 Review Article
Review Article
Review of Diabetes, Glycemic Control, and Dental Implant Therapy in Patients with Type 2 Diabetes Mellitus
Hakob Khachatryan1 and Gagik Hakobyan2*
1Maxillofacial surgeon, Central clinical Military hospital (Ministry of Defence of The Republic of Armenia), Department of Oral and Maxillofacial Surgery, Yerevan State Medical University after M. Heratsi, Armenia
2Department of Oral and Maxillofacial Surgery, Yerevan State Medical University after M. Heratsi, Armenia
Professor Gagik Hakobyan, Head of Department of Oral and Maxillofacial Surgery, University of Yerevan State Medical University, Yerevan State, Armenia.
Received Date: November 06, 2023; Published Date: November 14, 2023
Abstract
Over the past 20 years, the use of dental implants has increased and is widely used in the rehabilitation of patients with various forms of edentulous. Dental implants become indispensable part of the practice of dentists. The success of dental implants can be influenced by various local and systemic factors, among which diabetes mellitus occupies a special place which determines the protocol of management and the stability of the results of prosthetics. According to notions, diabetes mellitus is considered a syndrome that includes interconnected vascular distal, biochemical and neurological disorders, directly and indirectly affect the microflora of the oral cavity, creating a favorable background for the development of microorganisms, contributing to the severe course of inflammatory diseases of the oral cavity, as well as often complicating the healing of postoperative wounds and the healing processes of bone tissue.
The efficacy of dental implants in patients with diabetes has always been a matter of debate among professionals, with some doctors considering diabetes a relative contraindication and others stating that implants can be used effectively if certain rules are followed.
Based on a literature review and based on many years of personal experience, the authors present their point of view on the use of implants in patients with diabetes and provides professionals with an excellent opportunity to develop and improve their skills in and obtain consistent and predictable clinical results in the complex rehabilitation of patients with diabetes and various dental defects.
Keywords:Dental implant, Osseointegration, Type 2 diabetes mellitus, UV photo functionalization, Bone metabolism marker, OC, β-Cross-Laps
Abbreviation:Diabetes mellitus (DM); World health organization (WHO); Type 2 diabetes (T2DM); Titanium dioxide (TiO2); Surface acid treatment (SLA); Ultraviolet (UV); Osteocalcin (OC); Glucagon-like peptide-1 (GLP-1); C-terminal telopeptide (CTx); Beta form (beta-CTx)
Introduction
Over the past few decades, the incidence and prevalence of diabetes mellitus have increased significantly [1]. The leading place in the structure of diseases of the endocrine system is occupied by diabetes mellitus, which is currently one of the main threats to the health of the population and one of the important priority problems of the health care system [2]. Diabetes mellitus is a chronic metabolic polyetiological disease, which is based on an absolute or relative insulin deficiency in the body, which leads to violations of carbohydrate, fat and protein metabolism. Research in recent years has shown that there is a high prevalence of diabetes in all countries of the world (from 1.5 to 3% of the total population) [3]. Over the past few decades, the incidence and prevalence of diabetes mellitus have increased significantly.
The International Diabetes Federation registered 451 million cases of diabetes in 2017 (global prevalence 8.4%) and an increase in incidence is predicted, which determines the high social significance of this disease [4, 5].
Global diabetes-related health expenditures were estimated at 966 billion USD in 2021, and are projected to reach 1,054 billion USD by 2045 [6].
According to WHO diagnostic criteria for diabetes, a fasting plasma glucose level is >7.0 mmol/L (126 mg/dL) or a 2-hour plasma glucose level is >11.1 mmol/L (200mg/dL) with an oral tolerance test to glucose or hemoglobin A1c. (HbA1c) >6.5%.
Depending on the various etiological signs, diabetes is usually classified into type 1 diabetes mellitus (DM 1) (insulin-dependent, juvenile or diabetes mellitus with onset at a young age), type 2 diabetes mellitus (DM 2) (non-insulin-dependent or diabetes mellitus with onset in adulthood), gestational diabetes, and other specific types. Type 1 diabetes usually results from insufficient secretion of insulin by the pancreas, while type 2 diabetes is mainly due to insulin resistance. The most common type of diabetes mellitus, type 2, which accounts for 90–95% of diabetic patients [7].
Worldwide, the prevalence of diabetes mellitus (DM) continues to increase, developing late complications that lead to disability of patients.
