 Research Article
Research Article
Revisiting Prenatal Dental Care Practices- A Comprehensive Review
Georgios Chrysochoou1*, Eleni Androulidaki1, Costas Panayotidis2 and Μaria Antoniadou1,3*
1Department of Dentistry, School of Health Sciences, National and Kapodistrian University of Athens, Greece
2O&G Consultant MRCOG, MSc, MD Attiki Iatriki Athens, Greece
3CSAP Executive Mastering program in systemic management, University of Piraeus, Greece
Georgios Chrysochoou, Department of Dentistry, School of Health Sciences, National and Kapodistrian University of Athens, Greece. Μaria Antoniadou, As. Professor, Department of Dentistry, School of Health Sciences, National and Kapodistrian University of Athens, Greece and CSAP Executive Mastering program in systemic management, University of Piraeus, Greece.
Received Date: February 05, 2024; Published Date: February 16, 2024
Abstract
Pregnancy represents a physiological state characterized by a myriad of transformative processes within the female body. Regrettably, the oral manifestations of these changes are frequently overlooked by both gynecologists and dental practitioners. Furthermore, empirical evidence has substantiated a correlation between inadequate oral hygiene in pregnant individuals and an elevated likelihood of encountering complications during pregnancy, including but not limited to low birth weight, premature delivery, and dental anomalies.
It is imperative for dentists to adhere to meticulously crafted protocols governing the utilization of X-rays, anesthetics, and antibiotics during dental procedures involving pregnant women. Additionally, dental professionals should possess a comprehensive understanding of common oral findings specific to pregnant individuals, such as pyogenic granuloma, and foster effective communication with gynecologists when pregnant patients seek dental consultations. In the field of gynecology, practitioners are strongly recommended to integrate oral health assessments into their antenatal care programs. Simultaneously, dentists are encouraged not to exhibit hesitancy in delivering dental interventions to pregnant women. This review article serves as a comprehensive professional guide for interdisciplinary collaboration between gynecologists and dentists. It presents a systematic algorithm delineating the nuances of dental treatment during pregnancy, with due consideration given to both gynecological and dental perspectives for enhancing the quality services in both fields.
Keywords:Pregnancy; Oral health; Dental care; Obstetricians; Pregnancy outcomes; X-rays; Anaesthetics; Antibiotics; Pyogenic granuloma; Antenatal care; Quality of dental services
Introduction
The intricate relationship between women’s health and oral care during pregnancy is a crucial aspect of comprehensive healthcare. Pregnancy induces profound physiological changes impacting oral health, yet there is a noticeable gap in addressing dental hygiene specific to this period [1]. Despite the unique confluence of biological changes in pregnant women, a sense of caution and reluctance prevails among patients, obstetricians, and dentists when addressing oral health during pregnancy. This hesitancy, compounded by the absence of well-defined clinical guidelines [2], underscores the need for a more comprehensive approach. The reluctance to address oral health issues during pregnancy not only jeopardizes the pregnant individual’s wellbeing but also perpetuates a gap in the continuum of care. Despite existing literature and clinical guidelines [3-9], a unified and comprehensive approach is notably lacking.
Physiological changes during pregnancy, such as hormonal fluctuations and alterations in immune response, significantly impact oral health. Kohlhepp, et al. (2018) [1] stress the importance of understanding these alterations to provide effective medical and dental care to pregnant women [1]. This fact underscores the need for healthcare providers to collaborate closely and integrate oral health considerations into overall prenatal care. Clinical guidelines provide valuable recommendations for pregnancy care [3-9], but the specific parameters of oral health are often not comprehensively addressed. The challenge extends beyond clinical guidelines to the attitudes and knowledge of healthcare professionals. Hashim and Akbar (2014) [10] for example, highlight gynecologists’ lack of comprehensive knowledge about the links between oral health and adverse pregnancy outcomes and they also emphasize the role of gynecologists in promoting and maintaining oral health during pregnancy [11]. It seems that the reluctance to address oral health issues during pregnancy is a multifaceted challenge. It derives not only from the lack of well-defined clinical guidelines but also from societal misconceptions and a historical separation between dental and maternal healthcare.
This article advocates a paradigm shift in approaching oral health during pregnancy, emphasizing collaborative efforts among general practitioners, obstetricians, the antenatal care team, and dentists. It aims to address gaps in current literature and clinical practice by providing a synthesized overview of dental care during pregnancy. By examining existing knowledge and proposing structured approaches, the article aims to contribute to the understanding and effective management of oral health, benefiting both mothers and their unborn children.
Methodology
This narrative review conducted an extensive exploration of pertinent scholarly literature from the years 2000 to 2023 in the English, French, and German languages. The search strategy employed for this review involved the exploration of the relevant literature using MeSH (Medical Subject Headings) keywords in the PubMed database, as well as complementary searches in Google Scholar and Science Direct. The MeSH keywords employed included #physiological changes, #pregnancy, #oral health, #dental care, #prenatal oral health care, #pregnancy microbiome, #maternal periodontitis, #maternal caries, #smoking, #diet, #x-rays, #antibiotics, and #anaesthetics.
Inclusion criteria for this review, addressing the intersection of dentistry and pregnancy for ensuring the relevance and quality of the selected studies were the following:
1) Publication type: Systematic reviews, narrative reviews, practice guidelines, comparative studies and meta-analyses were considered.
