 Review Article
Review Article
Leveraging Human Oral-Derived Neural Crest-Derived Stem Cell Homing (hoNCSC-Homing) for Therapeutic Procedures in Regenerative Implant Dentistry-A Critical Analysis
T Fritsch1,3, M A Vukovic2 and Wolf D Grimm1,2,3*
1Salzburg NAM Research Institute, Austria
2DGParo Clinical Competence Center Hasslinghausen, Germany
3DGParo Clinical Competence Center Bayerisch Gmain, Germany
Wolf D Grimm, Salzburg NAM Research Institute, DGParo Clinical Competence Center Hasslinghausen, Germany, DGParo Clinical Competence Center Bayerisch Gmain, Germany.
Received Date:November 22, 2022; Published Date: November 28, 2022
Abstract
Resident stem cell pools in many tissues/organs are responsible not only for tissue maintenance during physiologic turnover but also for the process of bone healing following dental implant insertion. Recent advances have been made toward inducing stem cell mobilization and directing patients’ own cells to sites of interest for treating a broad spectrum of diseases in regenerative dentistry. An evolving body of work corroborates that delivering guidance cues can mobilize stem cells from the palate and drive these cells toward a specific region. In addition, the transplantation of cell-friendly biomaterials incorporating certain biomolecules has led to the regeneration of lost/damaged tissue without the need for delivering cellular materials manipulated ex vivo. Recently, cell homing has resulted in remarkable biological discoveries in the laboratory as well as great curative successes in preclinical scenarios. Here, we review using the results of an initial clinical- and histological-controlled study the biological evidence underlying in vivo cell mobilization and homing with the aim of leveraging endogenous reparative cells for therapeutic applications in dental regenerative implantology. Considering both the promise and the obstacles of this approach, we discuss the results how oral-derived neural crest-derived stem cells of the in vivo milieu can be modified to promote the native regenerative process and inspire future tissue-engineering design.
Introduction
Stem cell-based therapy has been one of the best documented approaches in regenerative medicine including the regenerative periodontology and implantology (Grimm et al. 2014) [1], promising cures for a multitude of diseases and disorders. However, the ex vivo expansion of stem cells and their in vivo delivery are restricted by the limited availability of stem cell sources, the excessive cost of commercialization, and the anticipated difficulties of clinical trans lation and regulatory approval. An alternative to adoptively transferred stem cells is cell populations already present in a patient’s body, including stem/progenitor cells, which can be actively attracted to sites of injury. This technique, known as endogenous stem cell homing, has the potential to provide new therapeutic options for in situ tissue regeneration. Such options would be less costly and complex than approaches that require substantial ex vivo cell ma- nipulation and that use artificial vehicles for cell delivery. Tissue regeneration methods that rely on endogenous stem/progenitor cell homing, local tissue responses, and functional stimulation thus offer new insights into in vivo tissue engineering and hold great promise for the future of translational medicine.
Cell homing has been regarded as a process of exit of hematopoietic
stem cells from blood vessels by transendothelization and
subsequent migration. Here we broadly define cell homing as active
recruitment of endogenous cells, including stem/progenitor cells,
into an anatomic compartment and relay on our recently published
results from the “Effect of Human Neural Crest-Derived Stem Cell
Homing (hNCSCs homing) on the Mineralization of Newly Formed
Alveolar Bone using an Allogen Bone Substitute” (Grimm et al.
2019) [2]. Cell homing offers a few advantages over cell transplantation
for vertical bone augmentation in combination with immediate
placement of implants:
•Induced homing of human oral-derived neural crest-derived
stem cells (hoNCSCs) from the palate of the patients overcomes
some of the key scientific, technical, commercialization, and
regulatory issues associated with stem cell transplantation,
such as potential contamination, excessive cost, immunorejection,
pathogen transmission, and a lack of training of current
clinicians to handle cells.
•Prior instances of regulatory approval for innovative cell products.
•Ease of clinical delivery of packaged and stored molecular delivery
products.
•Maximizes the body’s own regenerative capacity.
The purpose of this publication is to evaluate the results of our recently published initial clinical- and histopathological controlled study due to the potential of Adult Palatinum as a Novel source of stem cells in combination with transplantation of Spongiosa Bone Blocks (Osteograft®, Bingen, Germany) for one-stage bone augmentation and immediate implant placement. The aim of this patient observation study was to evaluate histologically the effect of a stem cell homing approach on the amount of newly formed bone mineralization using stem cell-supported human allogen bone as carrier material in vertical augmentation with immediate implant placement (Grimm et al. 2019) [2].
Thus, although our focus here is to describe progress in the field of homing resident reparative cells for therapeutic applications in Regenerative Implant Dentistry, the concept and design considerations that are discussed should be generally applicable to many, if not all, regenerative paradigms.
