 Review Article
Review Article
Dental Antibiotic Prophylaxis and Prosthetic Joint Infections: A Narrative Review
NS Jayalakshmi1*, Sheetal Saklecha1 and NS Harshavardhana2
1Santosh Dental Care, Bengaluru, India
2Dumfries & Galloway Royal Infirmary, Dumfries; United Kingdom
NS Jayalakshmi MDS, Santosh Dental Care, Bengaluru, India,
Received Date: February 20, 2021; Published Date: March 09, 2021
Abstract
Prosthetic joint infection (PJI) is the 3rd most common cause of revision in total hip arthroplasty (THA) and the most common cause of revision in total knee arthroplasty (TKA). It is associated with high costs/economic burden and mortality. The most common cause of late PJI is due to haematogenous seedling of bacteria into the joint from remote site infection (RSI). Oral flora constitutes 6-13% of burden in PJI. Dental interventions in patients who have undergone joint replacement surgery (JRS) are at increased risk of PJI due to bacteraemia. Dental antibiotic prophylaxes (DAP) are known to reduce this bacteraemia which in-turn may reduce the incidence of PJI. Significant controversy exists Re: the role and use of DAP in preventing PJI. This narrative review attempts to answer key questions surrounding its use based on extensive literature review in English language over past four decades.
The existing evidence is at best low and of poor quality with few comparative clinical trials that had assessed the incidence of PJI with DAP. Several systematic reviews have concluded that the existing evidence at best is inconclusive. The best existing guideline is the updated 2017 American Academy of Orthopaedic Surgeons (AAOS)-American Dental Association (ADA): Appropriate Use Criteria (AUC) that recommends shareddecision making taking individual risk factors into consideration with patients’ input. DAP are recommended only in at-risk patients and is neither cost-effective nor recommended for all cases. The risk of PJI is highest in the first two years of JRS. Regular 6-12monthly dental visits, good oral hygiene and collaboration between Surgeons and Dentists facilitate providing a high quality of care thus minimizing the incidence of PJIs.
Introduction
Prosthetic joint infection (PJI) following a joint replacement surgery (JRS) for primary or secondary arthritis can be devastating and is associated with high one and five-year mortality [1]. It is the third most common cause for revision following THA (after loosening and dislocations) [2]. The economic burden on the society and costs incurred in treating it are high. Patients with PJIs have lower satisfaction with residual stiffness, pain and reduced Quality of life (QoL) [3]. PJIs are classified into few categories based on the duration from the time of index surgery. This ‘duration’ is interpreted differently by Microbiologists and Orthopaedic surgeons as summarized in (Table 1) [4,5]. Suffices to say that most PJIs occur within the first two years of index surgery (at 0.14%) and the annual risk of PJI ≥2 years is 0.08% for Knees [6]. Infections in acute phase are most commonly caused by Staphylococcus aureus (a normal resident on skin and nasal cavity) [7]. Methicillinresistant staphylococcus aureus (MRSA) and Methicillin-resistant staphylococcus epidermidis (MRSE) are difficult to eradicate and is a major economic burden with high morbidity [8].
Most of the late/chronic PJIs are caused by haematogenous spread of organisms from a remote site (gastro-intestinal, genitourinary, oral cavity etc...). Oral flora account for 6-13% of PJI which are potentially avoidable with preventative measures and is a modifiable risk factor [9]. Much research has focussed on this subgroup to minimize the catastrophic consequences of infection and use of dental antibiotic prophylaxis (DAP) has a potential role in this regard. The oral flora are largely fastidious facultative anaerobes of low virulence causing sub-clinical infections [10]. Dental interventions are associated with bacteraemia that predisposes to haematogenous spread/seedling of these organisms into the prosthetic joint [11]. Given the indolent nature and non-specific signs, such PJIs are difficult to detect in early stages. Often the microbiological culture and sensitivity studies are largely negative or dismissed as likely contaminants. With advances in microbiological diagnostics and 16s/18s RNA studies, rare organisms are cultured on prolonged incubation and enrichment media [12]. Sophisticated methods like Matrix Assisted Laser Desorption Ionization-Time of Flight: Mass Spectrometry (MALDI-TOF MS) has further enhanced the diagnostic capabilities in identifying the causative organism and facilitated strain typing in PJI [13].