2) Study design: Primary studies employing rigorous designs such as randomized controlled trials (RCTs), cohort studies, case-control studies, and observational studies were included.
3) Population: The studies had to focus on pregnant individuals of any age and gestational stage. We included studies involving women with various medical conditions or risk factors related to pregnancy.
4) Intervention/exposure: The studies should encompass interventions or exposures related to dental care during pregnancy, evaluating oral health interventions, preventive measures, dental treatments, and behavioral interventions.
5) Outcome measures: Studies should emphasize outcomes related to both maternal and fetal health and include measures such as maternal periodontal health, pregnancy complications, birth outcomes, and the impact of dental interventions on these outcomes.
6) Language: We limited inclusion criteria to studies published in English, French, or German.
7) Time frame: We also specified a defined time frame for the inclusion of studies (e.g., 2000-2023) to ensure relevance to contemporary dental and obstetric practices.
8) Relevance to dentistry and pregnancy: The studies should directly address the relationship between dental health, dental care practices, and pregnancy outcomes. We further ensured that studies included in this review should explore relevant topics such as physiological changes, maternal periodontitis, impact of lifestyle factors, diagnostic procedures, and the use of medications during pregnancy.
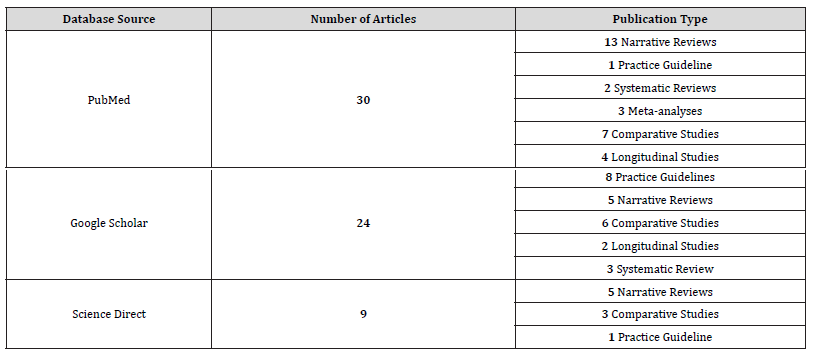
9) Database source: Studies were retrieved from reputable databases such as PubMed, Google Scholar and Science Direct. The distribution of articles collected from the three different databases are shown in Table 1.
Table 1:Presentation of articles from different databases.
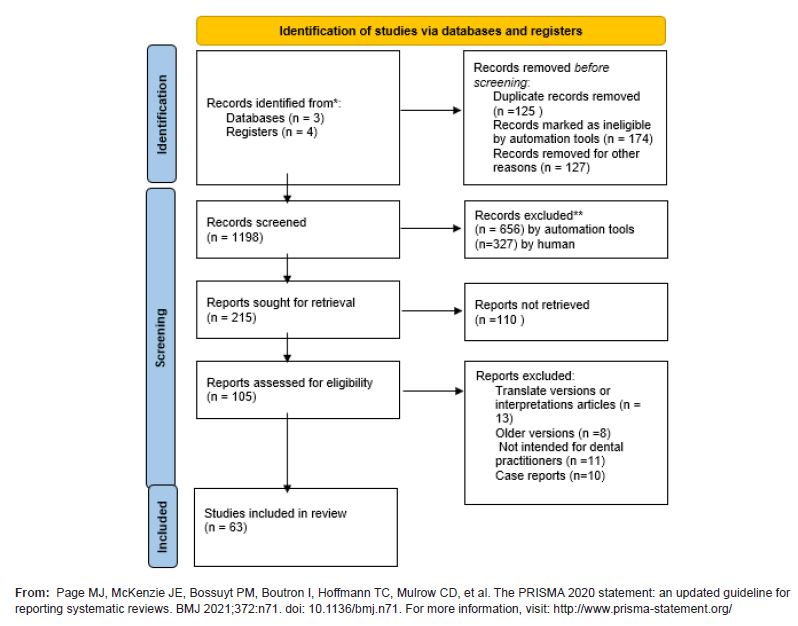
The initial phase retrieved 1198 papers. After eliminating duplicates and evaluating relevance, the procedure resulted in the inclusion of 63 papers for the final analysis as seen in the Prisma flow chart of the study using the PRISMA 2020 flow diagram for new systematic reviews (Table 2).
Table 2:Prisma chart.
Results
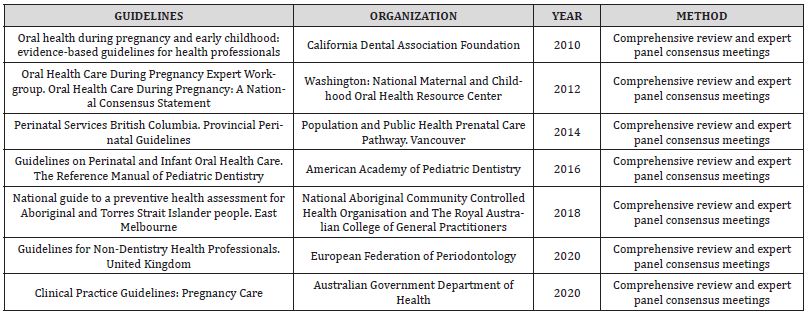
The results from the former described procedure revealed only seven guideline documents meeting the specified criteria. These guidelines originated from diverse geographical regions, comprising two from Australia, three from the United States of America, one from Canada, and one from Europe. In Table 3 we summarize the basic characteristics of the clinical guidelines included in our investigation.