Mobilization and Homing of oral-derived Neural Crest-derived Stem Cells (oNCSCs)
In cell science and biology, the term “homing” describes the ability of stem cells to find and return to their original “niche” (the “home” for native cells), and this term has recently been used to emphasize the ability of a cell to respond to extracellular signals (e.g., migratory stimuli and guidance cues) for targeted trafficking and migration (Yin et al. 2017) [3] (Figure 1).
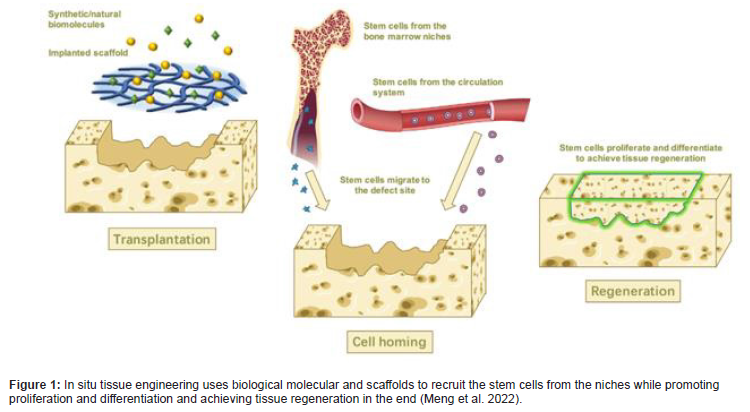
oNCSCs often referred to as the fourth germ layer, comprise a migratory, stem and progenitor cell population and are synonymous with vertebrate evolution and development (Le Douarin and Dupin, 2018) [4]. The cells follow specific paths to migrate to dif ferent locations of the body where they generate a diverse array of cell types and tissue (Achilleos and Trainor, 2014) [5]. Neural crest cells that form the vertebrate head skeleton migrate and interact with the surrounding tissues to shape the skull. The neural crest was first described as the ‘‘Zwischenstrang’ (German, ‘zwischen’: between; ‘Strang’: cord) by the Swiss anatomist and cardiologist Wilhelm His. He described the neural crest as a transient embryonic structure appearing between the neural tube and epidermis during avian embryonic development. After neurulation, embryonic neural crest cells (NCCs) migrate out of their niche and engender ectodermal cell types (e.g. Schwann cells, peripheral neurons, melanocytes and keratinocytes) in addition to mesenchymal cells (cranial bones, cartilage and fat cells). Due to this intrinsic potential to give rise to cells of two germ layers (ectoderm and mesoderm), NCCs possess an extraordinarily high developmental potential surpassed only by totipotent cells of the zygote and pluripotent embryonic stem cells (Widera et al. 2009) [6]. There are oNCSCs which are maintained in an undifferentiated state throughout the life in the oral tissues (Figure 2).
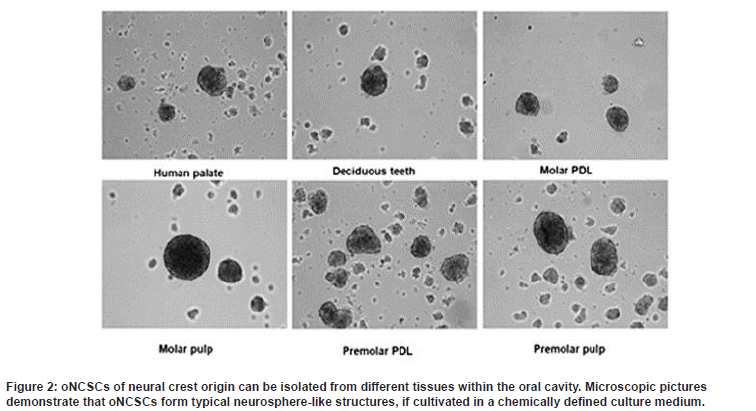
Consequently, we suggest the term oral-derived Neural Crest-derived Stem Cells (oNCSCs) as a new category that would include most adult dental stem cells described so far (see overview Grimm et al. 2011) [7].
The mechanisms underpinning the regenerative capabilities of oral-derived Neural Crest-derived stem cells were originally thought to reside in their ability to recognize damaged tissue and to differentiate into specific cell types that would replace defective cells. Although stem cell transplantation has been widely adopted for tissue repair, therapeutically modulating the stem cell niche to facilitate regeneration via a patient’s own cells is gaining an increasing amount of attention (reviewed in Lane et al. 2014) [8]. However recent work has shown that molecules produced by oNCSCs (secretome), particularly those packaged in extracellular vesicles (EVs), rather than the cells themselves are responsible for tissue repair (Haque et al 2021) [9]. Recently, regenerative outcomes of cellbased therapies have been linked to paracrine factors and extracellular vesicles [EVs] released by the transplanted cells rather than the transplanted cells themselves (Widera and Grimm 2019) [10].