Several systematic reviews have been published in the recent five years evaluating the evidence for ‘DAP in preventing PJIs’ [14-16]. Unfortunately these have added very little to the body of existing evidence and repeating the same message that ‘Existing evidence is low and of poor quality’. A narrative review such as this is an attempt to fill this lacuna and attempts to summarize the existing evidence in one piece. It attempts to answer the following key questions with evidence-based answers:
1. What are the common oral flora and their microbiological characteristics?
2. What is the consensus on preferred antibiotic prescription prior to dental surgery?
3. How are dental procedures risk stratified and what are the risks of bacteraemia from them?
4. What is the key summary from published comparative studies Re: DAP?
5. What are the recommendation/guidelines of national professional societies Re: DAP?
6. What is the key summary from published studies on costeffectiveness with use of DAP?
Methods
The authors included a group of two Dentists (NSJ and SS) and an Orthopaedic surgeon (NSH). We undertook a comprehensive search using PubMed, MedLine, EMBASE, Google Scholar and Cochrane Review in the months from October 2020 to December 2020 (3months) with the following Medical Subject Headings (MeSH) terms: Dental procedure/intervention/surgery; Prosthetic joint infection; Prophylactic antibiotics; Hip/Knee/Shoulder/ Ankle; Joint replacement surgery; Arthroplasty; Cost-effectiveness of Prophylaxis; Professional Guidelines; Consensus position statement and downloaded all articles related to the topic of interest in an attempt to answer ‘Six’ key questions and summarize ‘Ten’ key points. We only analyzed the existing evidence published in English language over four decades (1981-2020). We specifically looked for any published White paper and professional societies position statement documents that addressed Dentists and Orthopaedic Surgeons. We also reviewed the results of surveys administered on this topic to understand the perspective of both groups (i.e. Orthopaedic surgeons and Dentists). We excluded all studies published in any language other than English given our inability in comprehending and drawing meaningful conclusions from such publications. We also excluded the role/recommendations on DAP for implants/devices other than JRS (i.e., heart valves, stents etc.).
Discussion
Oral Flora & their Microbiological Characteristics
At least 500 organisms of different phyla inhabit the oral cavity of which>280 have been isolated from standard culture [9]. These are either native residents that are protective against invasion or source of dental infections (plaques, caries, gingivitis or periodontitis and dental abscess). A fine balance exists between native flora and infectious organisms. Disturbance of this balance results in plaque formation that causes breakdown of enamel. The acid production leads to teeth discolouration with cavity formation that gets colonized by bacteria. These could be either primary or secondary colonizers. The organisms on the surface are usually aerobic and those in-depths/at the core are anaerobic. Gingivitis refers to the inflammation of the gums and periodontitis is a severe form that is associated with bone loss and detachment of gums from teeth. If left untreated a dental abscess evolves. Antibiotics help in eradicating the infection/restoring the balance of oral flora. Good oral hygiene with regular dental review ensures healthy gums and early evaluation/management of plaques. The common oral flora and their microbiological characteristics are summarized in (Table 2) [17].
Table 1: Classification of prosthetic joint infection (PJI) based on duration.
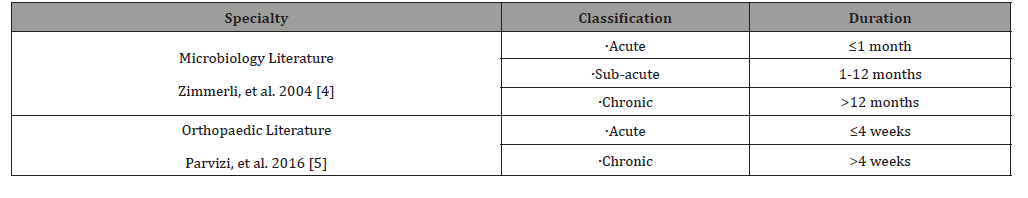
Table 2: Common oral flora and their microbiological characteristics [10].
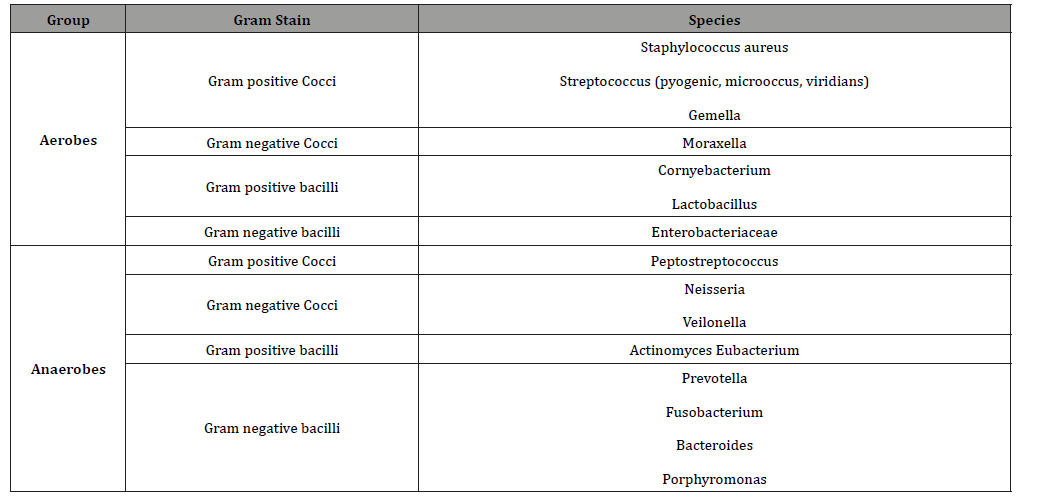
Risk stratification of Dental Procedures & Risk of Bacteraemia
Dental interventions that involve cleaning, filling of cavity, manipulation of periodontal tissues, drainage of dental abscess and extraction of tooth induce bleeding have the potential to introduce bacteraemia. Simple physiological activities like brushing, vigorous flossing also induce bacteraemia to a tune of up to 50%. Routine dental procedures produces 10-240 colony forming unit (CFU)/ml of blood and the bacteraemia load needed to cause PJI was at least 1000 CFU/ml [18]. Bacteraemia is merely a surrogate measure and there exists no direct evidence linking it to PJI. Some experts believed the prosthetic joints are to some degree protected by fibrotic scar and capsule from circulating less virulent oral flora. These interventions are classified as either low or high-risk procedures based on the severity of bacteraemia and (Table 3) summarizes such procedures along with percentage of bacteraemia load [19]. It is evident periodontal procedures and dental implantology/ extractions are associated with significant bacteraemia and constitute ‘high risk’ for haematogenous dissemination.