Long-term hyperglycemia in diabetes often leads to damage and/or dysfunction of many tissues and organs, including bone, causing significant clinical morbidity [8, 9]. These manifestations of diabetes usually result from a combination of negative consequences of the disease, which include delayed wound healing, microvascular complications, impaired response to infection, and impaired bone metabolism.
According to the results of studies, along with the development of late vascular and neurological complications, musculoskeletal problems in patients with diabetes, the occurrence of osteopenic syndrome is noted [10].
Diabetes Mellitus is classified as a metabolic polyetiologic disease characterized by chronic hyperglycemia with disturbances in carbohydrate, fat, and protein metabolism as a result of impaired insulin secretion, insulin action, or both [4].
Type 1 diabetes mellitus is based on absolute insulin deficiency caused by autoimmune destruction of β-cells in the pancreatic islets [11]. Type 2 diabetes mellitus is caused by a combination of two causes: muscle and liver resistance to insulin and its insufficient secretion.
Long-term diabetes causes significant clinical morbidity and results in damage and/or dysfunction of many tissues and organs of the human body.
These diabetes consequences are usually the result of a combination of negative consequences of the disease, which include delayed wound healing microvascular complications impaired response to infection impaired bone metabolism and strength among others [12].
Diabetes mellitus patients are more susceptible to infections. Hyperglycemia in diabetes mellitus causes dysfunction of the immune response through many mechanisms, which include suppression of cytokine production (cytokines induce innate immune response, inflammation and adaptive immune response), impaired phagocytosis, inhibition of complement effectors, dysfunction of immune cells and reduced recruitment of leukocytes.
In people who develop type 2 diabetes at a young age, the risk of microvascular and other complications increases steadily over time and affects most individuals by adolescence. Glycemia, the blood sugar level, may play an important role in these outcomes, as no correlation has been observed between glycemic control and the development of microvasculature and macrovascular complications [13]. Tight and intense glycemic control in diabetic patients can delay the onset and progression of many microvascular disorders.
Elevated blood glucose levels increase the risk of microvascular complications in diabetic patients, leading to severe complications in many organs of the body, including the heart, eyes, kidneys, and nervous system, as well as further disability and premature death [14].
Hyperglycemia adversely affects wound healing, periodontal health, and bone metabolism; therefore, diabetes has long been considered as a relative contraindication to dental implantation [15]. Thus, the possibility of implantation in patients with diabetes largely depends on good control of blood glucose levels.
The dental status of patients with diabetes mellitus is characterized by the presence of pathological changes in almost all organs and tissues of the oral cavity [16].
This prevalence increases the frequency of dental visits for diabetic patients, which is on the rise. Diabetes mellitus adversely affects periodontal health and bone metabolism, immunological response, patients with diabetes increases the risk of microvascular complications [17].
After the loss of teeth, the effectiveness of treatment with removable lamellar prostheses in patients with diabetes mellitus is low, which leads to impaired chewing function in this group of patients, which can lead to poor nutrition and metabolic disorders [18].
The development of dental implantation opens new opportunities for prostetic rehabilitation of patients with diabetes [19, 20]. The use of dental implantation has significantly improved the quality of orthopedic care for patients with various forms of adentia, which maximizes the quality of life patients.
Since diabetes mellitus has a high risk of complications after installing implants in patients with diabetes mellitus, until recently dental implantation was considered a relative contraindication for this category of patients since it is associated with delayed wound healing and impaired infection [21, 22].
Given the many potential postoperative complications of the oral cavity in diabetic patients, it is important to manage and prevent risks by ensuring the functional and esthetic efficacy of comprehensive oral care.
Despite the increased risk, dental implantation remains the optimal treatment option for partially or fully edentulous patients with diabetes, especially those with good glycemic control. Since implant prosthetics are quite stable and comfortable, they can effectively restore chewing function and create conditions for a wider choice of food products in patients with edentulous diabetes compared to traditional prosthetics.
Successful implant rehabilitation in patients with wellcontrolled diabetes improves nutrition and metabolic control, and patients benefit from oral rehabilitation.