Table 3:Basic characteristics of clinical guidelines for oral health care during pregnancy.
Notably, all identified guidelines uniformly recommend gynecologists to incorporate dental considerations into their clinical assessments [3, 4]. This entails obtaining a thorough dental history, vigilant observation for indications of oral health issues such as bleeding gums and untreated tooth decay, and proactive encouragement for pregnant women to undergo dental check-ups without hesitation [5-7]. Moreover, gynaecologists are advised to counsel pregnant individuals on the importance of regular tooth brushing, advocating a minimum frequency of twice daily with fluoride toothpaste [8-9].
However, a noteworthy observation pertains to the absence of organized guidelines for dental management during pregnancy in many countries. Consequently, this deficiency contributes to a reluctance among dentists to administer dental services to pregnant women. The underlying cause is identified as a lack of interdisciplinary communication between gynaecologists and dentists in the context of pregnancy management.
Collaboration between obstetrician-gynecologists and dental professionals
As already mentioned previously, maintaining optimal oral hygiene and dental care is of paramount importance during pregnancy, as inadequate oral care practices may predispose pregnant women to endure pain, infections, dental caries, and even tooth loss. Remarkably, the available information regarding the interplay between pregnancy and dental care appears to be suboptimal, with a notable lack of awareness among healthcare professionals regarding the physiological changes in oral health during pregnancy [10]. Moreover, a noteworthy trend emerges where women, particularly those in the early stages of pregnancy, frequently seek counsel primarily from their general practitioners (GPs) for the initiation of their prenatal care [11]. Although direct consultations with gynaecologist obstetricians are common, yet these healthcare providers may lack specialized training or express limited interest in dental care matters. An erroneous perception prevails, relegating dental visits to emergency situations, thereby delaying, or neglecting routine dental check-ups [12].
For women contemplating pregnancy, it is imperative to prioritize a dental examination as a crucial component of preconception medical assessments. This examination should encompass sophisticated cleaning technologies such as ultrasound and air polishing for removal of biofilms from teeth surfaces [13]. Also, rigorous evaluation of gum tissue health enables the identification and proactive management of any oral health issues before the onset of pregnancy, facilitated by a comprehensive treatment plan.
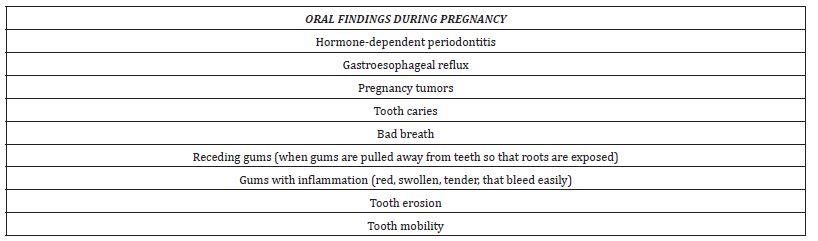
From the dentist’s perspective, pregnancy should not serve as a justification to abstain from providing dental care to pregnant women. This is crucial as their oral microbiome is transferred to the fetus, potentially correlating with adverse outcomes such as preterm birth, low birth weight, pre-eclampsia, and the occurrence of early childhood caries [14-17]. Moreover, hormonal changes and alterations in the oral microbiome of pregnant women have been associated with the development of gingivitis, periodontitis, and tooth mobility [18]. While some studies have shown an association between periodontal disease and adverse pregnancy outcomes such as preterm birth and low birth weight, others have reported no such correlation [19-20]. Ongoing research examines the pathophysiology of the cause-and-effect relationship between oral health and pregnancy outcomes [21]. Table 4 enumerates selected signs and symptoms observed in the oral cavity of pregnant women.
Table 4:Common oral findings during pregnancy.
The role of the antenatal team
Nurses, nurse practitioners, and nurse midwives are strategically positioned to impart comprehensive education to pregnant women concerning the advantageous implications of maintaining optimal oral health. Furthermore, they possess the capacity to discern and appropriately refer women necessitating dental interventions [2-4].
Conducting an oral examination assumes heightened significance for pregnant women who do not routinely avail themselves of dental care services. In instances where a pregnant woman reports symptoms such as bleeding gums, tooth mobility, toothache, tooth sensitivity, or other oral issues (Table 4), a prompt referral for dental care is recommended. Similarly, if the healthcare provider observes manifestations like bad breath, inflammation, discharge, or signs indicative of infection, a referral for dental care is deemed necessary [22]. Thus, an effective preventive strategy could be implemented during the woman’s initial visit to the gynecological clinic.