Osteogenic properties of oral-derived Neural Crest-derived Stem Cells (oNCSCs)
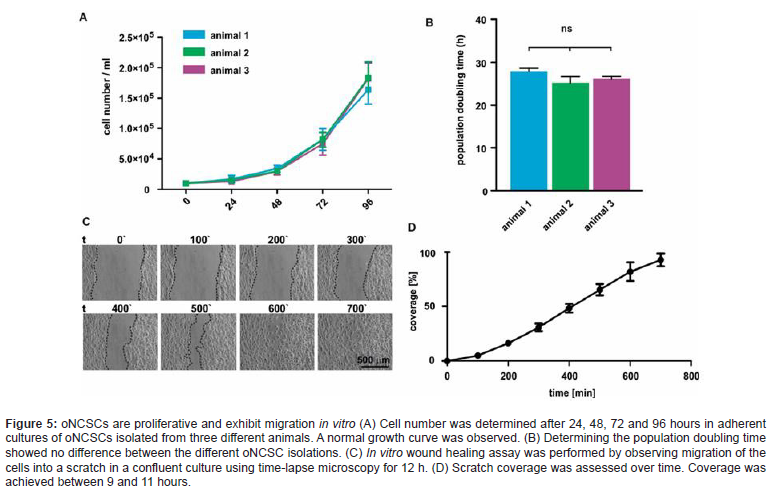
Although the mechanisms that control such endogenous regenerative processes are largely unknown, the development of cell-free strategies based on biomimetic devices and growth factor delivery has been established as a viable concept for endogenous regeneration (Zhao et al. 2015) [11]. A profound knowledge on the biological differences and shared characteristics between the oNCSC populations are critical for design and future application of regenerative (Figure 3) bone therapies (Widera et al., 1997) [12]. This unique plasticity encourages using of these cells to reconstruct lost bone tissues (Figure 3). We and other groups reported similar expression of stem cell associated cell surface antigens in all oNCSC populations (Zeuner et al., 2017) [13].
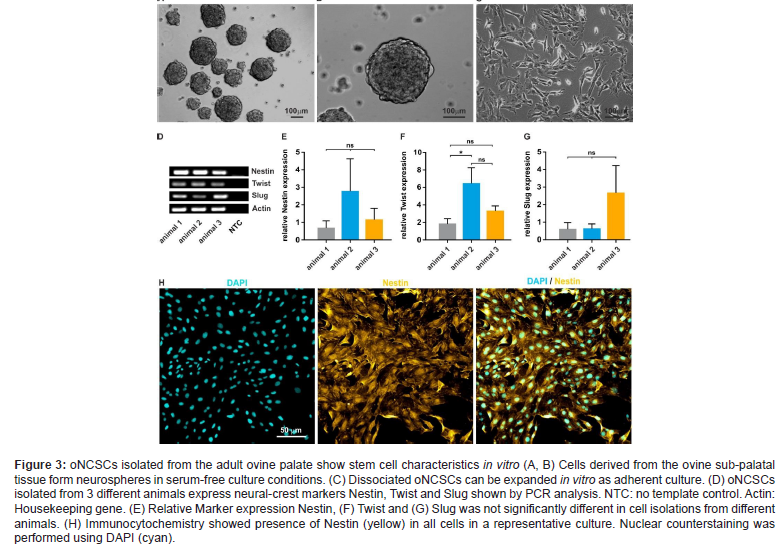
In our hands, oNCSCs express higher levels of cytokeratin 18 and 19 (CK-18 and CK-19). In addition, they seem to possess odontoblast differentiation potential, but not to be able to generate functional neurons or muscle cells. Further, oNCSCs showed faster proliferation rate and a higher telomerase activity. We also showed that oNCSCs express higher level of BMP-2 and BMP-6 mRNA. Further underlining the neural crest origin of these cells, all oNCSCs described so far have the intrinsic ability to give rise to osteogenic cell types. In particular, a combination of dexamethasone, ß-glycerophosphate and L-ascorbic acid-2-phosphate in a FCS containing medium has been widely used for osteogenic induction of various oNCSC populations. Osteogenic induction at early phase can be assessed by determination of the activity of alkaline phosphatase (ALP) or by RT-PCR or qPCR-based measurement of marker gene, o.g. Osteopontin, Osterix, Osteonectin.