Favorable bone quality and quantity contribute to good primary implant stability, while an efficient cellular response and bone remodeling process are critical for osseointegration and survival of dental implants [23]. The causes of implant failure in diabetic patients are not fully understood, and the available literature reports conflicting results. It has been suggested that hyperglycemia impairs osseointegration in patients with type 2 diabetes, as decreased bone formation has a detrimental effect on the bone matrix and may significantly affect long-term implant survival. In patients with diabetes, protein synthesis in the surgical site worsens and tissue healing slows down.
The feasibility of dental implantation in diabetic patients largely depends on good blood glucose control; glycemic control is critical to the success of dental implantation in diabetic patients. Patients with poorly controlled diabetes experience delayed osseointegration after implantation [24].
Among the factors that play an important role in the osseointegration of titanium dental implant is the implant surface, and it is known that with the use of various methods of implant surface modification, the osseointegration process can be optimized [25].
Since the efficiency of osseointegration in diabetic patients is lower than in healthy patients, it is very important to use implants with improved surfaces in these patients to improve efficiency and osseointegration. The fact that modification of the implant surface can affect the success of osseointegration has been proven in various studies. The modification of the implant surface is used to change its surface energy, resulting in improved hydrophilicity, increased cell proliferation and growth, and accelerated osseointegration process [26].
Currently, various methods of surface modification of titanium implants are used to promote osseointegration for successful implant therapy, such as plasma deposition of titanium powder, deposition of hydroxyapatite and calcium phosphate, surface acid treatment (SLA), electrochemical treatments (anodic oxidation) [27]. The aforementioned methods lead to an increase in the surface of the implant, while contributing to the improvement of the osseointegration process.
As is known, over time, changes occur on the surface of a titanium implant; the affinity for cells and the histocompatibility of titanium decreases.
To accelerate early osseointegration and increase the efficiency of bone-to-implant contact, biomedical research is aimed at modifying the bioactive properties of the surface.
UV irradiation, or photo functionalization, is one of the latest surface treatment techniques to promote osseointegration of implants [28]. This method can change the wettability of the surface and eliminate hydrocarbons formed as a result of aging on the implant surface, can enhance cell migration, attachment and proliferation, promote osseointegration and compaction of coronal soft tissues, and also has an antibacterial effect by preventing the formation of biofilm on the implant components, thereby playing vainut role in the prevention of peri-implantitis [29].
The phenomenon of photo functionalization, first described in 2009, is defined as a general phenomenon of titanium surface modification after ultraviolet (UV) irradiation, which changes the physical and chemical properties of the implant surface from hydrophobic to super hydrophilic, which positively influences osteointegration and strengthens the initial attachment of osteoblasts to the implant surface.
The biological effects of exposure to UV radiation on implant surfaces are defined as photo functionalization, which is a simple and effective method to promote osseointegration. UV irradiation transforms the naturally hydrophobic properties of Ti surfaces into super hydrophilic ones [30]. The change in the properties of the implant surface from hydrophobic to hydrophilic during photo functionalization was checked by lightly immersing the two implant surfaces in distilled water.
The accumulation of pathogenic microorganisms on dental implants and their components can stimulate inflammatory reactions in peri-implant tissues. Bacterial infections remain the leading cause of implant failure. It is important to note that among non-smokers with hyperglycemia, the risk of peri-implantitis was 3.39 times higher than with normoglycemia.
Dental implant placement takes place in an oral environment that harbors an abundance of biofilm-forming bacteria. Due to its transmucosal location, part of the implant structure is exposed to the oral cavity and there is no effective way to prevent bacterial attachment to the implant materials. The development of infections during the early healing phase is considered a risk factor for the osseointegration process, causing higher rates of early implant failure.
UV irradiation of titanium surfaces of implants demonstrated an antimicrobial effect, positively reduced the number of pathogenic bacteria in the periodontium due to enhanced photocatalytic properties, which. Photo functionalization also reduces the amount of attachment/accumulation of bacteria on the surface and components of the implant, thus may have an antimicrobial effect [31]. UV radiation reduces the adhesion of bacteria to the surface of the TiO implant and can increase the attachment of epithelial cells to TiO.
Photo functionalization of implants is currently also used in the complex of prevention and treatment of peri-implantitis [32]. UV treatment decomposes organic compounds and reduces bacterial adhesion of Streptococcus sanguinis. Photo functionalization not only increases the interaction of the implant surface with the surrounding bone, which promotes osteointegration, but at the same time minimizes bacterial colonization, reducing the risk of biofilm formation [33].