Diet during pregnancy and its implications on oral health
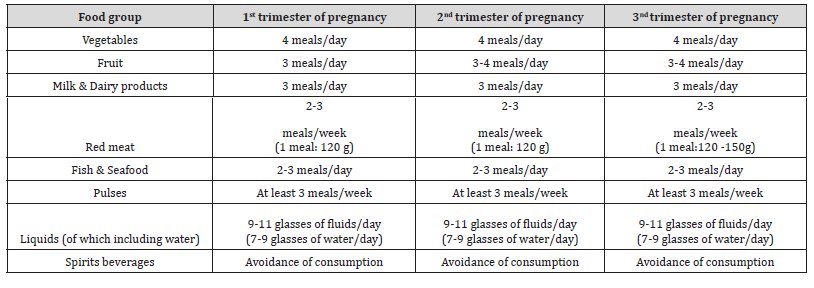
During pregnancy, the nutritional habits of women undergo significant modifications due to behavioral and hormonal changes. A notable shift is observed in the increased consumption of sugar at various intervals throughout the day [23]. Such dietary patterns, when combined with inadequate oral hygiene practices, contribute to an elevated risk of tooth decay. Tooth decay, a prevalent affliction, ensues when teeth are regularly exposed to sugar or carbohydraterich foods [24]. Bacterial action on sucrose generates acids, which gradually erode tooth enamel, leading to decay and structural weakening [24]. Pregnant women are therefore strongly advised to prioritize oral health, engaging in preventive dental assessments and concurrently minimizing the consumption of cariogenic foods attentively considering both the frequency and quantity [25, 26].
The ramifications of heightened sugar consumption extend beyond the maternal domain, impacting the fetus as well [27-29]. Evidence indicates a correlation between maternal and neonatal factors affecting calcium homeostasis during pregnancy and the development of enamel hypoplasia in newborns [30]. Calcium supplementation during pregnancy has demonstrated associations with reduced risks of gestational hypertension, pre-eclampsia, neonatal mortality, preterm birth, and low birth weight, with an optimal daily dose recommended at 2g/day [31]. The World Health Organization recommends an intake of 1.5-2.0g of elemental calcium/day, administered in three divided doses from 20 weeks’ gestation until the end of pregnancy. Additionally, monitoring vitamin D levels during pregnancy is extremely essential [32-34].
Moreover, maternal dietary patterns characterized by high adherence to a prudent diet significantly impact offspring bone health. Such diets, rich in fruits, vegetables, whole grains, fish, and low in processed foods, correlate with greater bone size and density in offspring [35-39]. Importantly, adherence to these dietary patterns is associated with a decreased likelihood of dental defects in children, such as incomplete enamel formation and enamel hypoplasia, both statistically linked to preterm birth and low birth weight [40-41]. The extent of information provided to low-risk pregnancies remains uncertain [42]. All relevant guidelines are presented in Table 5.
Table 5:Dietary guide by trimester of pregnancy.
Smoking during Pregnancy and Potential Dental Health Implications
Smoking during pregnancy presents a multifaceted concern, encompassing not only direct health risks for the expectant mother but also potential repercussions for fetal growth and, intriguingly, dental health in offspring. Research suggests a tentative association between systemic smoking during pregnancy and the occurrence of premature caries in childhood [43]. The complexity of establishing a direct causal relationship is underscored by the potential confounding factor of maternal neglect of children’s oral hygiene education rather than a direct consequence of fetal development. The need for additional prospective studies is evident to validate these associations and disentangle influencing factors. These findings highlight the imperative of including information on the detrimental consequences of smoking in tailored preventive programs designed for pregnant women and those of reproductive age [44].
Furthermore, several studies have contributed to our understanding of the potential link between maternal smoking during pregnancy and its impact on dental health in children. For instance, research by Tanaka, et al. (2010) indicates that maternal smoking during pregnancy and postnatal household smoking may influence dental caries in young children, emphasizing the importance of considering both prenatal and postnatal environmental exposures [43]. Moreover, Williams, et al. (2000) [44] explored parental smoking practices and their connection to caries experience in pre-school children, providing insights into the broader environmental context influencing oral health outcomes in early childhood [44]. A recent systematic review and meta-analysis by Zhong, et al. (2021) [45] further corroborated the correlation between maternal smoking during pregnancy and dental caries in children, adding to the growing body of evidence supporting the need for comprehensive preventive measures targeting maternal smoking in the context of oral health promotion [45].
X-rays during pregnancy
One of the reasons that some pregnant women are reluctant to go to the dentist is because of their fear about possible consequences on foetal development from the use of x-rays and local anaesthetics. Many dentists are not aware of the guidelines for the treatment of a pregnant woman. The teratogenicity of radiation depends on fetal age and radiation dose [46]. During the first 10 days after conception, the fetus is at greatest risk of teratogenicity and death. The cumulative exposure of the foetus to radiation should not exceed 0.20Gy, which is the dose that can cause microcephaly and mental retardation. The amount of ionising radiation produced by dental x-rays is so small that it is unlikely to reach the teratogenic threshold. Thus, it is unlikely that dental ionising radiation will be a cause of birth defects in utero because the effect has been shown to be deterministic (not stochastic) [47]. A bitewing and panoramic radiographic study generates about onethird the radiation exposure associated with a full-mouth series with E-speed film and a rectangular collimated beam. The dental staff must adapt to the principles of ALARA (As Low as Reasonably Achievable) and only X-rays necessary for the diagnosis will be performed, especially during the first 12-14 weeks of pregnancy, when fetal tissue is particularly sensitive to X-rays [48]. Pregnant women should wear a lead apron and a thyroid collar during X-rays, and dentists should use high-speed films to reduce the amount of time exposed to radiation [49].