Immunomodulatory properties oNCSCs
Our data also suggested that oNCSCs can be a good candidate for allogeneic SC-based therapies due to their superior immunomodulatory properties, which are regulated by different cytokines, particularly by interferon-γ (IFN-γ). These properties of oNCSCs can be beneficial for defect healing processes of oral tissues. In contrast, less information is provided about the effect of toll-like receptors ligand on immunomodulatory properties of these cells. Our data shows potential interaction between IFN-γ and TLR2 responses in hoNCSCs, which might be involved in regulation of immune response in inflammatory processes, and particularly after immediate implant insertion. Since oNCSCs themselves can be polarized into pro- and anti-inflammatory phenotypes, it is of major interest whether the pro- or antiinflammatory polarized oNCSCs secrete the anti-inflammatory cytokines. The literature data show potential interaction between IFN-γ and TLR2 responses in oNCSCs, which might be involved in regulation of immune response in acute inflammatory processes. Immuno-regulatory functions of oNCSCs were further reported in the literature (Király et al. 2009) [14]. Thus, we suggest that oNCSCs could represent a highly immunoprivileged stem cell type.
Pre-clinical large animal model allowing short term study of safety and efficacy of endogenous NCSC transplantations
According to the revised International Society for Stem Cell Research (ISSCR) [15] guidelines animal models should be used since they better simulate the human anatomy and pathology (2021). The route of administration, the optimal cell numbers and dose of biologicals can be better extrapolated to the human system based on large animal studies. In this context, sheep models offer the advantage of a body weight like humans and a significantly lon ger lifespan allowing long-term studies. In our recently published large animal studies (overview see Zeuner et al. 2017) [13], we focused on approving the standardized Alveolar Bone Defect Model in sheep, further developed as Iliac sheep model (Figure 4).
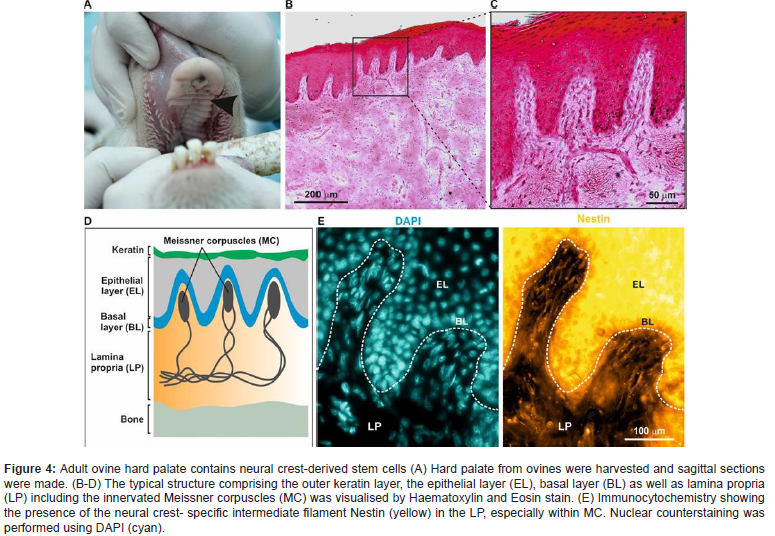
These studies included the isolation and characterization of oNCSC from sheep palate tissues and the development of adequate assessment methods for clinical and histo-pathological efficiency for evaluation of the alveolar bone regeneration process using appropriate X-ray, MicroCT, and non-demineralized histological methods. Briefly, primary ovine oral-derived neural crest-derived stem cells were obtained from the sheep’s palate for comparison with human derived oNCSC. Notably, all these oNCSC populations can be isolated and enriched using magnetic cell separation based on their expression of the cell surface marker CD271, see following chapter “CD 271-based magnetic isolation of animal and human palate-derived NCSCs”). Osteogenic potentials of ovine-derived NCSCs were evaluated in our studies by different histological measurements on non-demineralized and demineralized alveolar bone (Figure 4) tissue.
The different histological measurements on non-demineralized bone tissues used in our sheep studies have showed a high potential for evaluating the regenerative bone processes taking place by using homing processes. Based on these findings, we could have shown that ovine-derived NCSCs might serve as an appropriate cell source for Homing and for evaluating the regenerative bone processes taking place in the Sheep Models developed by our interdisciplinary and international research group.
Neural crest-derived stem cell sheets: a new cell-based strategy for bone repair and regeneration
However, the limitations of conventional tissue engineering methods (cell suspensions, scaffolds and/or growth factors) restrict its application in bone regeneration. We and other research groups have shown that oral Neural Crest-derived Stem Cells (oNCSCs), a class of adult stem cells, are considered a promising source for bone regeneration. Although combining NCSCs with biomaterial scaffolds offers an interesting clinical strategy for bone tissue engineering, the presence of the scaffolds could induce an undesirable effect on cell-cell interactions. Moreover, before the application of scaffold materials in bone tissue reconstruction, cells must be manipulated with proteolytic enzymes, such as trypsin or dispase, that degrade extracellular matrix (ECM) molecules and cell surface proteins, which can result in the cell damage and loss of cellular activity (Figure 5).
