 Research Article
Research Article
Regenerative Properties of a Manuka Honey Incorporated Membrane in a Porcine Model
Joshua A Evensky1, Martyn S Green2, Sidney H Stein3*, Gary L Bowlin4 and Wainscott Hollis5
1Main Line Periodontics and Dental Implants, P.C., USA
2Department of Periodontology, Tufts University School of Dental Medicine, USA
3Department of Periodontology, The University of Tennessee Health Science Center, USA
4Department of Biomedical Engineering, The University of Memphis, USA
5Department of Prosthodontics, The University of Tennessee Health Science Center, USA
Sidney H Stein, Department of Periodontology, The University of Tennessee Health Science Center, Memphis, TN, USA.
Received Date: January 22, 2019; Published Date: February 04, 2019
Abstract
The novel use of a Manuka honey incorporated dental membrane for tissue regeneration warrants investigation. In this study, we analyzed the use of the SweetBio Manuka honey incorporated membrane for epithelialization, bone maturation, or any adverse effects compared to Salvin Renovix (a clinically available product control) in a porcine model. Ridge preservation was performed with allograft plus experimental membrane with primary closure, experimental membrane without primary closure, control membrane, or neither. At 2, 4, 8, and 12 weeks, all dental defect sites were trimmed, embedded in methlymethacrylate (MMA), sectioned, stained with hematoxylin and eosin (H&E) and evaluated. Specifically, epithelialization, bone maturation, membrane remnants, healing, and inflammation were examined. Although the results demonstrated no significant differences between treatments groups, it was noticed that all sites were fully healed or nearly fully healed at 4 weeks and beyond. The results also showed variable amounts of new bone growth present with evidence of a normal progression in healing of the extraction sites. Furthermore, all SweetBio membrane sites were covered by epithelium at 4 weeks. While no ascertainable difference was seen at any time point, additional studies are needed to explore various regenerative parameters, including the slight increase in epithelialization seen at week 4 of the experimental membrane. Nonetheless, this study did conclude that there is no adverse effect to the regeneration of the defect site by using the Manuka honey incorporated membrane.
Introduction
According to the National Health and Nutrition Examination Surveys, half of the United States population 30 years or older has periodontitis [1]. One of the possible consequences of untreated periodontal disease is tooth loss. Tooth loss leads to necessary replacements. Options are contingent on the events that occur biologically after extraction. Despite improvement in restorative options and implant designs, placement of implants in a compromised position could lead to functional and esthetic complications.
The sequence of events that follow a tooth extraction plays a crucial role in the bone remodeling process. These stages involve hemorrhage, coagulation, thrombosis, organization of fibrin clot, proliferation of epithelium over the surface of the wound, resorption of the damage tissue, and formation of new bone. Extraction sites often do not fill completely with bone but with fibrous connective tissue or scar formation, leaving a deficient ridge [2].
Most bone resorption occurs in the first 6 to 24 months after extraction [3]. The width of the alveolar ridge can be reduced by 50% during a 12-month period with an average of 5 to 7mm of horizontal volume reduction [4]. Results have shown that alveolar bone continues to resorb creating atrophied edentulous areas that are problematic to restore even with extensive bone grafting [5]. In order to reduce the inevitable resorption from occurring, preservation or enhancement of bone volume is critical. Based on the same principles of guided tissue regeneration (GTR), guided bone regeneration (GBR) involves the use of a barrier membrane to exclude epithelial down growth while maintaining space for bone formation [6]. When combining GTR with GBR, statistically greater bone regeneration is found when compared to using a barrier membrane alone [7].