Administration of local anesthetics during dental procedures in pregnancy
The administration of local anesthetics during dental procedures to pregnant women is generally safe when performed judiciously with appropriate dosage considerations [15, 50]. The potential risk of toxicity to the fetus associated with anesthetic administration is contingent upon multiple factors, including the quantity of free drug reaching the fetus, the total dose administered, the route of administration, the concurrent presence of vasoconstrictors, and the metabolic levels of the mother. Typically, the total amount of local anesthetics utilized, even for extensive dental procedures, remains below the maximum recommended doses, mitigating the likelihood of complications [51, 52].
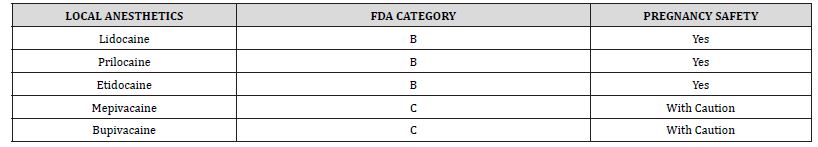
The addition of vasoconstrictor agents, such as adrenaline and norepinephrine, may induce reduced blood flow to the placenta due to alpha-receptor stimulation. Notably, the local anesthetic with an optimal balance of safety and efficacy for pregnant women is two percent (2%) lidocaine with epinephrine 1:200,000 [53]. Organogenesis is active during weeks 4-10 (gestational age) and therefore teratogenic effects may occur if the fetus is exposed to drugs during this period. It is therefore generally recommended that dental treatment be postponed until the end of the first trimester. In terms of gestational age, the second trimester is between 14 and 27 weeks. The risk of drugs causing teratogenesis is lower during this period than during the first trimester. In the third trimester of pregnancy, aortocaval compression is even more likely to occur in the supine position due to the enlargement of the uterus. The use of low doses of local anesthetics is possible for pregnant women in the third trimester. This may reduce the toxic effects of local anesthetics [54]. Table 6 classifies some anaesthetics according to their toxicity risk.
Table 6:Classification of anaesthetics by toxicity.
Administration of antibiotics during dental procedures in pregnancy
The administration of antibiotics during dental procedures in pregnancy necessitates careful consideration to ensure both maternal and fetal well-being. The safety of antibiotic use during pregnancy hinges on various factors, including the specific antibiotic employed, its dosage, the timing of administration during gestation, and the overall health status of the pregnant woman. Amoxicillin and cephalosporins are generally considered safe options during pregnancy, posing minimal risk to the fetus [55]. Clindamycin, although generally safe, is recommended with caution, considering the potential risk of developing Clostridium difficile infection [56].
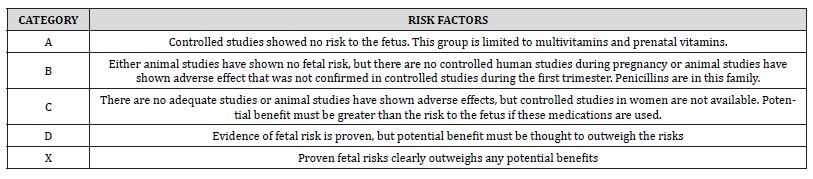
In general, during pregnancy the indications for the administration of antibiotics remain unchanged, the dosage does not alter, nor does the duration of administration. The only difference is the choice of appropriate antibiotics based on the risk of toxicity as categorized by the FDA. Table 7 sets out this classification. The risk–benefit ratio for the patient should be determined and the obstetrician consulted before prescribing these drugs [57]. According to the statement of the FDA, no relation has yet been reported linking the implementation of amoxicillin during pregnancy to miscarriage, the main birth defects, or fetal and maternal side effects. It is worthy to point that the danger of a severe disease called necrotizing enterocolitis in the newborn is increased using the combination of amoxicillin and clavulanic acid (co-amoxiclav) in the third trimester [58]. Metronidazole is a very effective category B drug against anaerobic bacteria, usually in combination with penicillin, to treat odontogenic infections [59]. Tetracyclines are all broad-spectrum antibiotics classified in category D. Traditionally, they have been contraindicated in pregnant and lactating women because of the possibility of permanent tooth staining in the developing fetus and because of the risk of hepatotoxicity in pregnant women. Regarding analgesics, neither aspirin nor ibuprofen (NSAI) should be given to the pregnant woman during the 3rd trimester of pregnancy, as they have been associated with rare fetal kidney problems and premature closure of the ductus arteriosus [60]. In any case, multidisciplinary discussion is needed between the dentist, the obstetrical team and the pharmacologist, in order to establish the best therapeutic plan in terms of drugs needed during and after dental surgery.
Table 7:FDA categories-Teratogenic risks of drugs.
Pregnancy and Oral Tumors
The manifestation of oral tumors during pregnancy, particularly in the form of pregnancy granulomas, underscores a notable facet of oral health intricacies in expectant women [61] Pregnancy granulomas are characterized by the development of a sizable lump adorned with deep red markings, typically situated on the labial surface of the papilla. The resulting inflammation may induce bleeding, impede eating and speaking, and elicit discomfort [62]. Occurring predominantly in the second trimester, pregnancy granulomas are discerned as an extreme inflammatory response to local irritants, such as food particles or plaque. Despite their formidable appearance, these granulomas are non-cancerous and lack the propensity to metastasize.
