 Research Article
Research Article
Improvement in Wellness Following Periodontal Therapy
David M Harris*, Wonseok Jang, Ranjitha R Padhiar and Andrew Sullivan
Department of Periodontics, Rutgers School of Dental Medicine, 110 Bergen Street Newark, NJ 07101-1709, USA
David M Harris, Bio-Medical Consultants, Inc., Canandaigua NY 14424, USA.
Received Date: December 21, 2021; Published Date: January 17, 2022
Abstract
Background: Periodontal disease is linked to numerous systemic diseases and it is known that periodontal therapy (PT) is effective in reducing the severity of those diseases. We tested the idea that PT also has a positive effect on quality of life and general wellness.
Methods: Patients scheduled for PT at the Rutgers Periodontics Clinic (Newark, NJ 07101) were administered the RAND-36 quality of life questionnaire before and at least 3-months after the procedure.
Results: The Wilcoxin signed ranks test demonstrates a significant improvement in wellness following Periodontal Therapy with a p-value=0.02.
Conclusion: In this sample most patient’s general wellness improved following PT. Improvement was in the categories of physical functioning, energy and emotional well-being.
Keywords: Periodontitis; Periodontal therapy; Wellness; RAND-36; Questionnaire; Systemic disease
Introduction
Periodontitis is an infectious disease of the periodontium leading to alveolar bone loss, and the formation of periodontal pockets. Osseous resective surgery became the basis of surgical periodontal therapy since it addressed the importance of alveolar bone reshaping [1]. Barrington [2] identified access for root debridement and pocket elimination as the two major benefits of resective treatment. Ramfjord and Nissle [3] introduced the modified Widman flap. A few years later Yukna and Lawrence [4] introduced excisional new attachment procedure (ENAP) and confirmed its success. Minimally invasive laser-assisted procedures have recently been introduced that are effective at bacterial reduction, [5, 6] reducing inflammation [7] and have recently gained FDA clearance for “true periodontal regeneration” (510(k): K151763) [8].
Transient and recurrent bacteremias of oral origin also generate systemic inflammation and metabolic stress that may initiate or exacerbate systemic disease [9-11]. The impact of oral infection on numerous medical conditions is well documented [12-15]. Conditions linked to oral infections include diabetes; [16-20] cardiovascular diseases such as atherosclerosis, acute myocardial infarction and stroke; [21-27] rheumatoid arthritis; [28, 29] obstructive pulmonary disease; [30, 31] osteopenia [32, 33] and low birth-weight preterm pregnancies [34-36]. There are indirect benefits of periodontal therapy (PT) for these patients including lower health care costs and fewer hospital visits [37].
It follows that oral infections may also have a negative impact on general wellness and, therefore, we conducted a study to test the hypothesis that PT will also affect general wellness. Patient responses to the RAND-36 questionnaire before and after PT were used to test this idea. The RAND-36 is a self-administered 36 question survey that measures quality of life before and after a medical or surgical intervention. It has been a well validated and accepted instrument in several different medical specialties [38- 44].
We conducted a pilot study to test the feasibility of a larger trial. However, in this small sample size results demonstrate an improvement in wellness following PT that is statistically significant (p=0.02).
Methods
Patients included were 18 years of age or older, diagnosed with moderate to severe periodontitis and were scheduled for PT. Excluded were patients that had periodontal therapy within the previous 12 months. Subjects signed an informed consent and were administered the RAND-36 questionnaire. The questionnaire responses were scored and scores were entered into Case Report Forms (CRF) along with demographics, medical history, medications, vital signs and PT details. Patients were offered a free choice of either osseous resective surgery or the laser-assisted LANAP procedure [45, 46]. Patients were scheduled for follow up appointments at 3 and 12 months. Following each visit the CRFs were scanned and uploaded to the statistician for data entry and analysis.