Table 3: Low vs. High-risk dental procedures [11,19].
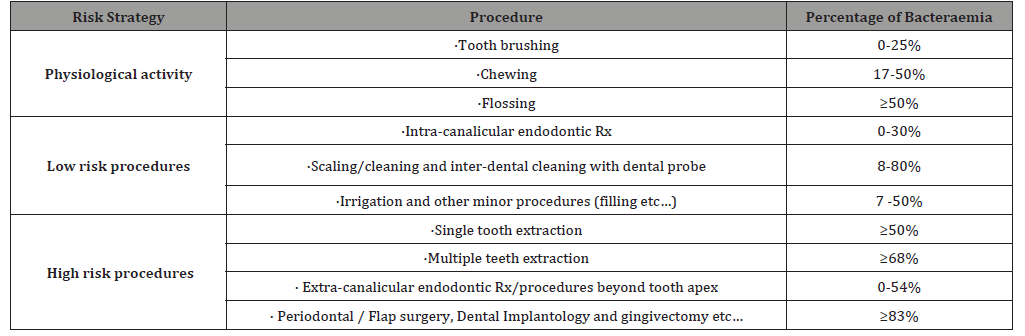
Antibiotic Susceptibility Pattern of Oral Flora
The infections from oral flora are usually treated by empirical antibiotics in the first instance. They are usually sensitive to β-Lactam Penicillins (i.e., Ampicillin and Amoxicillin) and Cephalosporins (i.e., first generation agents-Cephalexin and Cephradine). They are bactericidal and act by inhibiting the cell wall formation. Clindamycin is the preferred antibiotic in patients who are allergic to Penicillin and is bacteriostatic. Amoxicillin reduces the risk of oral bacteraemia from 63% to 35% in one of the studies and Erythromycin was used in 1980s [20]. Most publications recommend a single dose administered an hour prior to dental procedure with few proposing 24hr cover in severe infections/ extensive surgery. Key class of patients who had the highest benefit from DAP were:
i) On immunosuppressive therapy
ii) Dermatological infection and
iii) Obvious focal infection (i.e., Urosepsis etc.).
Table 4 summarizes preferred DAP to be used when a decision is made to administer one [19].
Table 4: Summary of dental antibiotic prophylaxis (DAP) of choice for procedures [19].
Published Comparative Studies (LoE II & III)
Five comparative studies have been published evaluating PJI in patients undergoing dental procedures since 1986 (Table 5) [21-25]. with the most recent study involving a large cohort of 255,568 Taiwanese patients undergoing THA or TKA over 12years by Kao, et al. [25]. The sub-set of dental cohort was 57,066 patients and the number of PJI was 328 (incidence of 0.57%). This was retrospectively matched to a cohort of 348 non-dental PJI (incidence of 0.61%) accounting for all confounding factors and had robust research methodology. They found no correlation between PJI and dental intervention (p=0.31). They did not recommend any DAP and recommended shared decision-making seeking patients’ input along the lines of AAOS-ADA: AUC [26]. This remains the largest sample size to date amongst all published studies and was retrospective (i.e. Level of evidence [LoE] III). The best available highest LoE study is that of Berbari, et al. [22] from 2010 that involved a prospective comparative 1:1 matched review (i.e., LoE II) in a cohort of 339 PJI (THA and TKA) in patients who had dental procedures within the 6months and 2years following index surgery who had no dental intervention [22]. They found no correlation for Low vs. High-risk procedures and with or without DAP. They concluded dental procedures not to be a risk factor for subsequent PJI and that administration of DAP did not reduce the risk of PJI.
Table 5: Summary of comparative studies evaluating the role of prophylactic antibiotics and prevention of prosthetic joint infections (PJI).