Ridge preservation goals include limitation of soft tissue collapse while simultaneously maintaining alveolar dimensions post extraction. Failure to use a site preservation technique inevitably increases the complexity of the procedures required to achieve acceptable esthetics results [8]. Lasella et al. [9] compared ridge preservation with freeze-dried bone allograft (FDBA) and a collagen membrane versus extraction alone. Width of socket preservation was 9.2 to 8.0 ± 1.4mm while height of socket preservation gained 1.3 ± 2.0mm of height. With no ridge preservation, these sockets had a width change from 9.1 to 6.4 ± 2.2mm with a height differential of 0.9 ± 1.6mm. This study determined that ridge preservation with FDBA plus collagen membrane improved ridge height and width when compared to extraction alone.
The next decision involves which materials will achieve optimal results. Barrier membranes need to be biologically acceptable with the ability to protect the blood clot while preventing epithelium migration. Ideal properties of barrier membranes include biocompatibility, space maintenance, ease of handling, and predictable resorption times. One of the first membranes used in a GTR procedure involved a non-resorbable membrane (expanded polytetrafluoroethylene) [10]. While these non-resorbable membranes are capable of space maintenance, they require a second surgery for membrane removal. If non-resorbable membranes become exposed, the site has an increased risk of infection and graft failure. To overcome some of these possible complications, resorbable collagen membranes made from porcine/bovine sources are popular treatment options [11].
To achieve ideal membrane requirements, this investigation trial uses a Manuka honey incorporated membrane manufactured by SweetBio, Inc. (not yet FDA cleared). Historically, honey has been applied to skin for its medicinal properties, specifically for burns and wound healing. Manuka honey is a monofloral honey derived from the Manuka flower (Leptospermum scoparium) exclusively in New Zealand and southeastern Australia. It has antimicrobial and antibacterial capabilities without the hydrogen peroxide found in other honeys [12]. Its mechanism may be related to the low pH level (3.2 to 4.5) of honey and its high sugar content which can hinder the growth of certain microbes [13]. Manuka honey, a natural antiseptic alternative to synthetic chemicals, also has shown antimicrobial effect against periodontal pathogenic bacteria such as P. gingivalis, Camplyobacter spp, and A. actinomycetemcomitan [14,15]. Methylglyoxal is the key antibacterial constituent in Manuka honey, which can target P. gingivalis [16,17]. While providing a natural option that has potential antibacterial properties, Manuka honey as an ingredient in a resorbable membrane certainly requires investigation. The aim of this study is to examine the regeneration potential of a membrane composed of cross-linked medical grade collagen derivative, hydroxyapatite nanoparticles, and Manuka honey in a porcine model via histological analysis.
Materials and Methods
After receiving Institutional Animal Care and Use Committee (IACUC) approval at the University of Memphis, female farm porcine all roughly 21 days of age with initial weight of 3.6- 4.5kg were obtained due to bone regeneration rates comparable to humans [18-20]. Animals were anesthetized via injection of tiletamine/zolazepam (4.4-6.6 mg/kg) then given isofluorane (2- 4%) via inhalation to maintain depth of sedation. Once adequate sedation had been achieved, 36 mg of 2% lidocaine with 0.018mg of 1:100000 epinephrine was given via infiltration at each surgical site.
Table 1:Methodology for study included four different groups with either experimental membrane, control membrane, or neither. Each group included suturing with primary closure, suturing without primary closure, or no suture. Four different time periods per each group with two subjects per time period. Total subjects per group were eight.