The bone has an intrinsic capacity to regenerate itself. However, if the lesion size or degree of degeneration is too severe to be regenerated via endogenous repair mechanisms, the use of stem cells provides an elegant and promising way to replace the lost tis sue or to boost the organism’s regenerative capacity. Importantly, several adult progenitor and stem cell types have been described to be able to generate bone cells. This includes osteoblast progenitor cells, mesenchymal stromal cells (MSCs) of different origin and adult neural crest-derived stem cells (NCSCs). Thus, all those cell type could be potentially applied to regenerate bone tissue after transplantation via engraftment, differentiation and functional integration (Zhao et al. 2015) [11].
Therefore, the development of alternative strategies for bone regeneration is required to solve these problems. Recently, a novel tissue engineering technology named “cell sheet” has been efficaciously utilized in the regeneration of bone, corneal, cardiac, tracheal and periodontal ligament-like tissues. The cell sheet is a layer of cells, which contains intact ECM and cell surface proteins such as growth factor receptors, ion channels and cell-to-cell junction proteins. The differentiation of neural crest cells towards osteogenic lineage is at least partly mediated by the topology. Cell-material interactions are of particularly interest in biomedical implants because the initial contact between the cells and the biomaterial can define the success of the device. Indeed, appropriate nanoscale topology has been reported to induce osteogenic differentiation of a variety of cell types including osteogenic cell lines, immature human osteoblasts, and mesenchymal stem cells (MSCs). As part of the tissue microenvironment presented to the cells, the surface morphology and chosen material are integral in this interaction and the cellular response-adhesion, spreading, migration, proliferation, and differentiation-ultimately contributing to the fate of the cells and tissue formation. Textured surface features are of specific interest because cells interact with the extracellular environment through micro- (e.g., organelles) and nanoscale (e.g., protein complexes, such as focal adhesions). When a surface demonstrates a characteristic dimension on the same order of magnitude as protein complexes up to organelles, the response of the cell can be modulated through a myriad of intracellular signaling and mechanotransduction events, leading to altered gene transcription and potentially regulating the differentiation of stem and progenitor cells, see overview Sheard et al. (2019) [16]. (Figure 5)
The use of allogeneic bone grafts with a stabilizing stem cell-containing subepithelial connective tissue graft is not limited to mature, fully mineralized bone. We chose to use stem cell-containing subepithelial connective tissue graft and allogeneic human bone. We used a sterile, highly safe (donor selection, viral testing, chemical purification, processing, and sterilization) allogeneic bone product derived from donor human bone (OsteoGraft™ block) as the carrier of our stem cell-containing soft palatal tissue. The high biological regenerative capacity of this allogeneic bone block leads to a predictable clinical and histo-pathological outcomes.
CD 271-based magnetic isolation of animal and human palate-derived NCSCs
Based on some oNCSCs migratory property, we successfully developed a separation strategy to isolate and identify a population of oral-derived neural crest-derived stem cells with neural crest stem cell features in different animal and human tissues within minimally- invasive surgical procedures (see overview Grimm et al. 2019) [2]. As we have shown these cells have a high degree of multi-potency and self-renewal capabilities, can be cultured and maintained in feeder-free adhesion conditions as monolayer cells, and also be able to grow in the suspension condition in the form of neurospheres. Taken together our results describes a readily accessible source of multipotent oNCSCs for autologous tissue engineer and cell-based therapeutic research supported by newest publications in the field (Meng et al. 2022) [21]. Culture-expanded neural stem cells (NCSCs) are used in current clinical trials, but these interventions are associated with significant costs and concerns about culture-related loss of efficacy (Figure 6). Our results showed the feasibility of a new clinical technology to obtain uncultured NCSC isolates from animal and human palatal tissue sources based on immunomagnetic selection for CD271-positive cells. MACS MicroBeads ® are 50-nm superparamagnetic particles conjugated with highly specific antibodies against the CD271 on the NCSC surface. Due to their small size, they do not activate cells or saturate cell surface epitopes. CD271, also known as LNGFR (low-affinity nerve growth factor receptor) or p75NTR, belongs to the low-affinity neurotrophin receptor and tumor necrosis factor receptor superfamily. CD271 is a known marker on neural crest-derived stem cells (Figure 6).
