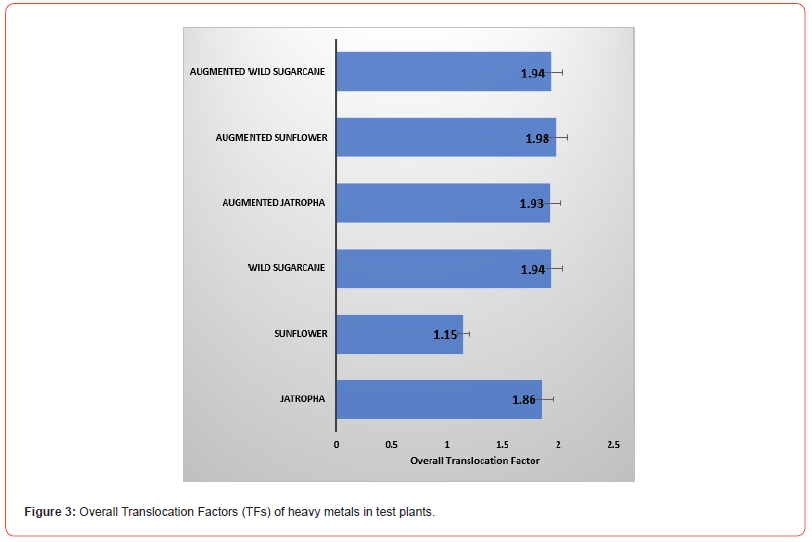
Computed Translocation Factors (TFs) are presented in Table 5. In the non-augmented experiments, the highest TFs of heavy metal were found in wild sugarcane (TFs of Cr was 0.63 and Hg was 0.36), Jatropha (TFs of Pb was 0.46) the two plants (wild sugarcane and Jatropha where TFs of Cd was 0.56). In the augmented experiments, the highest TFs of heavy metal were found in Sunflower (TFs of Cr was 0.77; Hg was 0.36), wild sugarcane (TFs of Cd was 0.62) and Jatropha (TF of Pb was 0.46).
As a result, all experimental trials had OTFs (overall Translocation Factors) values of >1.0 as shown in Figure 4. The augmented sunflower had the highest OTF of 1.98 while the lowest was found in sunflower (1.15). Augmentation of pots containing the test plants increased OTF values. In descending order, OTFS are given as follows: augmented sunflower (1.98) > augmented and non-augmented wild sugarcane (1.94) > augmented Jatropha (1.93) > Jatropha (1.86) > sunflower (1.15).
Table 1:Computation of pollution index (PI) for Jatropha treated soil.
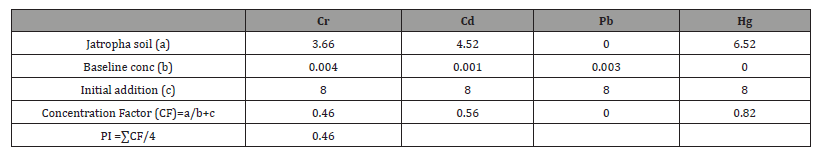
Table 2:Computation of pollution index (PI) for wild sugarcane treated soil..
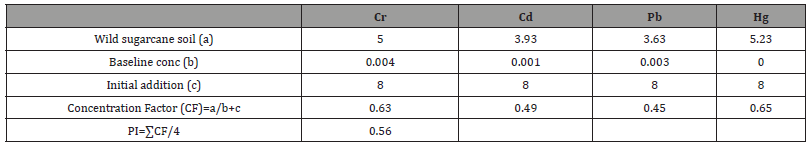
Table 3:Computation of pollution index (PI) for sunflower treated soil.
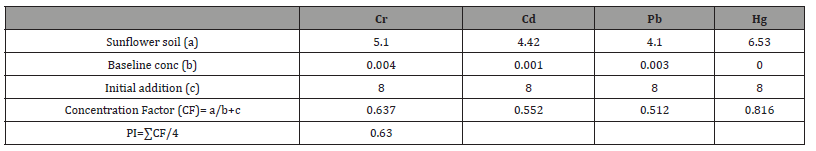
Table 4:Computation of Bio-Concentration Factors (BCFs) of each heavy metal in test plants..
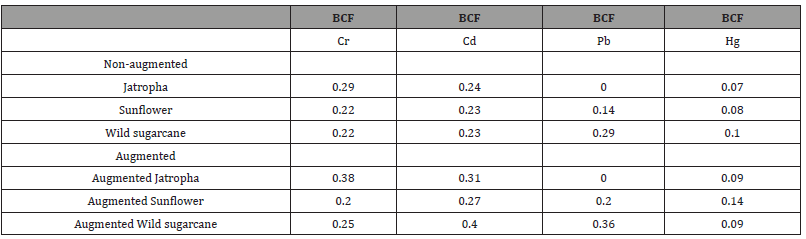
Table 5:Computation of Translocation Factors (TFs) of each heavy metal in test plants.