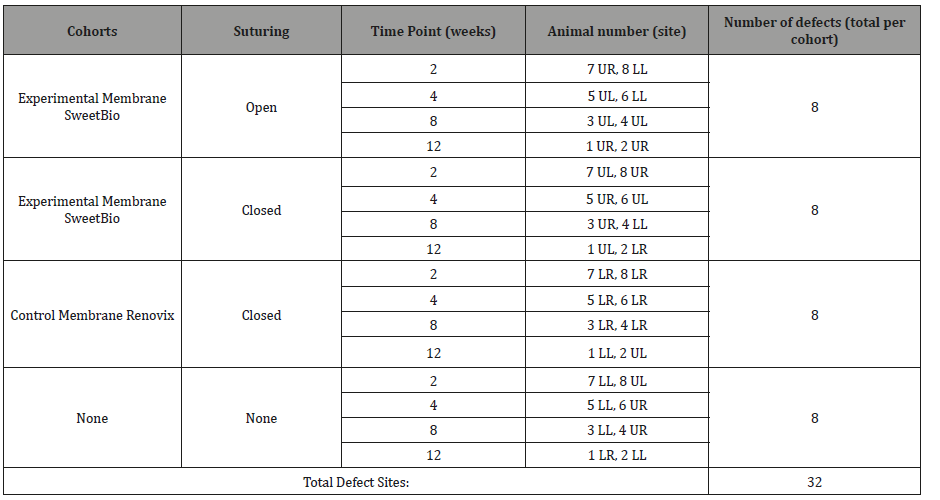
LL: Lower left; LR: Lower right; UL: Upper left; UR: Upper right
Either canine or 1st premolars were extracted due to ease of location and size of socket. Study design can be seen in Table 1. After the animal was sedated, the mouth was then prepped with 0.12% chlorhexidine and rinsed with sterile saline. Intrasulcular incisions were used for flap design followed by reflection passed the mucogingival junction. Once the designated teeth were atraumatically extracted, the sockets were irrigated and curetted.
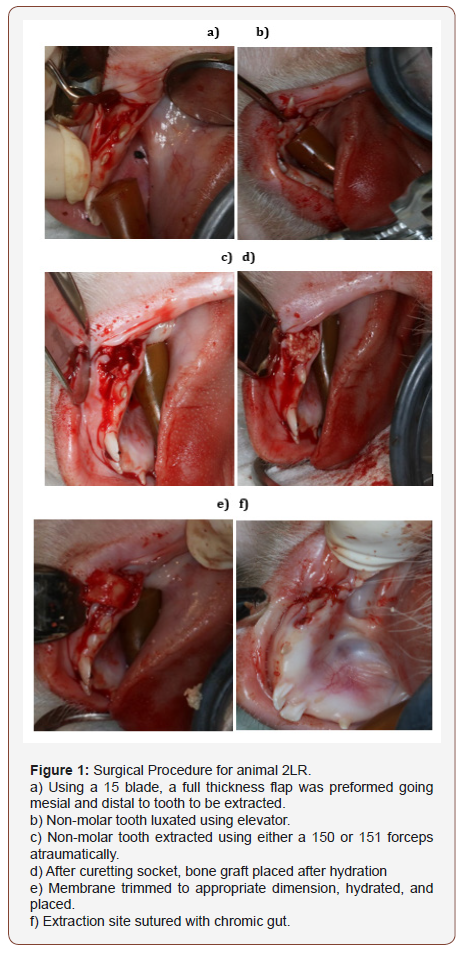
Cortical-cancellous particulate allograft (Zimmer Puros®) was placed into the extraction sockets and membranes (control or experimental) were placed over the sockets followed by suturing using 4-0 vicryl (Ethicon). All four sites were randomly assigned for each animal. One extraction site receiving the experimental Manuka honey incorporated membrane (SweetBio) was fully closed (Figure 1a-f) while the other site was sutured leaving the experimental membrane exposed. Another site was given the control membrane (Salvin Renovix®) and sutured with primary closure. The final extraction site was left without preservation. A photographic record was taken at each surgery. The measurements were with a #12 UNC periodontal probe and taken at the widest portion of the exposed membrane.
Animals were monitored twice daily for pain, swelling, lethargy, lack of appetite, or any other abnormal signs. Animals were weighed twice per week and diets adjusted by veterinarian accordingly to controlled growth protocol. At the end of each designated time point, animals were euthanized with Somnasol (1mL/4.5kg) via injection.