Analysis of the RAND-36 answers to 36 questions yields scores that range from 0-100 for eight health categories: general health, physical functioning, limitations due to physical health problems, limitations due to emotional problems, emotional well-being, social functioning, energy/fatigue, and pain. The eight scores are averaged to provide an over-all score for each visit. Scores for each category are averaged across subjects for baseline and follow-up visits to examine the magnitude and direction of change in the population for each condition. The Wilcoxon signed-rank test was used to compare two repeated measurements on a single sample to assess whether the population means differ from baseline to followup. The secondary outcome was to compare the mean difference between the minimally invasive laser procedure and the invasive scalpel surgery.
Results
The COVID-19 pandemic epicenter in Essex County, New Jersey affected recruitment, follow-up times and, perhaps, responses to the questionnaire. Recruitment was halted at 20 subjects when the clinic closed due to lock down. There were 5 males and 12 females with a mean age of 57.9 (range 23-85) with follow-ups and three patients lost to follow-up. Local population quarantine disrupted the planned follow-up schedule. The mean follow-up was 163 days with a range of 64-376 days. Also, the resulting sample size was inadequate to statistically evaluate secondary outcome variables.
Overall, thirteen (13) patients improved their RAND-36 scores, two (2) remained the same and two (2) worsened. Patients with baseline scores <80 improved by 14.2, whereas healthy individuals with baseline scores >80 only improved by 1.1. The Wilcoxon signed ranks test was applied to the entire group to see if there was a significant change in wellness scores from baseline to followup. Analysis demonstrates a significant improvement in wellness following PT with p<0.05 (Table 1).
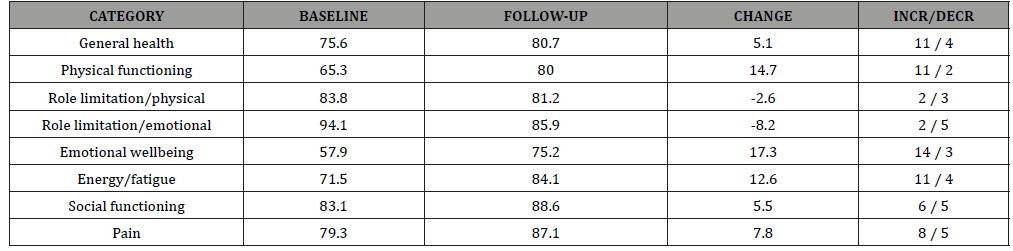
Averaged responses to the eight wellness categories are listed in (Table 2) for baseline and follow-up visits. A positive value for change indicates an improvement in wellness. In this sample PT led to improvement mainly in physical functioning, emotional wellbeing and energy. Negative values for role limitations, both physical and emotional, indicate a worsening of the condition. Eleven patients indicated an improvement in general health, two stayed the same and four reported a decrease. Most patients (82%) experienced a substantial improvement in their emotional wellbeing (Table 2).
Table 1: RAND-36 wellness summary scores for all subjects (n=17). The positive change (improvement) and percent change are significant with p-values = 0.022 and 0.015
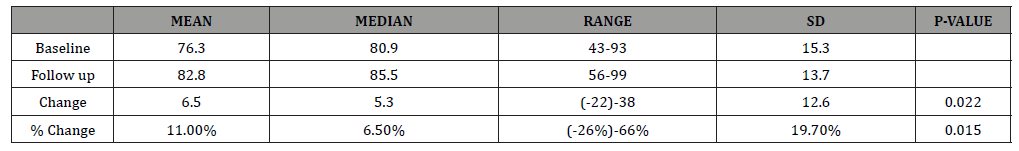
Table 2: Change in RAND-36 scores by category (n=17).
Discussion
The goal of periodontal surgical therapy is to eliminate periodontal disease and restore the periodontium to normal function so the dentition can be maintained [47]. The success of periodontal therapy can be evaluated by observing the clinical signs of inflammation, bone loss, probing depths, bleeding and suppuration. However, it is important to consider patient-reported outcomes, since what may appear to be successful clinically might not correlate with the patient’s own perceptions.