The analysis showed that the risk of peri-implantitis was approximately 50% higher in patients with diabetes mellitus compared with healthy people [34].
UV radiation reduces the adhesion of bacteria to the surface of the TiO implant, it can increase the attachment of epithelial cells to the surface of the implant and the formation of a biofilm, suppressing the growth of bacteria.
Due to UV, the adsorption of plasma proteins is enhanced and the attachment, distribution and proliferation of osteogenic cells are improved, which can reduce the time of dental implantation [35, 36].
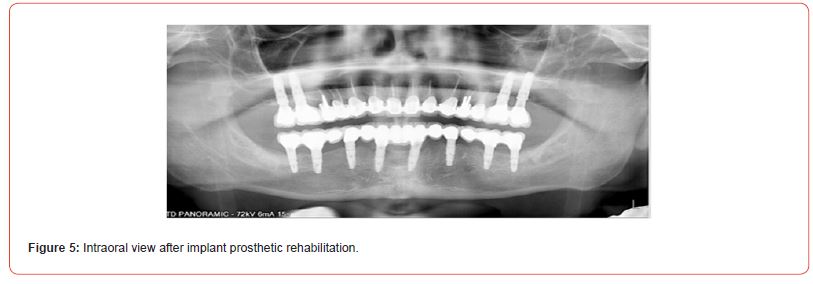
The using UV-photofunctionalized dental implants are a predictable and effective method with a minimum rehabilitation period (Figures 1-5 Clinical Case H. Khachatryan, G. Hakobyan)
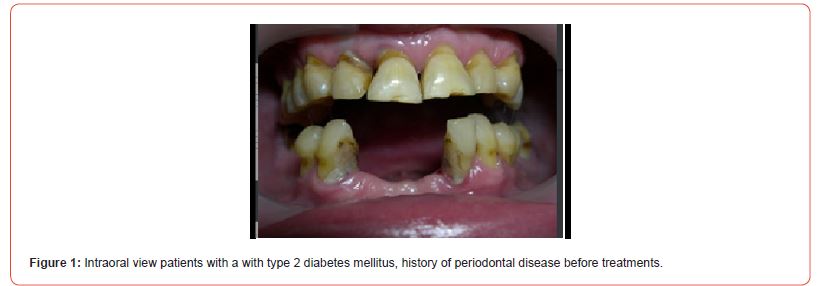
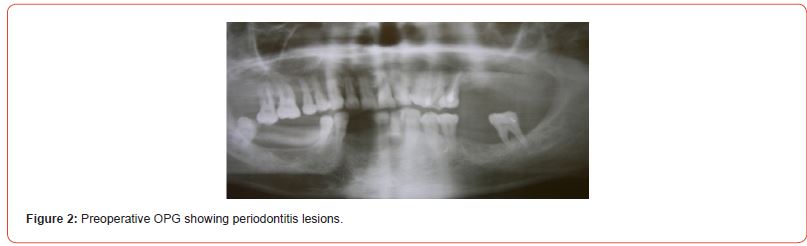
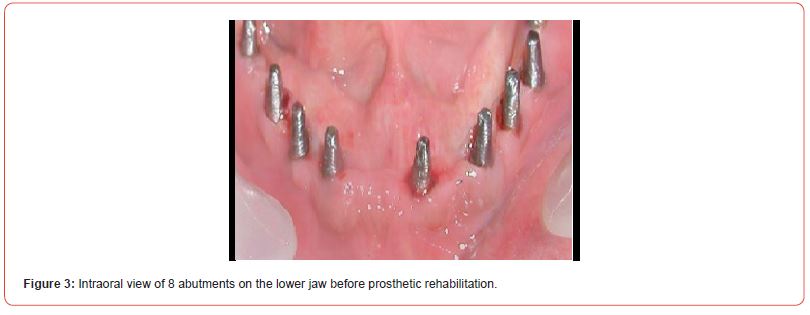
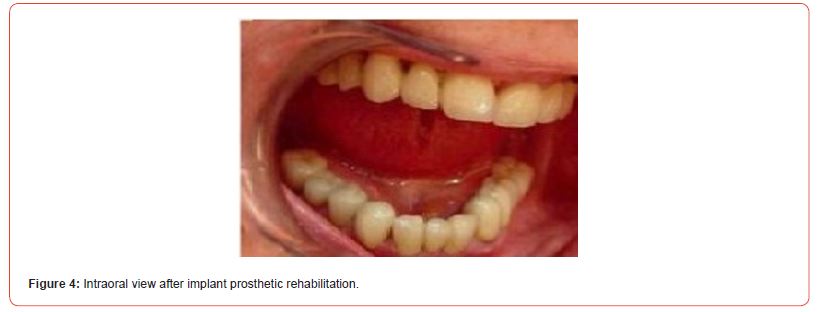
Hyperglycemia violated the process of osseointegration also since the decrease in bone formation has a detrimental effect on the bone matrix and bone formation is reduced such that can have a significant impact on the long-term survival of dental implants.