These oral structures are also identified by alternative nomenclatures including pyogenic granuloma, lobular capillary hemangioma, and epulis gravidarum [63]. While small lesions often respond well to localized debridement, chlorhexidine rinses, and enhanced oral hygiene practices, large lesions may necessitate deep excision. The intricacies associated with intraoperative bleeding management during surgery accentuate the importance of having clinicians with specialized training and experience perform such procedures [21].
Proposal of an algorithm for oral health assessment and counseling during pregnancy
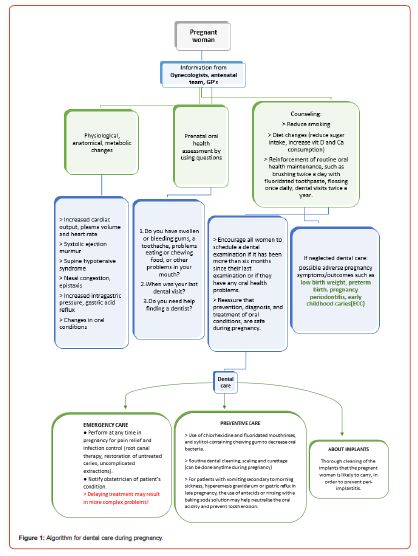
In figure 1 a concise guide is proposed in the form of an algorithm for the clinical management of the pregnant woman, with the collaboration of the gynecologist, dentist and antenatal team. It is written in a way to follow the path of pregnant women through the primary and secondary care and her interaction with the dental team. It points out, as a reminder, the information to be given and when dental treatment is necessary to be proposed during pregnancy. It incorporates details from various dimensions of dental care and oral health during pregnancy, shedding light on critical aspects ranging from preventive measures and safety guidelines to interdisciplinary communication and the psychological well-being of stakeholders.
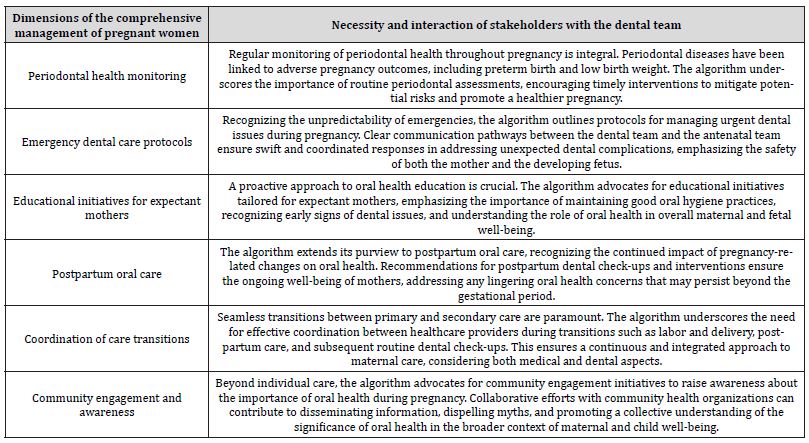
In the continuum of care during pregnancy, the proposed algorithm extends its focus to encompass additional dimensions for the comprehensive management of pregnant women and their interaction with the dental team (Table 8). The integration of these additional elements into the proposed guide significantly elevates the quality of services provided by the interdisciplinary team.
Table 8:Guide of comprehensive management of pregnant women from the dental team.
Conclusion
This review highlights the undeniable connection between dental care, oral health, and pregnancy outcomes. By incorporating additional dimensions into the proposed guide, such as early interventions, emergency preparedness, patient education, and seamless transitions between care stages, the interdisciplinary team can significantly enhance the quality of services provided. This comprehensive approach, guided by evidence-based protocols and accompanied by psychological support, and collective efforts not only underscore the importance of preventive measures but also contribute to positive maternal and fetal outcomes. The proposed algorithm embracing a multidisciplinary framework, grounded in both clinical expertise and community engagement, sets the stage for a holistic and supportive journey throughout pregnancy, ultimately fostering a positive impact on maternal and child health.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Kohlhepp LM, Hollerich G, Vo L, Hofmann Kiefer K, Rehm M, et al. (2018) Physiological changes during pregnancy. Anaesthesist 67(5): 383-396.
- (2013) Committee Opinion No. 569: oral health care during pregnancy and through the lifespan. Obstet Gynecol 122(2):417-422.
- Australian Government Department of Health (2020) Clinical Practice Guidelines: Pregnancy Care. Canberra: Australian Government.
- National Aboriginal Community Controlled Health Organisation and The Royal Australian College of General Practitioners (2018) National guide to a preventive health assessment for Aboriginal and Torres Strait Islander people. East Melbourne: RACGP.
- American Academy of Pediatric Dentistry (2016) Guidelines on Perinatal and Infant Oral Health Care. The Reference Manual of Pediatric Dentistry 252-256.
- California Dental Association Foundation (2010) Oral health during pregnancy and early childhood: evidence-based guidelines for health professionals. J Calif Dent Assoc 38(6): 391-440.
- Oral Health Care During Pregnancy Expert Workgroup (2012) Oral Health Care During Pregnancy: A National Consensus Statement. Washington: National Maternal and Childhood Oral Health Resource Center.
- Perinatal Services British Columbia (2014) Provincial Perinatal Guidelines: Population and Public Health Prenatal Care Pathway. Vancouver: Perinatal Services BC.
- European Federation of Periodontology (2020) Guidelines for Non-Dentistry Health Professionals. United Kingdom: EFP.
