 Research Article
Research Article
Understanding Antimicrobial Resistance and stewardship program regarding dental practice
Shaymaa Hussein Rafat Kotb*
Alazhar university, faculty of Dental Medicine, Department of Oral Medicine, Periodontology, oral Diagnosis and Dental Radiology, Assuit branch, Ministry of Health &Population, Egypt
Shaymaa Hussein Rafat Kotb, Alazhar university, faculty of Dental Medicine, Department of Oral Medicine, Periodontology, oral Diagnosis and Dental Radiology, Assuit branch, Ministry of Health &Population, Egypt.
Received Date: December 07, 2022; Published Date: December 16, 2022
Abstract
Abstract: Antimicrobials treatment a medical magical tool ‘which have a significant role on the prognosis of patients with severe infectious diseases over the past 60 years However, effectiveness of antimicrobials, it compromised by the growth and dissemination of resistant organisms. While the underlying reason behind this dynamic problem is the amount of antimicrobial use in general, massive consumption, abuse, and misuse of antimicrobials, which is influenced by several interrelated factors, that contributed to speed up the spread of resistant pathogens. Dental practitioner is one of the top specialty prescriber of antimicrobial treatment. Antimicrobial resistance is a major concern nowadays that rising the trend, impacting the whole world as leading to difficult-to-treat infections. WHO assume that there is a global role to combat the problem of antimicrobial resistance (AMR) by using antimicrobial stewardship programme.
Aim of this study:To thorough the light on the importance of antibiotic treatment and misuseof antibiotic which lead to antibiotic resistance and the newly antimicrobial stewardship programs (ASPs) that is show successful result as a preventive measures and a new guidelines in antimicrobial use to reduce antibiotic resistance.
Methodology: A systematic literature review depends on collecting data from an evidence-based studies. Searches were made of twenty electronic databases: the Cochrane Oral Health Group‘s Trials Register, The Cochrane Central Register of Controlled Trials (CENTRAL), EMBASE, PsycINFO, CINAHL, Scopus and Web of science, MEDLINE (PubMed).
Summary: Raise patient knowledge level and awareness regarding health care, healthy habits environmental impact on our health, infection control and prophylaxis concept rather than treatment, decrease antibiotic intake and so decrease incidence of antibiotic resistance. Future of medicine to direct the antibiotic producing industry to develop entirely new formulas for creating innovative antibiotics with unique action mechanisms to overcome AR.
Conclusion: Antibiotics widely prescribed prophylactically and therapeutically in dentistry. Antibiotic resistance considered a challenging global health problems, with a concerning lack of awareness from the health practitioners and the general public. there is a global attitude to the restrictive action should be taken to avoid overuse and inappropriate use of the antibiotics for treatment of patients. prescription of the antibiotics should be limited as much as possible and only used as a last resort to treat patients with microbial infection.
Keywords:Antibiotic; Antimicrobial; Antimicrobial resistance; Antimicrobial stewardship program; Patient education
Introduction
Antimicrobials one of the greatest advances in medical history, becoming an important tools for fighting against infectious diseases and life-threatening postsurgical infections. Bacterial infections are the commonest infection in dental practice; Dentist prescribe empirical antibiotics as the clinician does not know what microorganisms are responsible for the infection, since pus or exudate cultures are not commonly made. They are generally prescribed not only for acute odontogenic infection, nonodontogenic infection but also for a prophylaxis treatment for a limited periods of time. Although oral cavity hosted by broad range of organisms, but not all of them are potential pathogens. Dentist prefer to prescribe empiricale broad spectrum antibiotics to cover the wide range of organisms in oral cavity. The list of bacteria related with oral infections is relatively long (cocci, bacilli, gram positive and gram negative organisms, aerobes and anaerobes) [1].