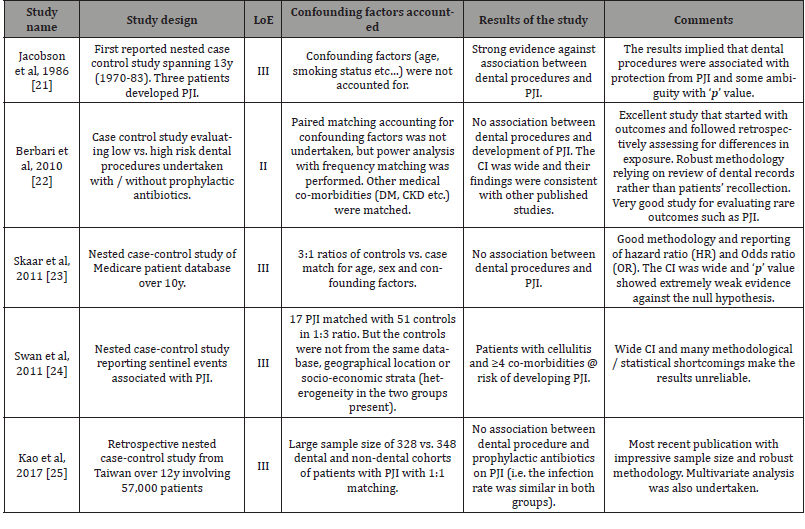
Couple of LoE III studies were published in 2011 by Skaar, et al. and Swan, et al. [23, 24]. Skaar observed 42 PJI in all JRS over 10years and case matched it 126 controls (1:3 ratio) [23]. They found no association between dental procedures and PJI. Patient profile risk factors that strongly correlated with PJI observed in literature were:
i) Morbid obesity
ii) Alcohol and nicotine
iii) Male sex
iv) Age >65years
v) Operative time of >2hours
vi) Diabetes mellitus and
vii) Rheumatoid arthritis (and immunosuppressed states).
Swan, et al. [24] observed 17 late PJI in a cohort of 1641 patients who underwent TKA. 15 PJIs were preceded by a sentinel event and only one patient had a dental source [24].
Published Recommendations of National Professional Societies
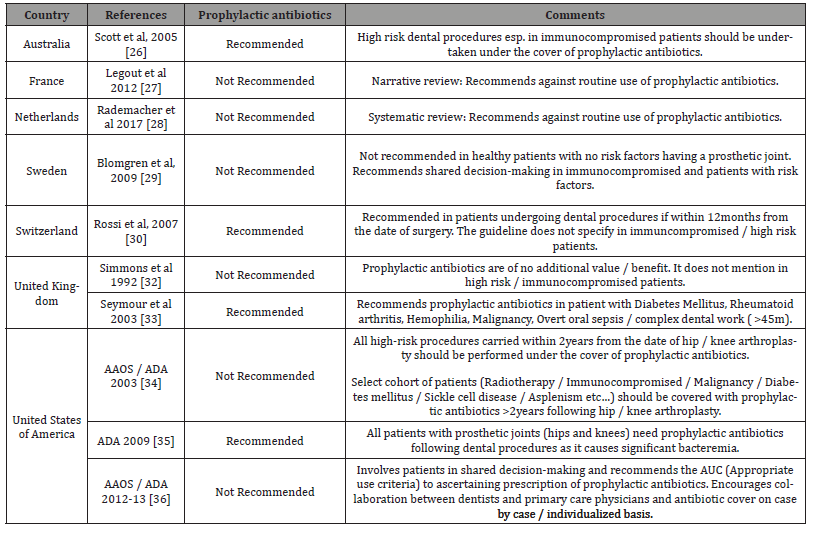
The professional Dentist and Orthopaedic scientific societies have jointly produced guidelines and recommended position statements regarding DAP in patients with prosthetic joints undergoing dental procedures and is summarized in (Table 6) [26- 36]. Despite best efforts and exhaustive review of literature, there is little consensus from these regulatory bodies regarding best practice. As bacteraemia was only an indirect surrogate marker for PJI, it was felt prophylactic antibiotics was not required. Australia along with European Union countries notably France, Netherlands, Sweden and Switzerland have also recommended against routine use of DAP [26-31]. This argument held ground and was also accepted by British orthopaedic association (BOA) and British dental association (BDA) [32,33]. The American association of orthopaedic surgeons (AAOS) and American dental association (ADA) did not recommend antibiotic proplylaxis in 2003 [34]. However the AAOS unilaterally made a decision in 2009 to strongly consider/ administer DAP in all patients with prosthetic joints with Amoxycillin [35]. In-summary the AAOS and ADA have gone back and forth reviewing and revising the recommendations on no less than three occasions over the past fifteen years [34-36]. The 2009 recommendation of the AAOS was strongly criticised by the Council of Scientific Affairs (CSA) of ADA who published a comprehensive evidence-based practice recommendation and found no evidence to support routine DAP [37]. The AAOS and ADA began working jointly to address this issue and came up with appropriate use criteria (AUC) in 2017 and was developed by Delphi consensus evaluating 64 scenarios [38]. The five key determinants of AUC were:
Table 6: Summary of published recommendations from national bodies, their guidelines with respect to the use of prophylactic antibiotics.
viii) Nature of underlying procedure
ix) Immunocompromised status of the patient
x) Presence of Diabetes mellitus
xi) Previous h/o PJI needing surgery and
xii) Planned dental procedure <12months from primary surgery.