The most widely used survey to measure health-related quality of life is the RAND-36. It is useful in analyzing a patient’s current physical and mental health status [48-51]. The RAND-36 is also available in multiple languages and can be used to summarize the outcome of cross-sectional and longitudinal studies [52]. We introduce the RAND-36 to evaluate the patient’s perceived outcomes of surgical PT. Using this tool, we discovered that most patient’s general wellness improved following PT. Improvement was in the categories of physical functioning, energy and emotional well-being. The survey was easy to administer and evaluate making it a potential asset for the periodontist in private practice.
A recent editorial in Science expressed concern about how to account for the impact of COVID-19 on ongoing clinical trial data [53]. The clinic is located at the New Jersey pandemic epicenter and the local spike in COVID cases occurred in the interval between baseline and follow up visits (April 3-24, 2020). (NJ website) [54] In this group of patients the categories of role limitations both physical and emotional did not improve but worsened slightly. It is likely that these role limitations were affected by the mandated lock down and quarantine of the COVID-19 pandemic.
Even though the pandemic exerted a negative effect on quality of life, the benefits of PT still prevailed in the overall outcome. In fact, the effects of PT on wellness must be quite strong to reach significance in this small sample. However, this was a pilot study and the experimental design was disrupted. Please interpret these results with caution. If our findings can be replicated one might add improvement in general wellness and emotional wellbeing to the list of possible benefits of Periodontal Therapy.
Acknowledgement
There was no external funding for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Standards and Human Rights
The patients’ rights and study design were reviewed by the Rutgers University Institutional Review Board (Newark Health Sciences IRB ID: Pro2018000955) and the study was performed in accordance with the 1964 Declaration of Helsinki. This was a noninterventional survey study so registration with Clinical trials.gov is not required.
Informed Consent
Informed consent was obtained from all study participants.
References
- Schluger S (1950) Osseous resection-a basic principle in periodontal surgery. Oral Surgery Oral Med Oral Path 2(3): 316-25.
- Barrington E (1981) An overview of periodontal surgical procedures. J Periodontol 81(52): 518-528.
- Ramfjord S, Nissle RR (1974) The modified Widman Flap. J Periodontol 45: 601-607.
- Yukna RA, Lawrence JJ (1980) Gingival Surgery for soft tissue new attachment. Dent Clin N Am 24: 705-718.
- Ben Hatit Y, Blum R, Severin C, Maquin M, Jabro MH (1996) The effects of a pulsed Nd: YAG laser on subgingival bacterial flora and on cementum: An in vivo study. J Clin Laser Med Surg 14(3): 137-143.
- McCawley TK, McCawley MN, Rams TE (2018) Immediate Effects of Laser-Assisted New Attachment Procedure (LANAP) on Human Periodontitis Microbiota. J Intl Acad Periodontology 20(4): 163-171.
- Schwarz GM, Harris DM (2020) Laser-assisted treatment of peri-implantitis: a retrospective cohort study. Gen Dent 68(3): 18-25.
- K151763: “Promotion of true regeneration of the attachment apparatus (new cementum, new periodontal ligament, and new alveolar bone) on a previously diseased root surface”
- Hunter W (1990) Oral sepsis as a cause of disease. Br Med J 1: 215-216.
- Bansal M, Rastogi S, Vineeth NS (2013) Influence of periodontal disease on systemic disease: inversion of a paradigm: a review. J Med Life 6(2): 126-130.
- Cullinan MP, Seymour GJ (2000) Periodontal disease and systemic illness: will the evidence ever be enough? Periodontol 2000 62: 271-286.
- Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K (2007) Relationship between Periodontal infections and systemic disease. Clin Microbiol Infect 13(suppl 4): 3-10.
- Scannapieco F, Dasanayake AP (2010) Does Periodontal therapy reduce the risk for systemic diseases?. Dental Clinics N Am 54: 163-181.
- Derouen TA (2015) Effect of periodontal therapy on systemic diseases. American Journal of Preventive Medicine 48(3): E4.
- Jeffcoat MK, Jeffcoat RL, Gladowski PA, Bramson JB, Blum JJ (2015) Periodontal Therapy and Systemic Disease: An Author’s View. Journal Evidence Base Dental Practice 15: 140-143.