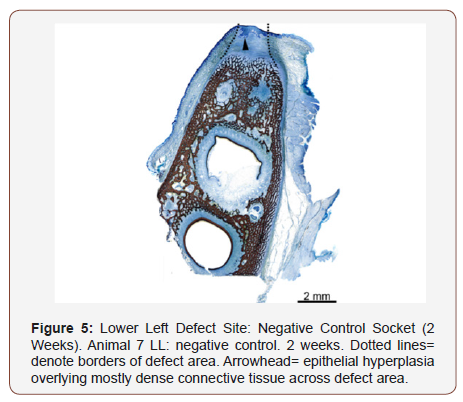
Imaging was performed using light microscopy to evaluate the healing and mineralization of the porcine tooth socket. The specimens were prepared after sacrificing each animal at specified time points. Block sectioning was preformed, and specimens were placed in 10% buffered formalin. The dental defects were trimmed, embedded in methylmethacrylate (MMA), and stained with hematoxylin and eosin. (H&E). Additionally, defect sites from the two-week cohort were stained with Stevenel’s blue (SB) to differentiate the membrane from surrounding collagen. Quantitative means were made based on subjects in each time period per group.
Results
Membrane remnants, epithelialization, bone maturation, inflammatory cells, and healing parameters for both experimental and control membranes were evaluated. Histological evidence of intact membrane remaining within extraction site was not found in either the experimental closed or experimental open treatments at any time point. One two-week sample in the control closed treatment (Figure 2) had possible histological evidence of membrane presence. This sample consisted of an epithelial rete peg that extended down into the connective tissue and surrounded by an aggregate of vascularized connective tissue that stains as if it has more proteoglycans than the surrounding connective tissue. All sites at 4, 8, and 12 weeks appeared to be mostly healed with no membrane visible.
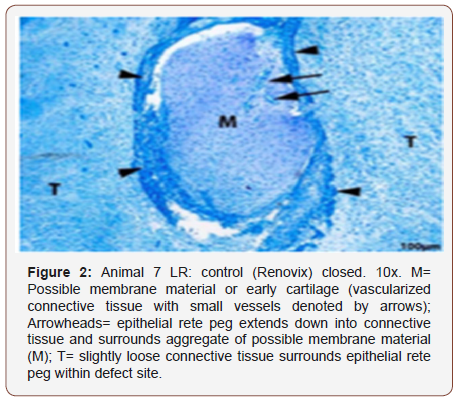
Table 2:Epithelialization was assessed with a quantitative scale ranging 0-4. 0 equaled absent, 1 being less than 1 mm of epithelial coverage on one or both defect sites, 2 being greater than 1 mm of epithelial coverage on both sides of defect site without full re-epithelialization, 3 being defect site is nearly completely re-epithelialized with few small gaps, and 4 equaled defect sites is fully re-epithelized.
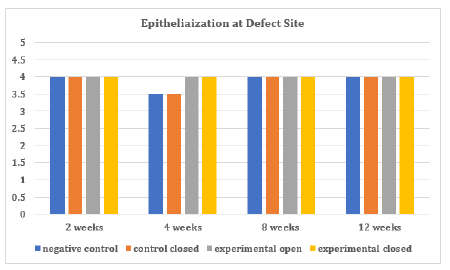
Table 3:Inflammation, which was determined by amount of polymorphonuclear leukocytes, lymphocytes, plasma cells, macrophages, and multinucleated giant cells, was assessed on the following 0-4 scale. 0 equaled no inflammation present, 1 equaled 1-4 cells per high power field, 2 equaled small number 5-10 cells, 3 equals heavy infiltrate, and 4 equaled packed with inflammatory cells.