Prophylactic antibiotics was not indicated in 61% and considered appropriate in only 12% of scenarios. The degree of consensus also varied from high (score of 7-9), maybe (score of 4-6) and low (score of 1-3). More recently, the AAOS also published a decision-making tree to simplify things and ascertain where DAP was appropriate [39].
Published Cost-effectiveness Studies
Economic modelling and cost-effectiveness studies are increasingly conducted since 2010 onwards to justify therapeutic intervention and seek insurance authorization. These are least common types of studies reported in Orthopaedic literature accounting for <2% of all publications. Four key studies have reported on Risk vs. Benefit and cost-effectiveness with routine use of DAP and their key findings are summarized in (Table 7) [40- 43]. The life-time risk of PJI is ≤2.0% and oral pathogens account for 6-13% of all PJIs. The number of patients who will need to be administered antibiotics before dental procedures to prevent one PJI is 625-1250. Such a blanket administration of antibiotics carries a risk of adverse event, intolerance or anaphylactic reaction in at least 37-80 patients for Amoxicillin alone. Penicillin is responsible for 75% of fatal anaphylaxis in the USA [44]. Dentists are the highest prescribers of Clindamycin amongst healthcare professionals and carried highest risk (odds ratio [OR] of 17-20) [45]. Its use risks causing Clostridium difficile infections (CDI) and annually 24,000 deaths are attributed to CDI alone. A single dose of 600mg Clindamycin could potentially cause 12 deaths/million due to CDI and Orthopaedic surgeons were unaware of this fact [46]. The DAP would only be cost-effective if the risk of HPJI was ≥0.7% and current best estimate/projections of PJI from oral flora was at best 0.03-0.10%.
Table 7: Summary of published cost-effectiveness studies on dental antibiotic prophylaxis.
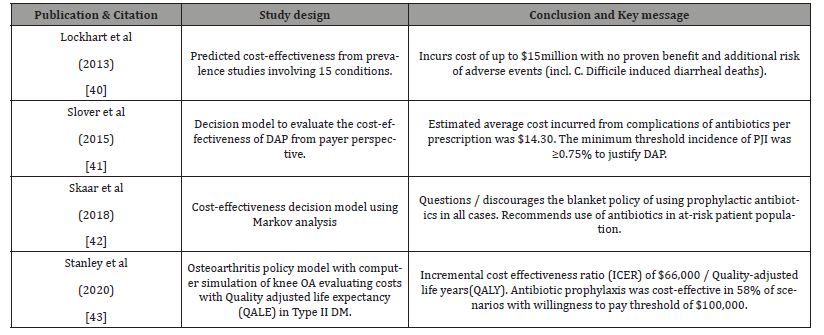
All published studies in Table 7 have rightfully questioned the routine use of DAP as did not prevent PJIs. Most recently study by Stanley et al in 2020 found that DAP was cost-effective in only 32% of scenarios with willingness to pay threshold of ≥$50,000 in select group of patients with Type II Diabetes Mellitus [43]. Skaar et al. recommended DAP in highly select group of ‘at-risk’ patients:
i) On renal dialysis
ii) Solid organ transplant (on immunosuppressive medication)
iii) Diabetes and Rheumatoid arthritis and
iv) Poor oral hygiene and severe periodontal disease [42].
Their Value of Information (VoI) projected cost estimate of $80m at an estimate of 700,000 TKA/year over 5-year time horizon.
Conclusion
The three components of evidence-based practice (EBP) includes:
i) Scientific evidence
ii) Clinical experience and
iii) Patient values
Professional judgement coupled with patients’ preferences and backed by EBP is needed for optimal treatment of PJIs. Surgeon vs. Dentist surveys from New Zealand, Australia and Canada observed that a majority of surgeons preferred to err on the side of administration of DAP despite existing evidence relying on practice experience and opinion papers (LoE V) [47-49]. Orthopaedic surgeons are concerned about PJI against a background of dental interventions and prefer to prescribe DAP as they see high value and low risk with it. Dentists exercise more discretion seeing this approach to be associated with low value and high risk of adverse effects. Patients are struck in the middle and are in a conundrum. We hope this article addresses this dilemma summarizing the existing evidence. 75% of the Dentists and 88% of Orthopaedic surgeons still prescribed DAP citing ‘Defensive Practice’ as the reason fearing lawsuit and allegations of negligence.