These factors can directly or indirectly impair the process of osseointegration, flap vascularization, cause soft tissue infection and delay healing, which will affect the survival of dental implants.
Studies have shown a relationship between diabetes mellitus and peri-implant inflammation, showing that the risk of implant rejection in patients with diabetes is 4.8% higher than in other patients however, this retrospective study showed that diabetic patients with good blood glucose control had a significantly higher success rate with implantation than those with poor control [37].
During the period of osseointegration and in the first year of loading in patients with diabetes, there is a progressive increase in the frequency of implant rejection, it has also been shown that patients with poorer glycemic control have more pronounced resorption of the marginal bone of the implant [38, 39].
The critical dependence of implant survival on bone metabolism markers requires an assessment of certain risk factors.
Biochemical markers of bone metabolism are classified as markers of bone formation and markers of bone resorption, and they are the product of bone cell activity [40, 41].
The bone metabolism system includes a cyclic process of resorption / formation of bone tissue, carried out by the corresponding cells: osteoclasts and osteoblasts. These processes are accompanied by the release into the bloodstream of substances called biochemical markers of bone remodeling.
Recent molecular genetic studies have shown that epigenetic modifications of gene expression, in particular, post-transcriptional DNA methylation, play an important role in the regulation of key signaling pathways for osteoblast and osteoclast differentiation. These processes determine the development and function of bone tissue and disorders of bone remodeling in pathological conditions.
In diabetes mellitus, negative changes in bone tissue are noted, including bone structure, bone density, and biochemical markers of bone metabolism [42].
An additional approach to study the effect of diabetes mellitus on bone metabolism is to assess markers of bone metabolism in blood serum, in particular osteocalcin and the amino-terminal propeptide type I procollagen, the blood levels of which decrease in patients with diabetes mellitus and are inversely correlated with blood glucose levels and the amount of adipose tissue.
To assess bone metabolism, a number of different bone markers in blood serum are used; the informativeness of Osteocalcin (OC) and Cross Laps (C-terminal telopeptide) in assessing bone formation is widely used in traumatology, orthodontics, periodontology and oral implantology [43]. Based on the foregoing, with diabetes, it is very important to study bone metabolism to predict and dynamically control implantation.
Osteocalcin stimulates pancreatic insulin production both by direct stimulation of gene expression and indirectly by increasing secretion of glucagon-like peptide-1 (GLP-1) in the small intestine and secretion of adiponectin in adipose tissue.
OC affects metabolic processes throughout the body and is a hormonally active peptide product of the bone tissue matrix, which is released by osteoblasts during bone remodeling [44, 45].
Transcription of the osteoblast gene is stimulated by vitamin D3 using a steroid-sensitive sequence. From 70 to 90% of the OC synthesized by osteoblasts is included in the bone matrix, and the rest enters the bloodstream. The level of OC in the blood may vary depending on age, the nature of metabolic disorders, and the efficiency of degradation in the renal tubules.
Osteocalcin plays a significant role in energy metabolism, in the humoral regulation of energy homeostasis. In its specific form, it stimulates insulin secretion and increases the sensitivity of both fat and muscle tissue to insulin [46].
An inverse relationship has been demonstrated between blood levels of osteocalcin and metabolic syndrome, indicating that its reduced levels may influence the pathophysiology of type 2 diabetes mellitus. Absolute deficiency of insulin reduces the production of collagen and alkaline phosphatase by osteoblasts, which are necessary for the formation of bone matrix and its mineralization, and reduces the stimulation of osteoblasts mediated through insulin-like and other growth factors.