- Graves DT, Liu R, Oates TW (2007) Diabetes-enhanced inflammation and apoptosis: impact on periodontal pathosis. Periodontol 2000. 45: 128-137.
- Gurav AN (2012) Periodontal Therapy-an adjuvant for glycemic control. Diabetes Metab Syndr 6(4): 218-223.
- Loe H (1993) Periodontal disease: the sixth complication of diabetes mellitus. Diabetes Care 16: 329-334.
- Saremi A, Nelson RG, Tulloch-Reid M, Hanson RL, Sievers ML, et al. (2005) Periodontal disease and mortality in type 2 diabetes. Diabetes Care 28: 27-32.
- Simpson TC, Needleman I, Wild SH, Moles DR, Mills EJ (2010) Treatment of periodontal disease for glycaemic control in people with diabetes. Cochrane Database Syst Rev 5: CD004714.
- Bahekar AA, Singh S, Saha S, Molnar J, Arora R (2007) The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: a meta-analysis. Am Heart J 154: 830-837.
- Humphrey LL, Fu R, Buckley DI, Freeman M, Helfand M (2008) Periodontal disease and coronary heart disease incidence: a systematic review and meta-analysis. J Gen Intern Med 23: 2079-2086.
- Holmlund A, Lampa E, Lind L (2017) Poor Response to Periodontal Treatment May Predict future cardiovascular disease. J Dent Res 96 (7): 768 -773.
- Lockhart PB, Bolger AF, Papapanou PN, Osinbowale O, Trevisan M, et al. (2012) Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association? A scientific statement from the American Heart Association. Circulation. 125: 2520-2544.
- Meurman JH, Sanz M, Janket SJ (2004) Oral health, atherosclerosis and cardiovascular disease. Crit Rev Oral Biol Med 15: 403-413.
- Matilla KJ, Nieminen MS, Valtonen VV, Rasi VP, Y A Kesäniemi (1989) Association between dental health and acute myocardial infarction. BMJ 298: 779-781.
- Syrjanen J, Peltola J, Valtonen V, Iivanainen M, Kaste M, et al. (1989) Dental infections in association with cerebral infarction in young and middle-aged men. J Intern Med 225: 179-184.
- Erciyas K, Sezer U, Üstün K, Pehlivan Y, SŞenyurt BK sac, et al. (2013) Effects of periodontal therapy on disease activity and systemic inflammation in rheumatoid arthritis patients. Oral Dis 19: 394-400.
- Potempa J, Mydel P, Koziel J (2017) The case for Periodontitis in the pathogenesis of Rheumatoid Arthritis. Nat Rev Rheumatol 13(10): 606-620.
- Scannapieco F (1999) Role of oral bacteria in respiratory infection. J Peridontol 70: 793-802.
- Scannapieco FA, Bush RB, Paju S (2003) Associations between periodontal disease and risk for nosocomial bacterial pneumonia and chronic obstructive pulmonary disease. A systematic review. Ann Periodontol 8: 54-69.
- Tezal M, Wactawski Wende J, Grossi SG, Ho AW, Dunford R, et al. (2000) The Relationship Between Bone Mineral Density and Periodontitis in Postmenopausal Women. J Periodontol 71: 1492-1498.
- Charles H Chestnut III (2001) The relationship between skeletal and oral bone mineral density: An Overview. Ann periodontal 6: 193-196.
- Chambrone L, Guglielmetti MR, Pannuti CM, Chambrone LA (2011) Evidence grade associating periodontitis to preterm birth and⁄or low birth weight: I. A systematic review of prospective cohort studies. J Clin Periodontol 38: 795-808.
- Jeffcoat MK, Geurs NC, Reddy MS, Cliver SP, Goldenberg RL, et al. (2001) Periodontal infection and preterm birth results of a prospective study. J Am Dent Assoc 132: 875-880.