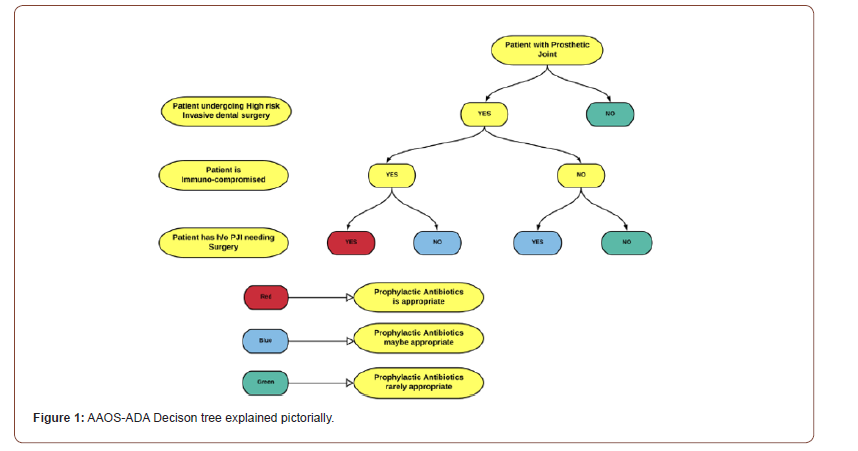
A recent survey found none of the 28 professionals (20 Dentists and 8 Orthopaedic surgeons) used AAOS-ADA risk calculator to aid them in decision-making re: DAP prescription. It is reasonable to adhere with AAOS-ADA: AUC guidelines and we strongly recommend incorporating it as a part of one’s practice. Dialogue between Surgeons, Dentists and patients in shared decisionmaking and need for ‘Dental Stewardship’ is proposed [50]. The AAOS-ADA: AUC decision tree represented pictorially in (Figure 1) aids in simplified decision-making [39].
We would refrain from administering antibiotics esp. if patients are immunocompetent or have minimal medical co-morbidities (Charleston index ≤4). Maintaining good oral hygiene and regular 6-12monthly dental assessments (i.e., enhanced preventive strategies) are believed to reduce the risk of PJI by ≥30%. Suffices to say that the existing evidence for using DAP in preventing PJI is weak and level of recommendation is at best ‘C’. Additional systematic reviews will be of very little benefit to the existing evidence.
Summary: Top Ten Key Points
1. In-addition to the existing evidence being low and of poor quality for use of DAP, a LoE I study (i.e., Randomized controlled trial) may be impractical/not feasible given several variables/ confounding factors and is unlikely to happen in the foreseeable future.
2. Oral pathogens are responsible for 6-13% of PJIs and are difficult to culture often needing enrichment media and prolonged incubation.
3. The risk of PJIs is high in the first two years of index/ primary surgery and Orthopaedic surgeons who were inpractice for >15y prescribed it most often than junior colleagues.
4. The incidence of PJI from dental interventions is due to bacteraemia that is only a ‘surrogate marker’ and at best an ‘indirect association’.
5. Dental procedures are classified as ‘Low vs. High risk’ interventions. Routine use of DAP for low risk procedures in healthy patients is not recommended.
6. ‘At-risk’ group of patients undergoing high risk procedures should be performed under prophylactic antibiotics cover.
7. Difference of opinion exists between Dentists and Orthopaedic surgeons Re: DAP. Orthopaedic specialists prescribed antibiotics more often independent of the nature of dental procedure whilst Dentists exercised more discretion in their prescriptions.
8. National recommendations differed widely and were mainly from Europe and North American countries. There were little/no guidelines from Asian (including India) and African countries.
9. Shared decision-making involving patients and their preferences guided by AAOS-ADA: AUC is the best available evidence at present to guide healthcare professionals Re: DAP.
10. DAP are neither cost-effective economically nor shown to be better with regards to ‘Benefit vs. Risk’ ratio when adverse events with their use was taken into consideration.
Acknowledgement
Mr. M Sarungi & Mr. M Changulani, Orthopaedic Surgeons: U.K.
Conflict of Interest
All authors do not have any CoI to disclose and no funding from any source was received by any of us for this research.
References
- Zmistowski B, Karam JA, Durinka JB, Casper DS, Parvizi J (2013) Periprosthetic joint infection increases the risk of one-year mortality. J Bone Joint Surg Am 95(24): 2177-2184.
- Sadoghi P, Liebensteiner M, Agreiter M, Leithner A, Bohler N, et al. (2013) Revision surgery after total joint arthroplasty: a complication-based analysis using worldwide arthroplasty registers. J Arthroplasty 28(8): 1329-1332.
- Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY (2004) Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am 86(5): 963-974.
- Zimmerli W, Trampuz, A, Ochsner PE (2004) Prosthetic-joint infections. N Engl J Med 351(16): 1645-1654.
- Parvizi J, Fassihi SC, Enayatollahi MA (2016) Diagnosis of Periprosthetic Joint Infection Following Hip and Knee Arthroplasty. Orthop Clin North Am 47(3): 505-515.
- Huotari K, Peltola M, Jämsen E (2015) The incidence of late prosthetic joint infections: a registry-based study of 112,708 primary hip and knee replacements. Acta Orthop 86(3): 321-325.
- Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, et al. (2016) Periprosthetic joint infection. Lancet 387(10016): 386-394.
- Parvizi J, Azzam K, Ghanem E, Austin MS, Rothman RH (2009) Periprosthetic infection due to resistant staphylococci: serious problems on the horizon. Clin Orthop Relat Res 467(7): 1732-1739.