The main target of prescribing the antimicrobial in dental practice is to provide the treatment for acute odontogenic infection, like with pulp origin as a complement to root canal treatment, in ulcerative necrotizing gingivitis, in periapical abscesses, in aggressive periodontitis, and in severe infections of the fascial layers and deep tissues of the head and neck. The preferable antibiotic for this acute condition are beta-lactam derivatives which provided with no allergies or intolerances. While in chronic situation, like chronic gingivitis or periodontal abscesses, they do not recommend antibiotic treatment (except in the presence of dissemination). Another target for prescribing antibiotic is to treat the non-odontogenic specific infections in the oral cavity (tuberculosis, syphilis, leprosy), and nonspecific infections of the mucosal membranes, muscles and fascias, salivary glands and bone. These types of infection need prolonged treatments, and the prescribed drug of choice include clindamycin, due to its capacity to reach high concentrations in bone, and fluorquinolones (ciprofloxacin, norfloxacin, moxifloxacin)-to extend the bacterial spectrum to include gram negative bacilli, gram positive aerobic cocci and, anaerobes [2, 3].
Moreover, antibiotics can be commonly used as a prophylaxis from the focal infection, and that is widely accepted in the dental profession, like in the prevention of bacterial endocarditis. Prophylaxis treatment in oral surgery with a healthy patient was also recommended in the case of the removal of impacted teeth, periapical surgery, bone surgery, implant surgery, bone grafting and surgery for benign tumors. In subjects with risk factors for local or systemic infection -including oncological patients, immune suppressed individuals, patients with metabolic disorders such as diabetes, and splenectomized patients, prophylactic antibiotic coverage should be provided before attempting any invasive procedure. Prophylaxis treatment can extend to include the traumatic injuries, that is taken to comprise the administration of antibiotics on a pre-, intra- or postoperative basis, to prevent bacterial proliferation and dissemination within and from the surgical wound [4].
Narrow spectrum antibiotic is the preferable antibiotics to be considered the first choice for AP purpose than the broad spectrum one as their drawbacks more than the benefit intended to given. Broad spectrum antibiotics play a role in spread of resistance across multiple bacterial species, and the detrimental effect they can have upon the host microbiome. Antibiotic Prescribing has to be timely administrated (30-60min preoperatively) and should not be continued after the procedure. Previous studies demonstrated that timing of AP was an important opportunity for improvement since longer durations have not shown more beneficial, and this practice may result in existence of a resistant strains and so increase incidence of raise AR [5].
Periodontal diseases is the most prevalent chronic multifactorial diseases caused by dental biofilm which constitute of multicomplex bacterial species. periodontitis shares common modifiable risk factors and social determinants with other major chronic noncommunicable diseases such as heart disease, diabetes, and hypertension. Treatment of periodontitis need to removal of the causative factor (i.e., dental biofilm) from the tooth surface. Initially, all patients are treated with non-surgical periodontal therapy where the diseased sites are treated with subgingival debridement using manual or ultrasonic instruments, individually or in combination, to eliminate the calculus which acts as a plaqueretentive factor. the European Federation of Periodontology clinical practice guidelines recommended the adjunctive use of systemic antibiotics in the treatment of periodontitis. Antibiotics are widely prescribed prophylactically and therapeutically Concurrently. Moreover unselective uses of antibiotics have contributed to the rise of antibiotic resistance [5].
As antibiotics widely prescribed prophylactically and therapeutically in dentistry so the clinical benefit observed following the adjunctive use of the antibiotics should be evaluated against possible severe risks and side effects. Antibiotic Risistance is one of side effect and considered a growing public health problem worldwide. In addition to increase antimicrobial resistance, the mutual use of systemic antibiotics has raised concerns about their impact on patients public health. They are associated with adverse events such as allergic reactions, gastrointestinal, cardiac, renal, and neurological effects. Restrictive action should be done to avoid overuse and inappropriate use of these antibiotics for treatment of patients. Hence, prescription of these antibiotics should be limited as much as possible and only used as a last resort to treat patients [6].