Hyperglycemia due to advanced glycation end products may enhance bone resorption by osteoclasts; due to reduced secretion of insulin, a lack of active vitamin D metabolites may develop, which reduces the absorption of Ca in the intestine and increases the secretion and activity of PTH, which creates a negative balance of Ca in the body and enhances bone resorption. This concept supports the idea that biochemical parameters of bone formation are lower in diabetic patients.
Among bone resorption markers, one of the most informative is C-terminal telopeptide (CTx) . More than 90% of the organic matrix of the bone consists of type 1 collagen, which is synthesized in it by regulated anabolism and catabolism [47, 48].
Throughout a person’s life, mature type 1 collagen is destroyed, while its fragments enter the bloodstream and are excreted through the kidneys. With physiological or pathologically increased bone resorption, type 1 collagen is destroyed more rapidly, and the level of collagen fragments in the blood increases accordingly. This is especially true for the C-terminal telopeptide (CTx), in which al-faaspartic acid passes into the beta form (beta-CTx). This telopeptide is the marker that characterizes the resorption of type 1 collagen in bone [49].
Bone metabolism disorder as a complication of diabetes mellitus can cause changes in the microstructure and deterioration of bone tissue quality leading to an increased risk of impaired osseointegration.
Hakob Khachatryan and Gagik Hakobyan (2023) based on an analysis of the results of treatment of 86 patients diabetec patients with defects of different localization and etiology who underwent rehabilitation with UV photo functionalization of the implants found the correlation between levels of bone markers OC and β-Cross-Laps in blood serum can be used as a predictor of dental implant evaluation and successful dental restoration. They conclusion; implant therapy can be successfully used in patients diagnosed with diabetes, blood glucose levels should be maintained at normal levels at all times and normal levels indicators of bone metabolism markers Osteocalcin and β-Cross- Laps [50].
Dental implants have now become the standard procedure for replacing lost teeth, providing many benefits, the long-term survival rates of dental implants are excellent. However, primary implant failure due to insufficient osseointegration occurs in 1-2% of patients during the first few months due to peri-implantitis, secondary implant failure develops several years after successful osseointegration in about 5% of patients.
• For the treatment of patients with diabetes mellitus, implants with improved biological surface properties are required. To improve osseointegrati in patients with diabetes mellitus, one of the methods can be UV photo functionalization of the implants used.
• UV-treated titanium implants enhance bone morphogenesis around the implants, leading to rapid osseointegration. UV photo functionalization may be an effective measure to improve implant therapy patients with diabetes mellitus.
• Poor metabolic control worsens the clinical behavior of peri-implant tissues, leading to peri-implant tissue disease. Dynamic monitoring markers of Osteocalcin and β-Cross-Laps in blood serum can be used as a predictor of dental implant evaluation and successful dental restoration.
• When dental implantation is planned and after dental implantation, it is imperative to constantly maintain the level of glucose in the blood at a normal level.
• There is a strong correlation between DM and periodontal disease, which is a common cause of tooth loss in the adult population. Untreated periodontitis is a risk for implant complications. Since most patients with diabetes have periodontitis of varying degrees, it is very important to carry out complex periodontal treatment and supportive periodontal therapy before dental implantation in such patients.
• Implant survival was relatively higher during the delayed implantation protocol in diabetic patients. Delayed implantation ensures sufficient healing of the alveolar sockets and stability of hard and soft tissues at the implant site before surgery.
Acknowledgement
None.
Conflict of Interest
No Conflict of interest.
References
- Deshpande AD, Harris Hayes M, Schootman M (2008) Epidemiology of diabetes and diabetes-related complications. Phys Ther 88(11): 1254-1264.
- Fan W (2017) Epidemiology in diabetes mellitus and cardiovascular disease. Cardiovasc Endocrinol 6(1): 8-16.
- He H, Liu R, Desta T, Leone C, Gerstenfeld LC, et al. (2004) Diabetes causes decrease osteoclastogenesis, reduced bone formation and enhanced apoptosis of osteoblastic cells in bacteria stimulated bone loss. Endocrinology 145: 447-452.
- Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 44(Suppl 1): S15-S33.
- Bjornstad P, Drews KL, Caprio S (2021) Long-Term Complications in Youth-Onset Type 2 Diabetes. N Engl J Med 385(5): 416-426.
- Sun H, Saeedi P, Karuranga S (2022) IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Research and Clinical Practice 183: 109119.