- Hashim R, Akbar M (2014) Gynecologists' knowledge and attitudes regarding oral health and periodontal disease leading to adverse pregnancy outcomes. J Int Soc Prev Community Dent 4(Suppl 3): S166–S172.
- Kobylińska A, Sochacki Wójcicka N, Dacyna N, Trzaska M, Zawadzka A, et al. (2018) The role of the gynaecologist in the promotion and maintenance of oral health during pregnancy. Ginekol Pol 89(3): 120-124.
- Şenyuva İ, Koca C (2020) Oral and dental health in pregnancy: Knowledge of gynecologists/obstetricians, dentists, family physicians and midwives. Nobel Med 16(2): 55-61.
- Morgan MA, Crall J, Goldenberg RL, Schulkin J (2009) Oral health during pregnancy J Matern Fetal Neonatal Med 22(9): 733-739.
- Rocha JS, Arima LY, Werneck RI, Moysés SJ, Baldani MH (2018) Determinants of dental care attendance during pregnancy: A systematic review. Caries Res 52(1-2): 139-152.
- Hartnett E, Haber J, Krainovich Miller B, Bella A, Vasilyeva A, et al. (2016) Oral Health in pregnancy. J Obstet Gynecol Neonatal Nurs 45(4): 565-573.
- Saadaoui M, Singh P, Al Khodor S (2021) Oral microbiome and pregnancy: A bidirectional relationship. J Reprod Immunol 145: 103293.
- López NJ, Smith PC, Gutierrez J (2002) Higher risk of preterm birth and low birth weight in women with periodontal disease. J Dent Res 81(1): 58-63.
- Boggess K (2003) Maternal periodontal disease is associated with an increased risk for preeclampsia. Obstet Gynecol 101(2): 227-231.
- Steinberg BJ, Hilton IV, Iida H, Samelson R (2013) Oral Health and dental care during pregnancy. Dent Clin North Am 57(2): 195-210.
- Sherif FM (2018) Maternal periodontal disease and pregnancy outcomes. Research in Medical & Engineering Sciences 4(4).
- Reda Mohamed E, Mohamed Fahmy N, Moussa Soliman S (2019) Pregnant women knowledge regard oral health care. Egyptian Journal of Health Care 10(1): 23-33.
- Dasanayake AP, Gennaro S, Hendricks Muñoz KD, Chhun N (2008) Maternal periodontal disease, pregnancy, and neonatal outcomes. MCN Am J Matern Child Nurs 33(1): 45-49.
- Graham JE, Mayan M, McCargar LJ, Bell RC (2013) Making compromises: A qualitative study of sugar consumption behaviors during pregnancy. J Nutr Educ Behav 45(6): 578-585.
- Vitale SG, Privitera S, Gulino FA, Rapisarda AM, Valenti G, et al. (2016) Dental Management in pregnancy: Recent trends. Clin Exp Obstet Gynecol 43(5): 638-642.
- Lagerweij M, van Loveren C (2019) Chapter 7: Sugar and dental caries. Monogr Oral Sci 68-76.
- Kawakubo Yasukochi T, Hayashi Y, Hirata M (2023) Effects of maternal nutrition on oral health in offspring. Current Oral Health Reports 10(3): 69-74.
- Neuman H, Koren O (2017) The pregnancy microbiome. Intestinal Microbiome. Nestle Nutr Inst Workshop Ser 1-9.
- Trumpff C, Sturm G, Picard M, Foss S, Lee S, et al. (2021) Added sugar intake during pregnancy: Fetal behavior, birth outcomes, and placental DNA methylation. Dev Psychobiol 63(5): 878-889.
- Wigen TI, Wang NJ (2011) Maternal health and lifestyle, and caries experience in preschool children. A longitudinal study from pregnancy to age 5yr. Eur J Oral Sci 119(6): 463-468.
- Reed SG, Miller CS, Wagner CL, Hollis BW, Lawson AB (2019) Toward preventing enamel hypoplasia: Modeling maternal and neonatal biomarkers of human calcium homeostasis. Caries Res 54(1): 55-67.
- Imdad A, Jabeen A, Bhutta ZA (2011) Role of calcium supplementation during pregnancy in reducing risk of developing gestational hypertensive disorders: A meta-analysis of studies from developing countries. BMC Public Health 11(S3).
- Tanaka K, Miyake Y, Sasaki S, Hirota Y (2012) Dairy products and calcium intake during pregnancy and dental caries in children. Nutrition Journal 11(1).
- Tanaka K, Hitsumoto S, Miyake Y, Okubo H, Sasaki S, et al. (2015) Higher vitamin D intake during pregnancy is associated with reduced risk of dental caries in young Japanese children. Annals of Epidemiology 25(8): 620-625.
- Suárez Calleja C, Aza Morera J, Iglesias Cabo T, Tardón A (2021) Vitamin D, pregnancy and caries in children in the inma-asturias birth cohort. BMC Pediatr 21(1).
- Chen X, Zhao D, Mao X, Xia Y, Baker P, et al. (2016) Maternal dietary patterns and pregnancy outcome. Nutrients 8(6): 351.
- Eshak ES, Okada C, Baba S, Kimura T, Ikehara S, et al. (2020) Maternal total energy, macronutrient and Vitamin intakes during pregnancy associated with the offspring’s birth size in the Japan Environment and Children’s study. Br J Nutr 124(6): 558-566.