There is a global emergence and spread concern regarding the antibiotic resistance (AR), due to decrease the efficacy of antibiotic treatment. A R contribute in lossing the most powerful tools for fighting life-threatening infection. Antibiotic resistance (AR) is a phenomenon consisting mostly of acquired adaptative mechanisms that aid bacteria to overcome and survive the aggression caused by antibiotics. Antibiotic resistance (AR) occure due to overprescription of antibiotics, the presence of faulty infectionprevention strategies, pollution in overcrowded areas, or the misuse of antibiotics in agriculture and with animal growth, together with a decreased interest from the pharmaceutical industry in researching and testing new antibiotics due to high cost of developing ones. Antimicrobial resistance (AMR) is a global health problem in both human and veterinary medicine [7].
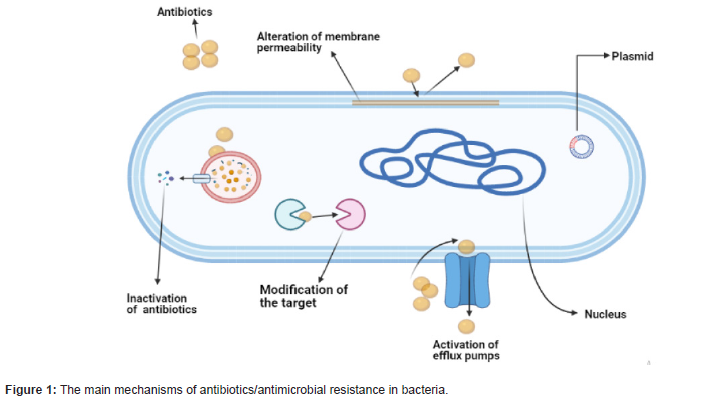
Mechanism of antimicrobial resistance is promoting the
bacteria to survive in the presence of antimicrobials treatment. This
survival is built on the ability of the bacteria to limit the penetration
of antimicrobials, modify the drug or the drug target, and express
an efflux system to reduce the drug concentration within the cells.
Bacteria possess a collection of genes that grant them resistance to
external aggressions. The resistance mechanisms specified by the
resistance genes fall into three major categories:
(i) Enzymatic inactivation,
(ii) Active efflux, or
(iii) (protection/modification/replacement of the cellular
target sites of the antimicrobial agents.
Mobile genetic elements, in particular plasmids and transposons, play a major role as carriers of antimicrobial resistance genes in staphylococci and also with other Gram-positive bacteria. This resistance develops either because of intrinsic or acquired mechanisms. The intrinsic mechanism occure when the bacteria is naturally resistant towards the antimicrobial, like the physical structure of the outer membrane of Gram-negative bacteria, which limits the penetration of antibiotic (vancomycin). Intrinsic resistance also includes the expression of general porins that efflux the antimicrobials from the bacterial cells. In contrast, bacteria can also develop antimicrobial resistance via acquired mechanisms through transfer the mobile resistance genes (for example, plasmid) from other resistance bacteria. Moreover, mutations in the gene could make changes in the drug target when the bacteria exposed to the antimicrobial [8].
World Health Organization reports that unnecessary and inappropriate antibiotics is amplifying antimicrobial resistance (AMR). Dispensing of non-prescribed antibiotics (DoNA) is a common phenomenon that promotes the spread of unneeded prescribed antibiotics in the community. Community pharmacies are considered as a main source of antibiotics‘ distribution, worldwide. The poor knowledge and lack awareness of the pharmacy staff and the inadequate supervision of pharmacies are considered a contributing factors in increase antibiotic resistance. it is estimated that 80% of medicines are distributed through community pharmacies. Inappropriate antibiotics use increases therapeutic costs so these pharmacies have the potential role to improve the rational use of antibiotics in the community [9].