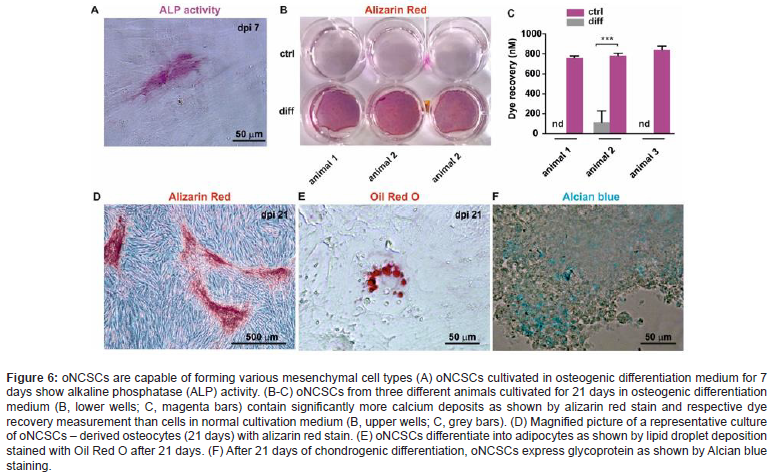
Palatal tissue was isolated and immediately processed under sterile conditions using the Rigenera® mechanical disgregator system. First, CD271+ cells were indirectly magnetically labeled with CD271 (LNGFR)-PE antibodies and anti-PE MicroBeads®. Then, the cell suspension was loaded onto a MACS® column located in the magnetic field of the MACS separator®. The magnetically labeled CD271+ cells are retained within the column. The unlabeled cells pass through; this cell fraction is thus depleted of CD271+ cells. After removing the column from the magnetic field, the magnetically retained CD271+ cells were eluted as a positively selected cell fraction. The obtained suspension rich in oNCSCs was grafted to the implant site using the allogeneic bone carrier.
Outlook and Crucial Barriers to Progress
Taken together, recent research topics in oral-derived neural
crest-derived stem cell homing-based regeneration include the following:
•developing release technologies that deliver signals to mobilize,
recruit, and dictate the behavior of endogenous oNCSCs;
•engineering therapeutic and degradable biomaterials that interact
with oral-(palate-) tissues and promote alveolar bone
defect healing;
•guaranteeing seamless biomaterial integration with the body
at the implant area;
and
•exploring stealth “sentinels” for monitoring and treating
wound healing processes after dental implant insertions and
after dental bone regenerative processes.
Isolation and prolonged expansion of oral stem cells under clinical grade, GMP-compliant conditions differentially affects “stemness” properties
Further developing safe and effective methodologies enabling the oral-(palate-)-specific homing of targeted oNCSCs subsets is pivotal for advancing in situ dental tissue engineering and regeneration to a clinical stage. A true requirement of in situ regeneration technologies and their biomedical applications is the ability to home live oNCSCs to, and accommodate them in, an engineered home, thus realizing genuine “tissue weaving.” To confirm therapeutic outcomes, special focus should be placed on the long-term evaluation of the biological and mechanical characteristics of these newly formed tissues. Continued and collaborative efforts among biologists, chemists, engineers, and clinicians in the field will initiate a new era of clinical regenerative dental medicine (Yue et al., 2022) [17].
However, we must eventually recognize that stem cell homing does not deliver a universal regenerative solution, as the recruitment of endogenous cells may be restricted in some cases by the intrinsically poor regenerative capacity of a given tissue, by the intrinsically poor migration potential of host cells in the body, and/ or by an age-associated low number of reparative cells that can be mobilized (Place et al. 2009) [18].
Secretome of oral-derived neural crest-derived stem cells (supported transplantation (oNCSCsec), a new secretome- based strategy for alveolar bone regeneration
Currently, there are no studies regarding the application of ovine NCSCs secretome per se in animal models of alveolar bone defect regeneration. Nevertheless, recently published reports from our research have shown robust effects on cellular processes that promote bone regeneration (Zeuner et al., 2017) [13]. Indeed, different studies showed that important neurotrophic factors, such as glial cell line-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), stem cell factor (SCF), and insulin growth factor (IGF), were found to be increased after NCSCs transplantation, thereby supporting NCSCs mediated effects (Haque et al. 2021) [9]. Having this in mind, it would be interesting to further explore the impact of the NCSCs secretome on the reversion of standardized alveolar bone defects, when compared to more conventional approaches like NCSCs transplantation. Following this, we will analyze the effects of ovineNCSCs secretome on bone defect regeneration in our Sheep model. To identify the soluble and EV-associated protein components potentially contributing to the effect of the ovineNCSC secretome, we will perform an ovine NCSC profiling with subsequent bioinformatic analysis, analyzing proteins in the soluble fraction in the ovineNCSCs secretome in accordance with Grimm and Widera (2019) [10], and highlighting possible new
bone regulatory molecules that mediate these actions. Primary Culture of ovineNCNCs and Collection of the Secretome ovineNCSCs cell culture will be performed as described by our international research group, and according to the protocols and strict ethical guidelines previously established and approved by the Health Research Ethics Boards of our research partners (IraSME application).