- Kumar A, Kaur S (2017) Calcium: A nutrient in pregnancy. J Obstet Gynaecol India 67(5): 313-318.
- Grieger J, Clifton V (2014) A review of the impact of dietary intakes in human pregnancy on infant birthweight. Nutrients. 7(1): 153-178.
- Prokocimer T, Amir E, Blumer S, Peretz B (2015) Birth-weight, pregnancy term, pre-natal and natal complications related to child’s dental anomalies. J Clin Pediatr Dent 39(4): 371-376.
- Nelson S, Albert JM, Lombardi G, Wishnek S, Asaad G, et al. (2010) Dental caries and enamel defects in very low birth weight adolescents. Caries Res 44(6): 509-518.
- Nelson S, Albert JM, Geng C, Curtan S, Lang K, et al. (2013) Increased enamel hypoplasia and very low birthweight infants. J Dent Res 92(9): 788-794.
- Xu S, Zhao C, Jia L, Ma Z, Zhang X (2022) Relationship between preterm, low birth weight, and development defects of enamel in the primary dentition: A meta-analysis. Front Pediatr 10.
- Tanaka K, Miyake Y, Sasaki S (2010) The effect of maternal smoking during pregnancy and postnatal household smoking on dental caries in young children. Obstetrical Gynecological Survey 65(1): 15-17.
- Williams SA, Kwan SYL, Parsons S (2000) Parental smoking practices and caries experience in pre-school children. Caries Res 34(2): 117-122.
- Zhong Y, Tang Q, Tan B, Huang R (2021) Correlation between maternal smoking during pregnancy and dental caries in children: A systematic review and meta-analysis. Front Oral Health.
- Nayak AG, Denny C, Veena KM (2012) Oral healthcare considerations for the pregnant woman. Dent Update 39(1): 51-54.
- Russell M, Grimwood H (2021) Dental Health during pregnancy-and beyond. Dental Nursing 17(2): 90-91.
- Flagler CK, Troici CM, Rathore SA (2022) A historical review of the effects of dental radiography on pregnant patients. J Am Dent Assoc 153(10) : 989-995.
- ADA Council on Scientific Affairs (2001) An update on radiographic practices: information and recommendations. ADA Council on Scientific Affairs. J Am Dent Assoc 132(2): 234-238.
- Giacoia GP, Mattison DR (2009) Obstetric and Fetal Pharmacology. The Global Library of Women’s Medicine.
- Manautou MA, Mayberry ME (2023) Local anesthetics and pregnancy. A review of the evidence and why dentists should feel safe to treat pregnant people. J Evid Based Dent Pract 23(2):101833.
- Zhou X, Zhong Y, Pan Z, Zhang J, Pan J (2023) Physiology of pregnancy and oral local anesthesia considerations. Peer J.
- Singh M (2012) The pregnant dental patient. J Mass Dent Soc 60(4): 32-34.
- Lee JM, Shin TJ (2017) Use of local anesthetics for dental treatment during pregnancy; safety for parturient. J Dent Anesth Pain Med 17(2): 81.
- Aragoneses J, Suárez A, Rodríguez C, Algar J, Aragoneses JM (2021) Knowledge, attitudes, and practices among dental practitioners regarding antibiotic prescriptions for pregnant and breastfeeding women in the Dominican Republic. Antibiotics 10(6): 668.
- Aliabadi T, Saberi EA, Motameni Tabatabaei A, Tahmasebi E (2022) Antibiotic use in endodontic treatment during pregnancy: A narrative review. Eur J Transl Myol.
- Kato H, Yamagishi Y, Hagihara M, Hirai J, Asai N, Shibata Y, et al. (2022) Systematic Review and meta-analysis for impacts of oral antibiotic treatment on pregnancy outcomes in chronic endometritis patients. J Infect Chemother 28(5): 610-615.
- Muanda FT, Sheehy O, Bérard A (2017) Use of antibiotics during pregnancy and risk of spontaneous abortion CMAJ 189(17).
- Skouteris CA (2018) General principles for the comprehensive treatment of the pregnant patient. Dental Management of the Pregnant Patient 33-69.
- VT Hemalatha (2013) Dental Considerations in Pregnancy-a Critical Review on the Oral Care. J Clin Diagn Res.
- Cardoso JA, Spanemberg JC, Cherubini K, Figueiredo MA, Salum FG (2013) Oral granuloma gravidarum: a retrospective study of 41 cases in Southern Brazil. J Appl Oral Sci 21(3): 215-218.
- Jafarzadeh H, Sanatkhani M, Mohtasham N (2006) Oral pyogenic granuloma: A Review. Journal of Oral Science 48(4): 167-175.
- Kamal R, Dahiya P, Puri A (2012) Oral pyogenic granuloma: Various concepts of etiopathogenesis. J Oral Maxillofac Pathol 16(1): 79.
-
Georgios Chrysochoou*, Eleni Androulidaki, Costas Panayotidis and Μaria Antoniadou*. Revisiting Prenatal Dental Care Practices-A Comprehensive Review. On J Dent & Oral Health. 7(4): 2024. OJDOH.MS.ID.000669.
-
Oral hygiene, Oral health, Dental interventions, Dental treatment, Dentists, Maternal periodontitis, Dental care, Periodontal disease, Dental defects, Dental procedures
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