Discussion
All pollution indices (PIs) were <1.0 benchmark. The highest pollution index was found in sunflower soil and the least in Jatropha soil. This suggests that the three test plants are good phytoremediants of polluted soils. The bioconcentration factor (BCF) and translocation factor (TF) are among the most important parameters to determine the potential of plants to be used in phytoremediation [26]. There are two tolerance mechanisms against metal stress. The first mechanism involves metal excluder species that have a TF value lower than 1 and accumulate toxic metals in the roots with low root-shoot transfer. The second mechanism is typical of hyperaccumulator plants that have TF and BCF values higher than 1.0 benchmark.
Based on the obtained results, the augmented wild sugarcane had overall bio-concentration factors (OBCFS) >1.0 benchmark, therefore a hyperaccumulator. All test plants (Jatropha, sunflower and wild sugarcane had TFs>1.0 benchmark, thus characterized with high heavy metal uptake from contaminated soil. Augmented sunflower had the highest TF value. This outcome is in agreement with previous phytoremediation studies on sunflower [27-29] although without the use soil amendments. Recently, Niu et al. [30] carried out a study on Sunflower (Helianthus annuus L.) under Citric and Glutaric acid-assisted phytoextraction. It was shown that the plant accumulated Cd and Pb excellently from polluted sites, hence that organic acids impacted the translocation of these heavy metals to aerial parts of the plants. Similarly, the application of organic manure to sunflower pots may have impacted in the translocation of Cr, Cd, Pb and Hg metals in the present work [31].
Conclusion
The study established that the three test plants are good phytoremediants, based on the low pollution indices shown in the result. Only the augmented wild sugarcane had BCF >1, therefore, a hyperaccumulator. All test plants had TFs>1 and therefore characterized with high heavy metal uptake. Augmentation of heavy metal polluted sites by cow dung during phytoremediation of the test plants produced excellent results. The organic manure used may have impacted the translocation of heavy metals in the test pants.
References
- Jadia C, Fulekar MH (2009) Phytoremediation of heavy metals: Recent techniques. Afr J Biotechnol 8: 921-928.
- Sanchez-Castro A, Lazaro MA, María-Angeles PB, Ana SA (2023) Past, present and future trends in the remediation of heavy-metal contaminated soil-Remediation techniques applied in real soil-contamination events. Heliyon 9(6): e16692.
- Burges A, Alkorta I, Epelde L, Garbisu C (2018) From phytoremediation of soil contaminants to phytomanagementof ecosystem services in metal contaminated sites. Int. J. Phytoremediat 20(4): 384-397.
- Ashraf S, Ali Q, Zahir ZA, Ashraf S, Asghar HN (2019) Phytoremediation: environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotox. Environ. Safe. 174: 714-727.
- Zhang Y, Li X, Zhang Y, Li Y, Zhang X, et al. (2021) Research Progress on Heavy Metals Pollution in the Soil of Smelting Sites in China. Int J Environ Res Public Health 18: 4704.
- Banfalvi G (2011) Heavy Metals, Trace Elements and Their Cellular Effects. In: Banfalvi G (Eds) Cellular Effects of Heavy Metals. Springer, Dordrecht.
- Wuana RA, Okieimen FE (2011) Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. International Scholarly Research Network Ecology Vol. 2011:
- Rius-Ayra O, Biserova-Tahchieva A, and Llorca-Isern N (2023) Removal of dyes, oils, alcohols, heavy metals and microplastics from water with superhydrophobic materials. Chemosphere 311 (Pt 2):137148.
- Nwoko CO, Mgbeahuruike L (2011) Heavy metal contamination of ready to use herbal remedies in South Eastern Nigeria. Pakistan Journal of Nutrition 10(10): 959-961.
- Popoola OE, Okonkwo OJ, Arowolo TA, Popoola AO, Awofalu OR (2012) Heavy metals content in classroom dust of some public primary schools in metropolitan Lagos Nigeria. Research Journal of Environmental and Earth Sciences 4(4):460-465.
- Shazia G, Alia N, Iftikhar F, Muhammad I (2015) Reducing Heavy Metals Extractionfrom Contaminated Soils Using Organicand Inorganic Amendments-a Review. Pol J Environ Stud 24(3): 1423-1426.
- Lone M, He Z, Stoffella PJ, Yang X (2008) Phytoremediation of heavy metal polluted soils and water: Progresses and perspectives. J Zhejiang Univ Sci 9(3): 210-220.