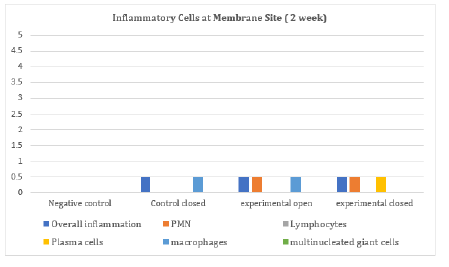
The epithelialization at the defect site in each group was found to be either completely epithelized or almost completely reepithelialized (Table 2). At four weeks, one negative control and one control closed group were not completely covered by epithelium. All experimental membrane groups on the other hand were covered completely by epithelium. Inflammation, which was determined by quantity of cells per high power field microscopy (400x) and type of inflammatory cell, was seen only at the two-week animal groups (Table 3). In all groups but the negative control, either none or less than 4 cells were visualized. In the experimental groups, less than 4 polymorphonuclear (PMN) cells were seen. Specifically, less than 4 macrophages were seen in the control closed and experimental open groups. Less than 4 plasma cells were seen in the experimental closed group. When present at the actual extraction site, inflammation was composed of few inflammatory cells such as lymphocytes and macrophages. In two of the two-week samples (control closed and experimental closed, Figure 3), inflammation occurred in an area with focal loss of epithelium. The third sample (experimental open, Figure 4) had inflammation surrounding preexisting bone that was encapsulated by several small rete pegs of epithelial hyperplasia.
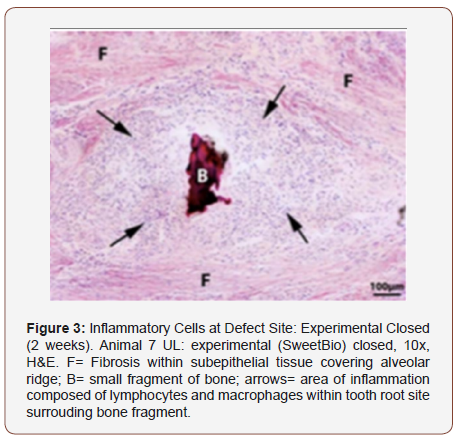
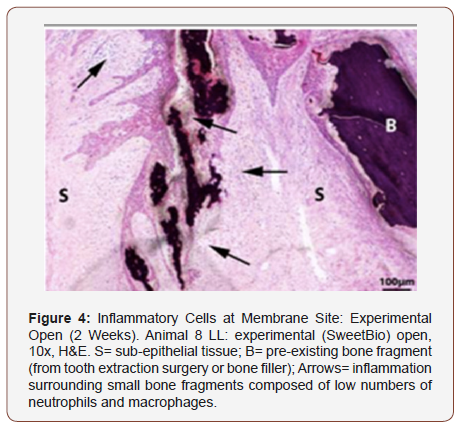
Table 4:Bone maturation was assessed on a quantitative scale ranging 0-4. 0 equaled predominately woven bone, 1 equaling more woven than mature, 2 being 50% mature alveolar and 50% woven, 3 being more mature than woven bone, and 4 being mature alveolar bone.
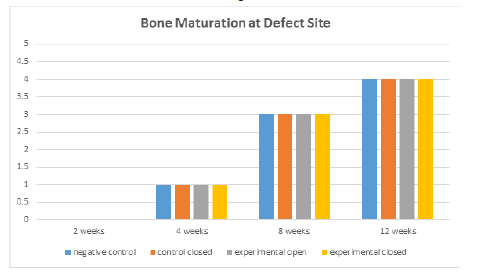
Bone maturation was quantified by ratio (woven vs mature alveolar bone). New bone growth (Table 4) was similar across all treatments and time points with minor variations. At least some new bone growth was found in each evaluated site at all time points; however due to advanced healing of all sites at 4 weeks and beyond, it is unclear if sections were consistently obtained in the center of the original defect site. At the two-week time interval, the defect site was definitively obtained in at least one slide using H&E and/or Stevenel’s blue (Figure 5). The new bone growth that was found in each site tended to become slightly more mature as the time point increased, as expected with normal healing process.
No evidence of necrosis was found in any sample at any time point. Fibrosis had mostly lower scores at two weeks, with the exception of a negative control animal, due to the absence of a membrane and thus greater amount of fibrous connective tissue.