- Young H, Hirsh J, Hammerberg EM, Price CS (2014) Dental disease and periprosthetic joint infection. J Bone Joint Surg Am 96(2): 162-168.
- Reynolds-Campbell G, Nicholson A, Thoms-Rodriguez CA (2017) Oral Bacterial Infections: Diagnosis and Management. Dent Clin North Am 61(2): 305-318.
- Hall G, Heimdahl A, Nord C E (1999) Bacteremia after oral surgery and antibiotic prophylaxis for endocarditis. Clin Infect Dis 29(1): 1-8; quiz 9-10.
- Mitreva M (2017) The Microbiome in Infectious Diseases. In J Cohen, WG Powderly, SM Opal (Eds.), Infectious Diseases (4th), p: 68-74.
- Singhal N, Kumar M, Kanaujia PK, Virdi JS (2015) MALDI-TOF mass spectrometry: an emerging technology for microbial identification and diagnosis. Frontiers in microbiology 6: 791.
- Rademacher WMH, Walenkamp GHIM, Moojen DJF, Hendriks JGE, Goedendorp TA, et al. (2017) Antibiotic prophylaxis is not indicated prior to dental procedures for prevention of periprosthetic joint infections. Acta orthopaedica 88(5): 568-574.
- Slullitel PA, Oñativia JI, Piuzzi NS, Higuera-Rueda C, Parvizi J, et al. (2020) Is there a Role for Antibiotic Prophylaxis Prior to Dental Procedures in Patients with Total Joint Arthroplasty? A Systematic Review of the Literature. J Bone Joint Infect 5(1): 7-15.
- Moreira AI, Mendes L, Pereira JA (2020) Is there scientific evidence to support antibiotic prophylaxis in patients with periodontal disease as a means to decrease the risk of prosthetic joint infections? A systematic review. Int Orthop 44(2): 231-236.
- Levi ME, Eusterman VD (2011) Oral infections and antibiotic therapy. Otolaryngol Clin North Am 44(1): 57-78.
- Fernandez-Sampedro M, Salas-Venero C, Fariñas Álvarez C, Sumillera M, Pérez-Carro L, et al. (2015) 26Postoperative diagnosis and outcome in patients with revision arthroplasty for aseptic loosening. BMC infectious diseases 15: 232.
- Kotze, MJ (2009) Prosthetic joint infection, dental treatment and antibiotic prophylaxis. Orthop Rev (Pavia) 1(1): e7.
- Coulter WA, Coffey A, Saunders ID, Emmerson AM (1990) Bacteremia in children following dental extraction. J Dent Res 69(10): 1691-1695.
- Jacobson JJ, Millard HD, Plezia R, Blankenship JR (1986) Dental treatment and late prosthetic joint infections. Oral Surg Oral Med Oral Pathol 61(4): 413-417.
- Berbari EF, Osmon DR, Carr A, Hanssen AD, Baddour LM, et al. (2010) Dental procedures as risk factors for prosthetic hip or knee infection: a hospital-based prospective case-control study. Clin Infect Dis 50(1): 8-16.
- Skaar DD, O'Connor H, Hodges JS, Michalowicz BS (2011) Dental procedures and subsequent prosthetic joint infections: findings from the Medicare Current Beneficiary Survey. J Am Dent Assoc 142(12): 1343-1351.
- Swan J, Dowsey M, Babazadeh S, Mandaleson A, Choong PFM (2011) Significance of sentinel infective events in haematogenous prosthetic knee infections. ANZ Journal of Surgery 81(1‐2): 40-45.
- Kao FC, Hsu YC, Chen WH, Lin JN, Lo YY, et al. (2017) Prosthetic Joint Infection Following Invasive Dental Procedures and Antibiotic Prophylaxis in Patients with Hip or Knee Arthroplasty. Infect Control Hosp Epidemiol 38(2): 154-161.
- Scott JF, Morgan D, Avent M, Graves S, Goss AN (2005) Patients with artificial joints: do they need antibiotic cover for dental treatment? Aust Dent J 50(4 Suppl 2): S45-53.
- Legout L, Beltrand E, Migaud H, Senneville E (2012) Antibiotic prophylaxis to reduce the risk of joint implant contamination during dental surgery seems unnecessary. Orthop Traumatol Surg Res 98(8): 910-914.
- Rademacher WMH, Walenkamp GHIM, Moojen DJF, Hendriks JGE, Goedendorp TA, et al. (2017) Antibiotic prophylaxis is not indicated prior to dental procedures for prevention of periprosthetic joint infections. Acta orthopaedica 88(5): 568-574.
- Blomgren J, Heimdahl A, Struwe J (2009) [Antibiotic prophylaxis seldom indicated in dental care: important that physicians and dentists agree]. Lakartidningen 106(52): 3485-3486.
- Rossi M, Zimmerli W, Furrer H, Zanetti G, Mühlemann K, Täuber MG (2005) [Antibiotic prophylaxis for late blood-borne infections of joint prostheses]. Schweiz Monatsschr Zahnmed 115(6): 571-579.