Discussion
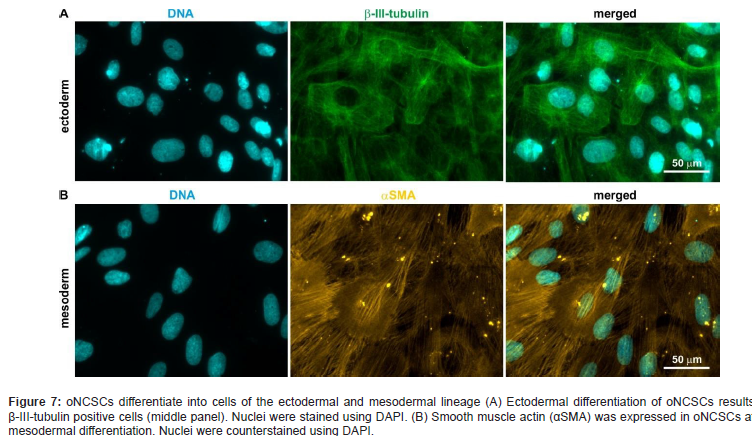
Stem cell homing plays an important role in tissue engineering. However, little is known about the proliferation and differentiation of oNCSCs on different types of materials. Several recent studies showed that oral-derived neural crest-derived stem cells can be isolated from mammalian craniofacial tissues such as periodontal ligament, dental pulp, and palate. These cells have in common that they form neurosphere-like clusters and proliferate in serum-free culture. From this perspective, the human palate has been shown to be of critical importance in the regenerative process. Although it is clear, that oNCSCs residing in the subepithelial soft tissues of the palate can achieve bone regeneration, this population is heterogenous, but it is not known which subpopulations can achieve regeneration. In 2007 our research group first describes the characterization and neuronal differentiation capacity of stem cells derived from inflamed periodontal tissues (Widera et al.).
These so called periodontal-derived neural crest- derived stem cells (pdNCSCs) were isolated by minimally invasive periodontal surgery and cultured as so-called dentaspheres under serum-free conditions (Figure 7).
Thus, we looked for a novel source of stem cells from the oral cavity being highly suited for regenerative purposes. In 2009 Widera et al. [6] described for the first time the presence of nestin-positive neural crest-derived stem cells within Meissner corpuscles and Merkel cell-neurite complexes located in the hard palate of adult Wistar rats. Characterization of oNCSCs by RT-PCR and flow cytometry revealed expression of several stem cell markers, such as Nestin and Sox2 as well as STRO-1 and CD146. Further differentiation studies proved this concept and revealed that oNCSCs exhibit an osteogenic differentiation capacity, too. The osteogenic differentiation capacity of oNCSCs was markedly developed suggesting that these cells might be a suitable cellular tool for Homing due to alveolar bone regeneration purposes.
Indeed, cells derived from palate were found to have specific properties, such as increased proliferation rates, and representative of a regenerative phenotype as shown by our research group and research groups worldwide (see overview by Moshy et al. 2020) [19].
Based on the hypothesis that the number of oNCSCs is secondary, the individual potential of oNCSCs to survive high stress and chemotactically attract osteogenic progenitor cells will dominate. As the augmentation material is far from local blood supply, it can be assumed that a natural selection procedure will be applied to the cells (Stem Cell Homing). As blood and oxygen supply in small standardized alveolar bone defects is reestablishing by yourself, the oNCSCs could then unfold their full potential. Therefore, stem cell-supported human allogen bone substitute as stem cell homing was analyzed. It was shown that the neoformation of mineralized bone occurred predominately around and adjacent to the stem cell-supported allogen bone substitute, which is consistent with the currently available literature (Yue et al., 2022 [17] Grimm et al., 2015) [20]. The histological results clearly point to the previously described osteoinductive potential of the applied neural crest-derived stem cells derived from the human palate (Figure 7).
Enhanced formation of unmineralized extracellular matrix and an enhanced mineral apposition rate adjacent to the degrading allo-gen bone scaffolds were accompanied by an increased osteoclastic bone surface, which resulted in higher bone mass and a tendency to a more mature trabecular bone structure around the allogen bone scaffolds. These results show that stem cell-supported allogen bone scaffolds induce extended peri-implant bone remodeling with a good biocompatibility. The influence of the healing time on the neoformation of mineralized bone was of special interest. Detailed descriptive evaluation of the histological samples revealed the presence of osteoid and ‘‘bone lining cells’’ as histological characteristics of mainly direct ossification. There was a close contact of the newly formed mineralized bone and the stem cell-supported allogen bone substitute after 3 months and after 4 months. Periimplant bone seemed to be less mature. Nevertheless oral-derived neural crest-derived stem cells of the in vivo milieu can be modified to promote the native regenerative process and inspire future tissue- engineering design [21, 22].
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Grimm WD, Dannan A, Giesenhagen B, Schau I, Varga G, et al. (2014) Translational research: Palatal-derived ecto-mesenchymal stem cells from human palate: A new hope for alveolar bone and cranio-facial bone reconstruction. Inter J Stem Cells 7 (1): 23-29.
- Grimm WD, N Didenko, T Fritsch, B Giesenhagen, MA Vukovic (2019) Effect of Human Neural Crest-Re-lated Stem Cell Homing (hNCSCs homing) on the Mineralization of Newly Formed Alveolar Bone using an Allogen Bone Substitute. Biomed J Sci & Tech Res 17(2).