- Soni S, Jain S (2014) A review on phytoremediation of Heavy metals from Soil by using plants to remove pollutants from the environment. International Journal of Advanced Research 2(8): 197-203
- Yan A, Wang Y, Tan SN, Mohd Yusof ML, Ghosh S (2020) Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front Plant Sci 11: 359.
- Suman J, Uhlik O, Viktorova J, Macek T (2018) Phytoextraction of Heavy Metals: A Promising Tool for Clean-Up of Polluted Environment? Front. Plant Sci 9: 1476.
- Priya AK, Muruganandam M, Ali SS, Kornaros M (2023) Clean-Up of Heavy Metals from Contaminated Soil by Phytoremediation: A Multidisciplinary and Eco-Friendly Approach. Toxics 11(5): 422.
- Sabreena A, Sultana R, Rahman MA, Islam MS, Hossain MA (2021) Phytoremediation of Toxic Metals: A Sustainable Green Solution for Clean Environment. Applied Sciences 11(21): 10348.
- Azubuike CC, Chikere CB, Okpokwasili GC (2016) Bioremediation techniques-classification based on site of application: principles, advantages, limitations and prospects. World Journal of Microbiology and Biotechnology 32(11): 180.
- Ghosh M, Singh, SP (2005) A review on phytoremediation of heavy metals and utilization of it’s by products. Asian J. Energy Environ 6: 214-231.
- Abah RC (2013) An application of Geographic Information System in mapping flood risk zones in a north central city in Nigeria. African Journal of Environmental Science and Technology 7(6): 365-371.
- Green DW, Perry RH (2008) Densities of aqueous inorganic solution Perry’s Chemical Engineers’ Handbook, McGra w-Hill (2) : 99-118.
- AOAC (1990) Official Methods of Analysis. Association of Official Analytical Chemists (11th edn ). Washington DC, USA.
- Paliza S, Korkmaz B, Josef HG (2019) Phytoremediation of Heavy Metal-Contaminated Soil by Switchgrass: A Comparative Study Utilizing Different Composts and Coir Fiber on Pollution Remediation, Plant Productivity, and Nutrient Leaching. International Journal of Environmental Research and Public Health 16(7): 1261
- Majolagbe AO, Paramole AA, Majolagbe HO, Oye WO, Sowemimo MO (2010) Concentration of heavy metals in tree barks as indicator of atmospheric pollution in Oyo Town, Southwest, Nigeria, Archives of Applied Science Research 2(1): 170-178.
- Ogbonna PC, Ukpai N, Obasi KO (2018) Assessment of metal contamination in Ubeyi River and accumulation in fish and sediment. Journal of applied science and environmental management 22 (8): 1151 -1157
- Saleem MH, Fahad S, Rehman M, Saud S, Jamal Y, et al. (2020) Morpho-physiological traits, biochemical response and phytoextraction potential of short-term copper stress on kenaf (Hibiscus cannabinus L.) seedlings. Peer Journal 8: e8321
- Allah EF, Hashem A, Alqarawi AA, Alwathnani HA (2015) Alleviation of adverse impact of cadmium stress in sunflower (Helianthus annuus L.) by arbuscular mycorrhizal fungi Pakistan Journal of Botany 47: 785-795.
- Farid M, Ali S, Rizwan M, Ali Q, Saeed R, et al. (2018) Phyto-management of chromium contaminated soils through sunflower under exogenously applied 5-aminolevulinic acid. Ecotoxicology and Environmental Safety 151: 255-265.
- Piracha MA, Ashraf M, Niaz A (2019) Arsenic fractionation and its impact on physiological behavior of sunflower (Helianthus annuus L.) in three texturally different soils under alkaline calcareous conditions. Environmental Science and Pollution Research 26:17438-17449.
- Niu Z, LI X, Mohammad M (2023) Accumulation Potential Cadmium and Lead by Sunflower (Helianthus annuus L.) under Citric and Glutaric Acid-Assisted Phytoextraction. International Journal of Environmental Research and Public Health 20(5): 4107
- Tyubee BT (2009) The influence of ENSO and North Atlantic sea surface temperature anomaly (SSTA) on extreme rainfall events in Makurdi, Nigeria. Meteorological Climate Science 7: 28 -33.
-
Kever D . G, Oluma H . O . A 1, Aguoru C . U1, Eneji I . S2 and Olasan J . O1. Comparative Phyto Extractive Potentials of Jatropha curcas, Saccharum spontaneum and Helianthus annuus in Heavy Metal Contaminated Soil Using Remediation Indices. Online J Ecol Environ Sci. 1(3): 2023. OJEES.MS.ID.000512.
-
Liquid chromatography, Loblolly pine, Shortleaf pine, Climate change, Ecological adaptability, Hybridization, Geographic information systems, Solvent extraction system, Chromatograms, Peaks
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