Discussion
The evaluation of dental extractions in a porcine model treated with an experimental membrane, control membrane, and nothing were examined at 2, 4, 8, and 12 weeks. There were no unexpected differences between all four groups at each time point in terms of bone maturation, membrane remnants, healing, and inflammation. Although not statistically significant, it was noticed that all experimental membranes were fully epithelialized by 4 weeks while half of the negative controls and half of the positive control products were not. Further studies are needed to explore if the experimental membrane has significant differences with respect to faster epithelialization compared to collagen-based commercially available membranes.
Considering bone maturation after a typical extraction occurs with an increase in time, this study exemplified what occurs in the healing process regardless of graft material. Control or experimental membrane remnants were not evident in any time interval with the exception of one sample in the closed control twoweek group. Considering the Stevenel’s blue stain confirmed that the histological sections were at the defect site and that the control membrane (Renovix) has a well-defined resorption period of three to four months, it was concluded that the lack of visualization of membrane remnants was attributed to the inefficiency of the histological staining to capture this data. While it is possible that all membrane materials remodeled over the span of two weeks via macrophage and fibroblast activity, the visual absence of membrane material may not correlate with the presence or absence of the membrane material in the site.
In a systematic review involving extractions and naturally healed sockets over a three to twelve month period, the mean alterations in width and height of the alveolar ridges were 3.87+/- 0.82mm and 1.67 +/- 1.11mm [21]. Utilizing ridge preservation procedures, a systematic review showed the amount of ridge alterations may be reduced but varied, depending on the type of materials used [22]. Failure to use a site preservation technique following tooth removal inevitably increases the number of complexity of the sitedevelopment procedures required to achieve acceptable esthetics results [23].
Examination of previous studies involving different animals following tooth extraction were analyzed. In a recent study, Araujo and Lindhe looked at ridge alterations following extraction in mongrels. The relative reduction of the height of the buccal bone between the one- and eight-week intervals was 2.2 +/- 0.2 mm, i.e. about 45 μm/day. This showed the speed of healing is faster in extraction sites in animal models than human extractions studies, i.e. 50-60μm/day, which is a possible explanation for the results obtained [24].
All the factors that influence healing and degradation are complex and can be difficult to replicate in vitro. Thus, the pig model became the in vivo model of interest based on the following analysis. The oral maxillofacial region of the pig is similar to that of humans in anatomy, development, physiology, pathophysiology, and disease occurrence [25]. The porcine model is also similar to human bone maturation and mineralization [20].
Another possible complication within our study design involved the difficulty isolating the tooth defect. Per pathology report, allograft material was seen within the soft tissue underlying the epithelium as well as evidence of new bone growth in the extraction socket. This confirmed that the actual sites of interest were evaluated in the two-week time groups. This was not confirmed in any of the other time frames groups. In order to insure a bigger defect size, future studies will focus on creating a more critically sized defect which would have potentially helped ascertain a larger difference in each site.
Manuka honey has multiple characteristics such as low pH, high osmolarity, high viscosity, and presence of methylglyoxal, bee defensin-1, and phenolic compounds that contribute to its medicinal benefits. These benefits include antioxidant, antimicrobial, bacteriostatic, antibacterial, anti-inflammatory, and overall wound healing properties which help contribute towards angiogenesis [12,26]. Healing time decreases after honey treatment due to inhibition of prolonged inflammatory responses and by suppressing production and propagation of inflammatory cells at the wound site. Consequently, the honey stimulates pro-inflammatory cytokines, which then stimulate proliferation of fibroblasts and epithelial cells [27]. There are also studies showing the topical use of honey accelerating wound healing from the bactericidal and energy producing properties [28]. A recent (2018) review article by Minden-Birkenmaier et al. highlights the value of incorporating honey within tissue engineering scaffolds and mentions SweetBio as leading the development of long-lasting resorbable scaffolds incorporated with Manuka honey [29]. Another 2018 review article by Rodriguez et al. further details how Manuka honey can be leveraged in dental applications. Furthermore, it highlights SweetBio’s development of the Manuka honey-incorporated barrier membrane used in this study [30]. While no ascertainable difference was seen at any time point, additional studies are needed to explore various regenerative parameters, including the slight increase in epithelialization seen at week 4 of the experimental membrane. Nonetheless, this study did conclude that there is no adverse effect to the regeneration of the defect site by using the Manuka honey incorporated membrane.