Antibiotics are also broadly used in veterinary medicine and agriculture. Antimicrobials treatments are administered to livestock either as therapeutics (to treat individual animals) to improve animal health, welfare and productivity. or in a metaphylactic approach, i.e., the presence of clinical illness in a small number of animals triggers drug administration of the whole herd or flock. The slaughtering process produces large amounts of wastewater, potentially contaminated with bacteria resistant against antimicrobials. In this regard, wastewater represents a source for environmental pollution with antibiotic-resistant bacteria during wastewater treatment occurs, it has to be assumed that significant bacterial loads are present in the production areas of the slaughterhouses. This fact is alarming in two respects: first, it results in the possibility of the pathogens entering downstream water sources and, thus, the environment and the food chain and second, the pathogens pose a risk to the employees of the slaughterhouse [10].
Foods can be contaminated with antimicrobial-resistant bacteria. Salmonella organisms can survive in undercooked food and transmitted by several ways: by the presence of antibioticresistant bacteria in foods using antibiotics during agricultural production; by the presence of resistance genes in bacteria added during food processing (probiotics); and by cross contamination with antimicrobial-resistant bacteria during food processing which causing pathological conditions, such as gastroenteritis and fever and so consequences complications. At the same time, when a raw food products consumed without prior processing or preservation so a significant risk of transmitting antimicrobial resistance to humans, as the resistant bacteria present are not killed. For this reason, raw sheep‘s milk and milk products may be potentially contaminated with such bacteria that survive during production process and subsequently introduce genetic bacterial resistance in the gastrointestinal tract that transmitted to the intestinal microflora. Gut microbiota is necessary for enhence development of immune and nervous system maturation. Eating contaminated food or intaking long course of antibiotics contribute in gut dysbiosis and so the microflora of the gastrointestinal tract transfere gene of antibiotic resistant to clinical pathogens, by gene transfer mechanisms (HGT) which lead to the acquisition of resistance in recipients strains. Mutations can lead to resistance to several antibiotics. The microbe become resistant to several different antibiotics, each with distinct mechanisms of action due to the same or related genetic determinant [11].
The increase burden of antimicrobial resistant has a great impact on management of life threatening infections, therapy of immunocompromised patients and hospitalized intensive care patients, which leading to high mortality rate that exceeds 50% that contribute to serious socioeconomic and health problems. AR led to the development of coordinated and comprehensive global action plans. the implementation of antimicrobial stewardship programs (ASPs) has encouraged the promotion of the prudent use of antibiotics and minimize the emergence and spread of antibiotic resistance. the positive impact of these bundle of educational and restrictive measures to optimize and reduce the incidence of AR in the hospitals. Moreover, these measures had increased the appropriate use of antibiotic without increasing the mortality risk and these guidelines were met with a high degree of acceptance by the prescribers [12].
Antimicrobial stewardship programmes (ASPs) generally refer to specific programmes of interventions to monitor and optimize AMU at the hospital or primary care level. These ASPs developed to be effective in optimizing AMU and reducing AMR levels. Hence, appropriate measures and new guidelines should be established to address the rational use of antibiotics and to prevent their abuse, since they represent a major healthcare challenge. Moreover, the new mission of Health Systems and Policy Approach to Antibiotic Resistance Containment: Coordination, Accountability, Resourcing, Regulation and Ownership (ABR CARRO), which aims to explore and describe howtional action plans on antibiotic resistance. This approach is defined as a -collaborative, multisectoral, and transdisciplinary approach-working at local, regional, national, and global levels-to achieve optimal health and well-being of people, animals, plants and their shared environment, recognizing the interconnections between them. The ADKAR® model is an acronym for successful behavior change. Monitoring of AMU in combination with regulations shown to be effective up to a certain level. Therefore, the Awareness, Desire, Knowledge, Ability, and Reinforcement (ADKAR®) change management model. These behavior change is difficult, coaching may be helpful. Coaching is the process of helping individuals to identify their goals through non-directive questioning and interaction [13, 14].