- Alao U, Pydisetty R, Sandiford NA (2015) Antibiotic prophylaxis during dental procedures in patients with in situ lower limb prosthetic joints. Eur J Orthop Surg Traumatol: 25(2): 217-220.
- Simmons NA, Ball AP, Cawson RA, Eykyn SJ, Hughes SP, et al. (1992) Case against antibiotic prophylaxis for dental treatment of patients with joint prostheses. Lancet 339(8788): 301.
- Seymour RA, Whitworth JM, Martin M (2003) Antibiotic prophylaxis for patients with joint prostheses-still a dilemma for dental practitioners. Br Dent J 194(12): 649-653.
- American Dental Association (2003) Antibiotic prophylaxis for dental patients with total joint replacements. J Am Dent Assoc 134(7): 895-899.
- Jevsevar DS, Abt E (2013) The new AAOS-ADA clinical practice guideline on Prevention of Orthopaedic Implant Infection in Patients Undergoing Dental Procedures. J Am Acad Orthop Surg 21(3): 195-197.
- (2021) https://www.orthoguidelines.org/go/auc/default.cfm?auc_id=224995&actionxm=Terms/.
- Quinn RH, Murray JN, Pezold R, Sevarino KS (2017) Management of Patients with Orthopaedic Implants Undergoing Dental Procedures. J Am Acad Orthop Surg 25(7): e138-141.
- Sollecito TP, Abt E, Lockhart PB, Truelove E, Paumier TM, et al. (2015) The use of prophylactic antibiotics prior to dental procedures in patients with prosthetic joints: Evidence-based clinical practice guideline for dental practitioners--a report of the American Dental Association Council on Scientific Affairs. J Am Dent Assoc 146(1): 11-16 e18.
- (2021) https://www.aaos.org/aaosnow/2020/mar/qualityresearch/quality-research02/.
- Lockhart PB, Blizzard J, Maslow AL, Brennan MT, Sasser H, et al. (2013) Drug cost implications for antibiotic prophylaxis for dental procedures. Oral Surg Oral Med Oral Pathol Oral Radiol 115(3): 345-353.
- Slover JD, Phillips MS, Iorio R, Bosco J (2015) Is routine antibiotic prophylaxis cost effective for total joint replacement patients? J Arthroplasty 30(4): 543-546.
- Skaar DD, Park T, Swiontkowski MF, Kuntz KM (2018) Is Antibiotic Prophylaxis Cost-effective for Dental Patients Following Total Knee Arthroplasty? JDR Clinical & Translational Research 4(1): 9-18.
- Stanley EE, Trentadue TP, Smith KC, Sullivan JK, Thornhill TS, et al. (2020) Cost-effectiveness of dental antibiotic prophylaxis in total knee arthroplasty recipients with type II diabetes mellitus. Osteoarthritis and Cartilage Open 2(4): 100084.
- Swift JQ, Gulden WS (2002) Antibiotic therapy--managing odontogenic infections. Dent Clin North Am 46(4): 623-633.
- Durkin MJ, Hsueh K, Sallah YH, Feng Q, Jafarzadeh SR, et al. (2017) An evaluation of dental antibiotic prescribing practices in the United States. J Am Dent Assoc 148(12): 878-886.e1.
- Goff DA, Mangino JE, Glassman AH, Goff D, Larsen P, et al. (2019) Review of Guidelines for Dental Antibiotic Prophylaxis for Prevention of Endocarditis and Prosthetic Joint Infections and Need for Dental Stewardship. Clin Infect Dis 71(2): 455-462.
- Tong D, Theis JC (2008) Antibiotic prophylaxis and invasive dental treatment in prosthetic joint patients. N Z Med J 121(1280): 45-52.
- McNally CM, Visvanathan R, Liberali S, Adams R J (2016) Antibiotic prophylaxis for dental treatment after prosthetic joint replacement: exploring the orthopaedic surgeon's opinion. Arthroplasty Today 2(3): 123-126.
- Colterjohn T, de Beer J, Petruccelli D, Zabtia N, Winemaker M (2014) Antibiotic Prophylaxis for Dental Procedures at Risk of Causing Bacteremia Among Post-Total Joint Arthroplasty Patients: A Survey of Canadian Orthopaedic Surgeons and Dental Surgeons. J Arthroplasty 29(6): 1091-1097.
- Lockhart PB, Thornhill MH, Zhao J, Baddour LM, Davis J, et al. (2020) Prophylactic antibiotic prescribing in dental practice: Findings from a National Dental Practice-Based Research Network questionnaire. J Am Dent Assoc 151(10): 770-781.
-
NS Jayalakshmi, Sheetal Saklecha and NS Harshavardhana. Dental Antibiotic Prophylaxis and Prosthetic Joint Infections: A Narrative Review. On J Dent & Oral Health. 4(2): 2021. OJDOH.MS.ID.000583.
-
Dental antibiotic prophylaxes, Oral flora, Dental procedures, Dental intervention, Dental assessments, Oral pathogens, Dental Stewardship, Periodontal tissues, Dental surgery.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