- Yin Y, X Li, XT He, RX Wu, HH Sun, et al. (2017) Leveraging Stem Cell Homing for Therapeutic Regeneration. J Dent Res 96(6): 601-609.
- Le Douarin NM, Dupin E (2018) The "beginnings" of the neural crest. Dev Biol 444(1): S3-S13.
- Achilleos A, PA Trainor (2012) Neural crest stem cells: discovery, properties, and potential for therapy. Cell Res 22: 288-304.
- Widera D, Zander C, Heidbreder M (2009) Adult palatum as a novel source of neural crest-related stem cells. Stem Cells 27: 1899-1910.
- Grimm WD, A Dannan, S Becher, G Gassmann, WH Arnold, et al. (2011) The Ability of Human Periodontium-derived Stem Cells to Regenerate Periodontal Tissues-A Preliminary in vivo Int J Periodontics Restorative Dent 31(6): e94-e101.
- Lane SW, Williams DA, Watt FM (2014) Modulating the stem cell niche for tissue regeneration. Nat Biotechnol 32(8): 795-803.
- Haque NH, D Widera, V Govindasamyd, P Soesilawatie, NHA Kasime (2021) Extracellular Vesicles from Stem and Progenitor Cells for Cell-Free Regenerative Therapy. Curr Mol Med 21: 1-12.
- Grimm WD, D Widera (2019) Secretome of stem cells as an alternative to stem cell transplantation. Genes and Cells 14(12).
- Zhao D, Lei L, Wang S, Nie H (2015) Understanding cell homing-based tissue regeneration from the perspective of materials. J Mater Chem B 3(37):7319-7333.
- Widera D, Grimm WD, Moebius J, Piechaczek C, Gassmann G, et al. (2007) Highly Efficient neural differentiation of human somatic stem cells, isolated via minimally invasive periodontal surgery. Stem Cells Dev 16(3): 447-460.
- Zeuner M Th, L Turner, D Humphries, S Stergiadis, T Morash, et al. (2018) Isolation and Characterisation of Adult Ovine Neural Crest-Derived Stem Cells from Palatal Tissue. Front Cell Dev Biol 6 (39): 1-10.
- Király M, B Porcsalmy, A Pataki, K Kádár, M Jelitai, et al. (2009) Simultaneous PKC and cAMP activation induces differentiation of human dental pulp stem cells into functionally active neurons. Neurochem Int 55(5): 323-332.
- International Society for Stem Cell Res (ISSCR) (2021) Version 1.0.
- Sheard JJ, Bicer M, Meng Y, Frigo A, Aguilar RM, et al. (2019) Optically Transparent Anionic Nanofibrillar Cellulose Is Cytocompatible with Human Adipose Tissue-Derived Stem Cells and Allows Simple Imaging in 3D. Stem Cells Int 2019: 3106929.
- Yue C, J Cao, A Wong, JH Kim, S Alam, et al. (2022) Human Bone Marrow Stromal Cell Exosomes Ameliorate Periodontitis. J Dent Res 101(9): 1110-1118.
- Place ES, Evans ND, Stevens MM (2009) Complexity in biomaterials for tissue engineering. Nat Mater 8(6): 457-470.
- Sara El Moshy, Israa Ahmed Radwan, Dina Rady, Marwa M S Abbass, AA El Rashidy, et al. (2020) Dental Stem Cell-Derived Secretome/Conditioned Medium: The Future for Regenerative Therapeutic Applications. Stem Cells Int 1-29.
- Grimm WD, B Giesenhagen, S Hakki, I Schau, S Sirak, et al. (2015) Translational Research and Therapeutic Applications of Neural Crest-Derived Stem Cells in Regenerative Periodontology. Curr Oral Health Rep.
- Meng L, Wei Y, Liang Y, Hu Q, Xie H (2022) Stem cell homing in periodontal tissue regeneration. Front Bioeng Biotechnol 10: 1017613.
- Yu M, Luo D, Qiao J, Guo J, He D, et al. (2022) A hierarchical bilayer architecture for complex tissue regeneration. Bioact Mat 10: 93-106.
-
T Fritsch, M A Vukovic and Wolf D Grimm*. Leveraging Human Oral-Derived Neural Crest-Derived Stem Cell Homing (hoNCSC-Homing) for Therapeutic Procedures in Regenerative Implant Dentistry-A Critical Analysis. On J Dent & Oral Health. 6(3): 2022. OJDOH.MS.ID.000640.
-
Dental regenerative implantology, Periodontology, Implant Dentistry, Immuno-regulatory functions, Human anatomy, Oral Neural crest derived stem cells, NCSCs transplantation, Dental pulp, Invasive periodontal surgery, Peri-implant bone.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