Sweden is a northern European high-income country with a population of about 10.3 million people. Sweden is a model for the containment of antibiotic resistance. Swedish Strategy to Combat Antibiotic Resistance 2020-2023. The strategy outlines antibiotic resistance as a problem of global dimensions that affects all, focus on Sweden‘s international leadership in action against antibiotic resistance, aiming for continued engagement in advocacy and setting a good example and highlights the complexity of antibiotic resistance and its transmission between humans, animals, food, and the environment, Most stakeholders feel that a lot has been achieved in Sweden regarding antibiotic resistance containment but that there is still room for increasing awareness and emphasising changes in behaviour and attitudes among prescribers and the public. With regards to the term-One Health‖ itself, knowledge among stakeholders was relatively low [15, 16].
Diverse global effort done to encourage to change the wrong practice by using prophylaxis concept rather than the treatment. So understanding different contributing factors to the continuous growth of AR, like Social, economic, and scientific factors were helpful. Therefore, knowledge on proper hygiene is mandatory for preventing bacterial infections. Appropriate hand hygiene alongside food safety measures can reduce the number of infections and lead to lower antibiotic usage. Proper disinfection and sterilization procedures represent helpful measures against nosocomial infections in hospital -acquired multiresistant bacteria. In addition, resistant bacteria hotspots should be located and eliminated to prevent further development of bacterial reservoirs. The rise in urbanization levels leads to increased pollution and overcrowded areas and, together with other factors such as the individual‘s susceptibility and pathogen‘s virulence increase susceptability to infection. Recurrent infectious episodes lead to the evolution of multi-resistant bacteria. Usage of antibiotics in the fodder fed to livestock affects humans as well. Animal excrements containing traces of antibiotics determine an environmental pollution which cause serious human infection and so the appearance of drugresistant bacteria. another possible factor for the emergence of AR bacteria is the antibiotic-producing industry which focused on generic drugs that resemble established, instead of trying to develop new formulas. Tourism is another factor for the emergence of AR bacteria. there is a higher chance of people becoming vectors for bacteria. Through this extensive spread, bacteria are responsible for creating new reservoirs that can transform into endemic outbreaks [17, 18].
Institutional Program for the Prevention and Control of Healthcare associated Infections and Antimicrobial Stewardship in Andalusia (PIRASOA) implemented other measures during the intervention period to optimize antimicrobial use. Antibiotic consumption indicators were implemented in each department, guidelines for empirical treatment and surgical prophylaxis were updated, training sessions were conducted with the antimicrobial team, and a program was carried out to validate antibiotic treatment duration. Our results suggest that there is a positive role toward the bundle of educational and restrictive measures to optimize antibiotics use and reduce the incidence of AR. Control measures and a strong monitoring system should be urgently advocated for and implemented in Malaysia to reduce AMR emergence. In addition, further research on alternatives to antimicrobials, good animal husbandry practices, and biosecurity should be encouraged in order to replace the application of existing antimicrobials in animal health [19].
Targeted therapy is a good tool to limit the spread of resistance in clinics and hospitals. It was tailored approach to the patients and their pathology. These aspects are important from a public health point of view and could lead to decrease AR. Establishing a rational working flow and prescribing antibiotic treatment only after receiving the antibiotic susceptibility test results seem to be a good way to treat the patient specifically and avoid recruitment. A working flow was established with the entire medical staff in order to pursue antimicrobial stewardship programs. This approach enhances the common ownership of the ASP. This aspect explained the importance of having an-ASP team‖. This ASP team should be able to write specific guidelines, according to national and international regulations, dividing drugs with specific pharmacokinetic and pharmacodynamic information that are related to the different pathologies. These guidelines should be revised and updated every year and tailored to specific contexts [20].