Conclusion
When a Manuka honey incorporated resorbable membrane was compared to control groups in a porcine model to evaluate ridge preservation, no adverse reactions or effects were observed. At four different time points, the soft and hard tissues showed epithelialization, bone maturation, membrane remnants, healing, and inflammatory cells via histologic staining with no perceptible differences between the four different treatments groups. Although the results demonstrated no significant differences between treatments groups, it was observed that all sites were fully healed or nearly fully healed at 4 weeks and beyond, with variable amounts of new bone growth present, and with evidence over time of a normal progression in healing of the extraction sites. Furthermore, unlike control membranes, all Manuka honey membrane sites were covered by epithelium at 4 weeks.
Acknowledgement
Special thanks to SweetBio, Inc. for providing their product and support throughout this study.
Disclosures
Gary Bowlin has a financial interest in SweetBio, Inc. This study was funded by SweetBio, Inc.
Conflict of Interest
The potential conflict of interest with Gary Bowlin was managed by him not playing a role in data gathering or analysis.
References
- Albandar JM (2011) Underestimation of periodontitis in NHANES surveys. J. Periodontol 82(3): 337-341.
- Amler MH, Johnson PL, Salman I (1960) Histological and histochemical investigation of human alveolar socket healing in undisturbed extraction wounds. J Am Dent Assoc 61: 32-44.
- Carlsson GE, Persson G (1967) Morphologic changes of the mandible after extraction and wearing of dentures: A longitudinal, clinical, and x-ray cephalometric study covering 5 years. Odontol Revy 18(1): 27-54.
- Schropp L, Wenzel A, Kostopoulos L, Karring T (2003) Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent 23(4): 313-323.
- Atwood DA (1963) Post extraction changes in the adult mandible as illustrated by microradiographs of midsagittal section and serial cephalometric roentgenograms. Journal of Prosthetic Dentistry 13(5): 810-824.
- Dahlin C, Lindhe A, Gottlow J, Nyman S (1988) Healing of bone defects by guided tissue regeneration. Plast Reconstr Surg 81(5): 672-676.
- Gher ME, Quintero G, Assad D, Monaco E, Richardson AC (1994) Bone grafting and guided bone regeneration for immediate dental implants in humans. J Periodontol 65(9): 881-891.
- Sclar AG (2004) Strategies for management of a single-tooth extraction sites in aesthetics implant therapy. J Oral Maxillofac Surg 62 (9 Suppl 2): 90-105
- Iasella JM, Greenwell H, Miller RL, Hill M, Drisko C, et al. (2003) Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: a clinical and histologic study in humans. J Periodontol 74(7): 990-999.
- Gottlow J, Nyman S, Karring T, Lindhe J (1984) New Attachment formation as the result of controlled tissue regeneration. J Clin Periodontol 11(8): 494-503.
- Hammerle CH, Jung RE, Yaman D, Lang NP (2008) Ridge augmentation by applying bioresorbable membranes and deproteinized bovine bone mineral: a report of twelve consecutive cases. Clin Oral Implants Res 19(1): 19-25.
- Alvarez-Suarez JM, Gasparrini M, Forbes-Hernandez TY, Mazzoni L, Giampieri F (2014) The Composition and Biological Activity of Honey: A Focus on Manuka Honey. Foods 3(3): 420-432.
- Mandal MD, Mandal S (2011) Honey: its medicinal property and antibacterial activity. Asian Pac J Trop Biomed 1(2): 154-160.
- Ahmed S, Othman NH (2013) Review of the medicinal effects of tualang honey and a comparison with manuka honey. Malays J Med Sci 20(3): 6-13.