- Cowie CC, Casagrande SS, Menke A (Eds.), (2018)Diabetes in America. (3rd), Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases (US).
- Kanazawa I, Sugimoto T (2018) Diabetes Mellitus-induced Bone Fragility. Intern Med 57(19): 2773-2785.
- Kalaitzoglou E, Fowlkes JL, Popescu I, Thrailkill KM (2019) Diabetes pharmacotherapy and effects on the musculoskeletal system. Diabetes Metab Res Rev 35(2): e3100.
- Sözen T, Başaran NÇ, Tınazlı M, Özışık L (2018) Musculoskeletal problems in diabetes mellitus. Eur J Rheumatol 5(4): 258-265.
- Katsarou A, Gudbjörnsdottir S, Rawshani A, Dabelea D, Bonifacio E, et al. (2017) Type 1 diabetes mellitus. Nat Rev Dis Primers 3: 17016.
- Spampinato SF, Caruso GI, De Pasquale R, Sortino MA, Merlo S (2020) The Treatment of Impaired Wound Healing in Diabetes: Looking among Old Drugs. Pharmaceuticals (Basel) 13(4): 60.
- Chawla A, Chawla R, Jaggi S (2016) Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J Endocrinol Metab 20(4): 546-551.
- Cade WT (2008) Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther 88(11): 1322-1335.
- Katyayan PA, Katyayan M, Shah RJ (2013) Rehabilitative considerations for dental implants in the diabetic patient. J Indian Prosthodont Soc 13(3):175-183.
- Mauri Obradors E, Estrugo Devesa A, Jané Salas E, Viñas M, López López J (2017) Oral manifestations of Diabetes Mellitus. A systematic review. Med Oral Patol Oral Cir Bucal 22(5): e586-e594.
- Wu YY, Xiao E, Graves DT (2015) Diabetes mellitus related bone metabolism and periodontal disease. Int J Oral Sci 7(2): 63-72.
- Bukhary DM (2023) Teeth Rehabilitation and Nutritional Influence on Diabetic Patients: A Review. Cureus 15(9): e46182.
- Alzahrani AS, Abed HH (2016) To what extent should dental implant placement be adopted as a standard for diabetic patients? Saudi Med J 37(11): 1179-1183.
- Oates TW, Huynh-Ba G, Vargas A, Alexander P, Feine J (2013) A critical review of diabetes, glycemic control, and dental implant therapy. Clin Oral Implants Res 24(2): 117-127.
- Morris HF, Ochi S, Winkler S (2000) Implant survival in patients with type 2 diabetes: placement to 36 months. Ann Periodontol 5(1): 157-165.
- Michaeli E, Weinberg I, Nahlieli O (2009) Dental implants in the diabetic patient: systemic and rehabilitative considerations. Quintessence Int 40(8):639-645.
- Huang YC, Huang YC, Ding SJ (2023) Primary stability of implant placement and loading related to dental implant materials and designs: A literature review. J Dent Sci 18(4):1467-1476.
- Nibali L, Gkranias N, Mainas G, Di Pino A (2022) Periodontitis and implant complications in diabetes. Periodontol 2000. 90(1): 88-105.
- Kligman S, Ren Z, Chung CH, Perillo MA, Chang YC, et al. (2021) The Impact of Dental Implant Surface Modifications on Osseointegration and Biofilm Formation. J Clin Med 10(8): 1641.
- Naujokat H, Kunzendorf B, Wiltfang J (2016) Dental implants and diabetes mellitus-a systematic review. Int J Implant Dent (1): 5.
- Jemat A, Ghazali MJ, Razali M, Otsuka Y (2015) Surface Modifications and Their Effects on Titanium Dental Implants. Biomed Res Int 2015: 791725.
- Jun JH, Oh KC, Park KH, Jung N, Li J, et al. (2021) Improvement of osseointegration by ultraviolet and/or simvastatin treatment on titanium implants with or without bone graft materials. Materials 14(13): 3707.
- Ogawa T (2014) Ultraviolet photo functionalization of titanium implants. Int J Oral Maxillofac Implants 29: e95-e102.
- Suzuki S, Kobayashi H, Ogawa T (2013) Implant stability change and osseointegration speed of immediately loaded photo functionalized implants. Implant Dent 22: 481-490.