ASP is the guide effort done to measure and improve how antibiotics are prescribed by clinicians and used by patients. Improving antibiotic prescribing and use is critical to effectively treat infections, protect patients from harms caused by unnecessary antibiotic use, and combat antibiotic resistance. Core Elements for Hospital Antibiotic Stewardship Programs assessment-2019, Accountability: Appoint a leader or co-leaders, such as a physician and pharmacist, responsible for program management and outcomes. Pharmacy Expertise (previously “Drug Expertise”): Appoint a pharmacist, ideally as the co-leader of the stewardship program, to lead implementation efforts to improve antibiotic use. Action: Implement interventions, such as prospective audit and feedback or preauthorization, to improve antibiotic use. Tracking: Monitor antibiotic prescribing, impact of interventions, and other important outcomes like C. difficile infection and resistance patterns. Reporting: Regularly report information on antibiotic use and resistance to prescribers, pharmacists, nurses, and hospital leadership. Education: Educate prescribers, pharmacists, and nurses about adverse reactions from antibiotics, antibiotic resistance and optimal prescribing [21].
Result
Table 1:Different rates of antibiotic prescription by dentists.
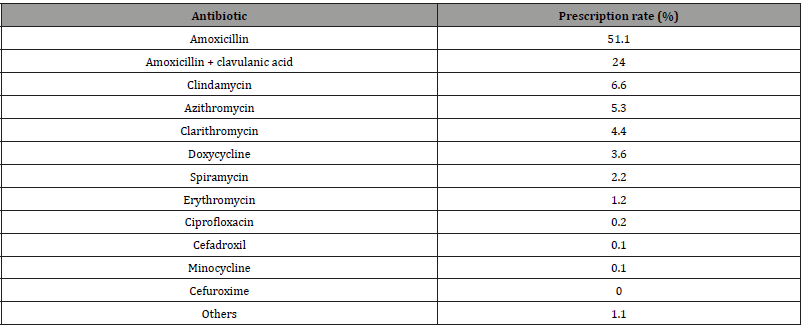
Table 2:Dispensing of non-prescribed antibiotics (DoNA).
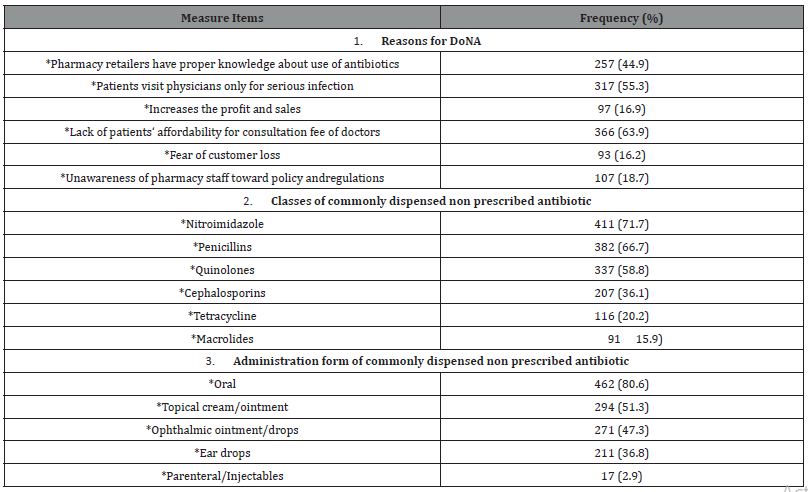
Table 3:Self-reported AP prescription in the explored dental procedures according to presence of an indication.
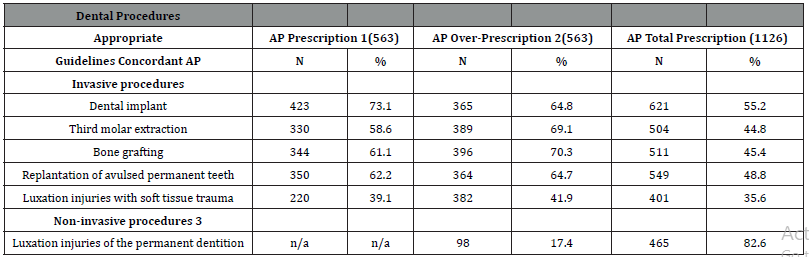
AP, antibiotic prophylaxis
1. AP prescription in high-risk patients (i.e., history of IE,
prosthetic cardiac valves, or prosthetic joint replacement in the
previous 6 months).