- Offenbacher S, Katz V, Fertik G, Collins J, Boyd D, et al. (1996) Periodontal infection as a possible risk factor for preterm low birth weight. J Periodontol 67: 1103-1113.
- Jeffcoat MK, RL Jeffcoat, PA Gladowski, JB Bramson, JJ Blum (2014) Impact of Periodontal Therapy on General Health Evidence from insurance data for five systemic conditions. Am J Prev Med 47(2): 166-174.
- Alakärppä AI, Koskenkorva TJ, Koivunen PT, Alho OP (2017) Quality of life before and after sinonasal surgery: a population-based matched cohort study. Eur Arch Otorhinolaryngol 274(2): 795-802.
- Ames SC, Jones GN, Howe JT, Brantley PJ (2001) A Prospective Study of the Impact of Stress on Quality of Life: An Investigation of Low-Income Individuals with Hypertension. Ann Behav Med 23(2): 112-119.
- Anderkova L, Elfmarková N, Svěrák T, Peterkova H, Brancikova D, et al. (2016) Change in Quality of Life Measured over Time in Czech Women with Breast Cancer. Klin Onkol 29(2): 113-121.
- Arnold R, Ranchor AV, DeJongste MJ, Köeter GH, Ten Hacken NH, et al. (2005) The relationship between self-efficacy and self-reported physical functioning in chronic obstructive pulmonary disease and chronic heart failure. Behav Med 31(3): 107-115.
- Bakker SF, Pouwer F, Tushuizen ME, Hoogma RP, Mulder CJ, et al. (2013) Compromised quality of life in patients with both Type 1 diabetes mellitus and coeliac disease. Diabet Med 30(7): 835-839.
- Assaf AR, Beresford SAA, Risica PM, Aragaki A, Brunner RL, et al. (2016) Low-fat dietary pattern intervention and health-related quality of life: The WHI randomized controlled Dietary Modification trial. J Acad Nutr Diet 116(2): 259-271.
- Aspinen S, Kärkkäinen J, Harju J, Juvonen P, Kokki H, et al. (2017) Improvement in the quality of life following cholecystectomy: a randomized multicenter study of health status (RAND-36) in patients with laparoscopic cholecystectomy versus minilaparotomy cholecystectomy. Qual Life Res 26(3): 665-671.
- Gregg RH, McCarthy D (2001) Laser periodontal therapy: case reports. Dent Today 20: 74-81.
- Harris DM, Gregg RH, McCarthy DK, Colby LE and Tilt LV (2004) Laser-assisted new attachment procedure in private practice. Gen Dent 52: 396-403.
- Kakehashi S, Parakkal P (1982) Proceedings from the state of the art workshop on surgical therapy for periodontitis. J Periodontol 53: 475-501.
- Ware JE Jr, Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30: 473-483.
- Ware JE, Kosinski M, Keller SD (1994) SF-36 physical and mental health summary scales: a user manual Massachusetts: Health Institute, New England Medical Center.
- Hemingway H, Stafford M, Stansfeld S, Shipley M, Marmot M (1997) Is the SF-36 a valid measure of change in population health? Results from the Whitehall II Study. BMJ 315: 1273-1279.
- Adams TB, Bezner J, Steinhardt MA (2010) The conceptualization and measurements of Perceived wellness: Integrating balance across and within dimensions. Am J Health Promot 48(4): 165-173.
- Hays, Morales (2001) The RAND-36 measure of health-related quality of life. Ann Med 33: 350-357.
- Servick K (2020) Pandemic could add noise to clinical trial data. Science 368: 693.
- (2020) State of New Jersey Department of Health Website, COVID-19 Dashboard.
-
David M Harris, Wonseok Jang, Ranjitha R Padhiar, Andrew Sullivan. Improvement in Wellness Following Periodontal Therapy. On J Dent & Oral Health. 5(3): 2022. OJDOH.MS.ID.000611.
-
Periodontal therapy, Periodontitis, Probing depths, Bone loss, Periodontal pockets, Exacerbate systemic disease, Inflammation, Periodontal disease, Bleeding.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