- Abdullatif FA, Al-Askar M (2022) Does Ultraviolet Radiation Exhibit Antimicrobial Effect against Oral Pathogens Attached on Various Dental Implant Surfaces? A Systematic Review. Dent J (Basel) 10(6): 93.
- Chang LC (2022) Clinical Applications of Photo functionalization on Dental Implant Surfaces: A Narrative Review. J Clin Med 11(19): 5823.
- Razali M, Ngeow WC, Omar RA, Chai WL (2021) An In-Vitro Analysis of Peri-Implant Mucosal Seal Following Photo functionalization of Zirconia Abutment Materials. Biomedicines 9: 78.
- Alberti A, Morandi P, Zotti B, Tironi F, Francetti L, et al. (2020) Influence of Diabetes on Implant Failure and Peri-Implant Diseases: A Retrospective Study. Dent J (Basel) 8(3): 70.
- Funato A, Yamada M, Ogawa T (2013) Success rate, healing time, and implant stability of photo functionalized dental implants. Int J Oral Maxillofac Implants 28: 1261-1271.
- Jin S, Yamamoto Y, Harada Y, Kaneko S, Oishi K, Ishibashi Y (2022) Effectiveness of photofunctionalized titanium alloy on osseointegration in rats with type 2 diabetes. J Orthop Surg Res 17(1): 445.
- Wagner J, Spille JH, Wiltfang J, Naujokat H (2022) Systematic review on diabetes mellitus and dental implants: an update. Int J Implant Dent 8(1): 1.
- Mellado Valero A, Ferrer García JC, Herrera Ballester A, Labaig Rueda C (2007) Effects of diabetes on the osseointegration of dental implants. Med Oral Patol Oral Cir Bucal 12(1): E38-43.
- Javed F, Romanos GE (2009) Impact of diabetes mellitus and glycemic control on the osseointegration of dental implants: a systematic literature review. J Periodontol 80(11): 1719-1730.
- Seibel MJ (2005) Biochemical markers of bone turnover: part I: biochemistry and variability. Clin Biochem Rev 26(4): 97-122.
- Christenson RH (1997) Biochemical markers of bone metabolism: an overview. Clin Biochem 30(8): 573-593.
- Sanches CP, Vianna AGD, Barreto FC (2017) The impact of type 2 diabetes on bone metabolism. Diabetol Metab Syndr 9: 85.
- Greenblatt MB, Tsai JN, Wein MN (2017) Bone Turnover Markers in the Diagnosis and Monitoring of Metabolic Bone Disease. Clin Chem 63(2): 464-474.
- Chen Y, Zhou Y, Lin J, Zhang S (2022) Challenges to Improve Bone Healing Under Diabetic Conditions. Front Endocrinol (Lausanne) 13: 861878.
- Zanatta LC, Boguszewski CL, Borba VZ, Kulak CA (2014) Osteocalcin, energy and glucose metabolism. Arq Bras Endocrinol Metabol 58(5): 444-451.
- Fernández Real JM, Ricart W (2011) Osteocalcin: a new link between bone and energy metabolism. Some evolutionary clues. Current opinion in clinical nutrition and metabolic care 14(4): 360-366.
- Kanazawa I (2015) Osteocalcin as a hormone regulating glucose metabolism. World J Diabetes 6(18): 1345-1354.
- Starup Linde J (2013) Diabetes, biochemical markers of bone turnover, diabetes control, and bone. Front Endocrinol (Lausanne) 4: 21.
- Khachatryan H, Hakobyan G (2023) Diagnostic and prognostic value of indicators of markers of bone metabolism in type 2 diabetes mellitus patients with UV functionalised dental implants. J Stomatol Oral Maxillofac Surg 28:101608.
- Khachatryan H (2023) Dental Implant Treatment in Diabetes Mellitus (DM) Patients: Review. Bulletin of Stomatology and Maxillofacial Surgery 19(3): 121-130.
-
Hakob Khachatryan and Gagik Hakobyan*. Review of Diabetes, Glycemic Control, and Dental Implant Therapy in Patients with Type 2 Diabetes Mellitus. On J Dent & Oral Health. 7(3): 2023. OJDOH.MS.ID.000662.
-
Dental implants, Dentists, Oral cavity, Dental defects, Periodontal health, Wound healing, Bone metabolism, Oral care, Type 2 diabetes, Osseointegration.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