2. AP prescription in healthy patients.
3. Procedures without manipulation of the gingival or
periapical region of the teeth or perforation of the oral mucosa
in which AP is never indicated.
4. Eligible procedures.
Conclusion
There is a global concern toward decreasing antibiotic resistance by using the prophylactic measures for combating AR. By implementing rigorous protocols that stop unnecessary antibiotics prescription, improving hygiene and sterilization procedures. Antimicrobial therapy prescriping being empiric, directed, and prophylactic. Empiric treatment is the treatment prescriped without identifying the infected organism. Directed therapy was a treatment done after identified organisms upon the microbiological culture , Prophylaxis was the use of an antimicrobial agent to prevent the patient from acquiring an infection following surgical procedure. A high proportion of AP prescriptions before dental procedures are unnecessary. This new guidance procedure incorporate recommendations of best practice into national and local protocols/ guidelines as soon as they are established. Moreover, the empirical and broad use of AP reported is clearly no longer accepted as it does not allow for the identification of the etiology or a pathology. Specific antibiotic stewardship strategies and prescribing tools targeted to DPs should be developed, for effectiveness in improving prescribing of antibiotics for infection prophylaxis.
Recommendations
Control measures and a strong monitoring system on antibiotic prescription should be urgently advocated for implementation in order to reduce AMR emergence. Moreover, warning to farmers to enhance the awareness of the appropriate uses of antibiotics in farms. In addition, other environmentally friendly treatments alternatives such as herbal therapy, phages, and vaccination should be encouraged to apply to reduce the antibiotic resistance, improve food quality, and minimize negative impacts to human and environment.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Vallano A, Izarra A (2006) Principles of antimicrobial therapeutics. Medicine 9: 3196-203.
- Bascones Martinez A, Aguirre Urizar JM, Bermejo Fenoll A, Blanco Carrion A, Gay Escoda C, et al. (2004) Consensus document on antimicrobial treatment of odontogenic bacterial infections. Med Oral Patol Oral Cir Bucal 9: 369-376.
- Parra J, Peña A, Martínez MA, Hernández J (2006) Quinolones, sulfonamides, trimethoprim, cotrimoxazole. Medicine 9: 3538-3543.
- Martínez Lacasa J, Jiménez, Ferrás VA, Garcia Rey G, Bosom M, et al. (2006) A double-blind placebo-controlled, randosmised comparative phase III clinical trial of pharmacokinetiocally enhanced amoxicillin-clavulanate 2000/125, as prophilaxis or as treatment versus placebo for infectious and inflammatory morbidity after third molar mandibular removal. Program and abstracs of the 43rd InterScience Conference on Antimicrobial Agents and Chemotherapy. Chicago 2003. American Society for Mocrobiology, Washington DC. Citado en: Gutiérrez JL, Bagán JV, Bascones A, Llamas R, Llena J, Morales A, et al. (2006) Consensus document on the use of antibiotic prophylaxis in surgery. Med Oral Patol Oral Cir Bucal 11: 119-136.
- Melander RJ, Zurawski DV, Melander C (2018) Narrow-spectrum antibacterial agents. Medchemcomm 9: 12-21.
- Abdulkareem A, Abdulbaqi H, Gul S, Milward M, Chasib N, et al. (2022) Classic vs. Novel antibacterial approaches for eradicating dental biofilm as adjunct to periodontal debridement: An evidence-based overview. Antibiotics (Basel) 11: 9.
- Rams TE, Sautter JD, van Winkelhoff AJ (2020) Antibiotic resistance of human periodontal pathogen parvimonas micra over 10 years. Antibiotics (Basel) 9: 709.