- Schmidlin PR, English H, Duncan W, Belibasakis GN, Thurnheer T (2014) Antibacterial potential of Manuka honey against three oral bacteria in vitro. Swiss Dent J 124(9): 922-924.
- Mavric E, Wittmann S, Barth G, Henle T (2008) Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol Nutr Food Res 52(4): 483-489.
- Eick S, Schafer G, Kwiecinski J, Atrott J, Henle T, et al. (2014) Honey - a potential agent against Porphyromonas gingivalis: an in vitro study. BMC Oral Health 14: 24.
- Martiniakova M, Grosskopf B, Omelka R, Vondrakova M, Bauerova M (2006) Differences among species in compact bone tissue microstructure of mammalian skeleton: use of a discriminant function analysis for species identification. J Forensic Sci 51(6): 1235-1239.
- Ma JL, Pan JL, Tan BS, Cui FZ (2009) Determination of critical size defect of minipig mandible. J Tissue Eng Regen Med 3(8): 615-622.
- Mardas N, Dereka X, Donos N, Dard M (2014) Experimental Model for Bone Regeneration in Ora and Cranio-Maxillo-Facial Surgery. J Invest Surg 27(1): 32-49.
- Van der Weijden F, Dell’Acqua F, Slot DE (2009) Alveolar bone dimensional changes of post-extraction sockets in humans: A systematic review. J Clin Periodontol 36(12):1048-1058.
- Ten Heggeler JM, Slot DE, Van der Weijden GA (2011) Effect of socket preservation therapies following tooth extraction in non-molar regions in humans: A systematic review. Clin Oral Implants Res 22(8): 779-788.
- Lekovic V, Camargo PM, Klokkevold PR, Weinlaender M, Kenney EB, et al. (1998) Preservation of alveolar bone in extraction sockets using bioabsorbable membranes. J Periodontol 69(9): 1044-1049.
- Araujo MG, Lindhe J (2005) Dimensional ridge alterations following tooth extraction: an experimental study in the dog. J Clin Periodontol 32(2): 212-218.
- Wang S, Liu Y, Fang D, Shi S (2007) Review Article The miniature pig: a useful large animal model for dental and orofacial research. Oral Dis 13(6): 530‐537.
- Yaghoobi R, Kazerouni A, Kazerouni O (2013) Evidence for clinical use of honey in wound healing as an anti-bacterial, anti-inflammatory, antioxidant, and anti-viral agent: a review. Jundishapur J Nat Pharm Prod 8(3): 100-104.
- Visavadia BG, Honeysett J, Danforf MH (2008) Manuka honey dressing: an effective treatment for chronic wound infections. Br J Oral Maxillofac Surg 46(1): 696-697.
- Bergman A, Yanai J, Weiss J, Bell D, David MP (1983) Acceleration of wound healing by topical application of honey: An animal model. Am J Surg 145(3): 374-376.
- Minden-Birkenmaier BA, Bowlin GL (2018) Honey-Based Templates in Wound Healing and Tissue Engineering. Bioengineering (Basel) 5(2): E46.
- Rodriguez IA, Selders GS, Fetz AE, Gehrmann CJ, Stein SH, et al. (2018) Barrier membranes for dental applications: A review and sweet advancement in membrane developments. Mouth Teeth 2(1): 1-9.
-
Joshua A Evensky, Martyn S Green, Sidney H Stein, Gary L Bowlin, Wainscott Hollis. Regenerative Properties of a Manuka Honey Incorporated Membrane in a Porcine Model. On J Dent & Oral Health. 1(4): 2019. OJDOH.MS.ID.000520.
-
Manuka honey, SweetBio, Tooth, Bone allograft, Mouth, Muco-gingival junction, Bone growth, Oral surgery, Maxillofacial surgery, Dental arch, Adjacent teeth, Odontogenic infection, Dental health, Dental treatments, Tooth brushing, Teeth, Dental school, Ceramics, Gingivitis, Supragingival calculus
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