- Watkins RR, Bonomo RA (2016) Overview: Global and Local Impact of Antibiotic Resistance. Infect Dis Clin N Am 30: 313-322.
- Reygaert WC (2018) An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol 4: 482-501.
- Hadi MA, Karami NA, Al-Muwalid AS, Al-Otabi A, Al-Subahi E, et al. (2016) Community pharmacists‘ knowledge, attitude, and practices towards dispensing antibiotics without prescription (DAwP): A cross-sectional survey in Makkah Province, Saudi Arabia. Int J Infect Dis 47: 95-100.
- Savin M, Bierbaum G, Hammerl JA, Heinemann C, Parcina M, et al. (2020) Eskape Bacteria and Extended-Spectrum-Beta-Lactamase Producing Escherichia coli Isolated from Wastewater and Process Water from German Poultry Slaughterhouses. Appl Environ Microb 86: e02748-19.
- Verraes C, Van Boxstael S, Van Meervenne E, Van Coillie E, Butaye P, et al. (2013) Antimicrobial resistance in the food chain: A review. Int J Environ Res Public Health 10: 2643-2669.
- Giacobbe DR; On behalf of the San Martino, Antimicrobial Stewardship Group; Del Bono V, Mikulska M, Gustinetti G, Marchese A, Mina F, Signori A, Orsi A, Rudello F et al. (2017) Impact of a mixed educational and semi-restrictive antimicrobial stewardship project in a large teaching hospital in Northern Italy. Infection 45: 849-856.
- Houben M, Caekebeke N, van den Hoogen A, Ringenier M, Tobias T, et al. (2020) The ADKAR® change management model for farmer profiling with regard to antimicrobial stewardship in livestock production. Vlaams Diergeneeskd. Tijdschr 89: 309-314.
- Molstad, S, Lofmark S, Carlin K, Erntell M, Aspevall O, et al. (2017) Lessons learnt during 20 years of the Swedish strategic programme against antibiotic resistance. Bull World Health Organ 95: 764-773.
- Roing M, Bjorkman I, Eriksen J, Stalsby Lundborg C (2020) The challenges of implementing national policies to contain antibiotic resistance in Swedish healthcare-A qualitative study of perceptions among healthcare professionals. PLoS ONE 15: e0233236.
- Curtis LT (2008) Prevention of hospital-acquired infections: Review of non-pharmacological interventions. J Hosp Infect 69: 204-219.
- Iskandar K, Murugaiyan J, Halat DH, El Hage S, Chibabhai V, et al. (2022) Antibiotic Discovery and Resistance: The Chase and the Race. Antibiotics (Basel) 11: 182.
- Rojo Martín MD, Peñalva G, Pinto C, Salcedo I, Fernández Urrusuno R, et al. (2018) The PIRASOA programme: Design, structure, organisation and indicators of a comprehensive regional Institutional Programme for the Prevention and Control of Healthcare-Associated Infections and Antimicrobial Stewardship for Hospitals and Primary Care Setti. WwwProtocolsIo.
- Weese JS, Blondeau J, Boothe D, Guardabassi LG, Gumley N, et al. (2019) International Society for Companion Animal Infectious Diseases (ISCAID) guidelines for the diagnosis and management of bacterial urinary tract infections in dogs and cats. Vet J 247: 8-25.
- Rohde JM, Jacobsen D, Rosenberg DJ (2013) Role of the Hospitalist in Antimicrobial Stewardship: A Review of Work Completed and Description of a Multisite Collaborative. Clin Ther 35(6):751-757.
-
Shaymaa Hussein Rafat Kotb*. Understanding Antimicrobial Resistance and stewardship program regarding dental practice. On J Dent & Oral Health. 6(4): 2022. OJDOH.MS.ID.000642.
-
Dental practice, Oral health, Dentist, Oral cavity, Non-odontogenic infection, Root canal treatment, Implant surgery, Eating contaminated food, Antibiotic resistance, Oral mucosa.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






