 Research Article
Research Article
Effects of Various Cleansing Methods on Bond Strength of Contaminated Zirconia, Lithium Disilicate and Metals
Liang Chen*, Chenye Zheng, Mengmeng Tao, Jialing Liu , Menglu He and Guohao Li
Changzhou Key Laboratory of Oral Disease Prevenetion and Treatment, China
Liang Chen, Changzhou Key Laboratory of Oral Disease Prevenetion and Treatment, 28 Yandanghe Road, Xuejia Xinbei, Changzhou, Jiangsu, China.
Received Date:March 18, 2024; Published Date:April 01, 2024
Abstract
PURPOSE: The purpose of this study is to investigate the effects of various cleansing methods on bond strengths of contaminated zirconia, lithium disilicate, and metal. MATERIALS AND METHODS: Zirconia and titanium metal blocks were air-abraded with alumina sand (50μm) for 20 seconds, and lithium disilicate blocks were etched with hydrofluoric acid etchants for 20 seconds. The specimens were rinsed with water, dried, and contaminated with saliva, blood or oil for 1 minutes. Ten groups of each restoration material were studied: 1) no contamination (NC); 2-4) saliva contamination followed by ultrasonic cleaning in water (SW), ultrasonic cleaning in ethanol (SE), or cleaning with a cleaning agent DashClean (SD); 5-7) blood contamination followed by ultrasonic cleaning in water (BW), ultrasonic cleaning in ethanol (BE), or cleaning with DashClean (BD); 8-10) oil contamination followed by ultrasonic cleaning in water (OW), ultrasonic cleaning in ethanol (OE), or cleaning with DashClean (OD). Zirconia and metal groups were treated with an MDP-containing primer, and lithium disilicate group was treated with a silane primer. All groups were subjected to shear bond strength test. RESULTS: For zirconia bonding, SW, SE, BW, BE, OW and OE were significantly lower than NC, SD, BD, or OD (p<0.05); for lithium disilicate bonding, SW and OW were significantly lower than NC, SD, BD, or OD (p<0.05); for metal bonding, SW, SE, OW and OE were significantly lower than NC, SD, BD, or OD (p<0.05). CONCLUSION: Within the limitations of this in vitro study, ultrasonic cleaning in water or ethanol were not able to remove the negative effects on resin bond strength of contaminated zirconia, lithium disilicate or metal, while the cleaning agent DashClean was effective in removing saliva, blood, and oil contamination and enhancing the resin bond strength.
Keywords:Cleaning; Zirconia; Lithium disilicate; Metal; Dental bonding; Saliva; Blood; Oil
Abbreviations:No contamination (NC); Saliva contamination followed by ultrasonic cleaning in water (SW), Ultrasonic cleaning in ethanol(SE), or Cleaning with DashClean (SD); Blood contamination followed by ultrasonic cleaning in water (BW), Ultrasonic cleaning in ethanol (BE), or cleaning with DashClean (BD); Oil contamination followed by ultrasonic cleaning in water (OW), Ultrasonic cleaning in ethanol (OE), or Cleaning with DashClean (OD)
Introduction
In contemporary restorative dentistry, the durability and longevity of prosthetic restorations are paramount considerations. The successful adhesion of restorative materials to tooth structures relies heavily on the bond strength achieved between the materials and the substrate. However, achieving optimal bond strength can be compromised in clinical scenarios where contamination occurs, such as during try-in procedures, saliva or blood exposure, or handling by clinicians.
Among the diverse range of restorative materials utilized in modern dentistry, zirconia, lithium disilicate, and metal alloys stand out for their mechanical properties, biocompatibility, and/or esthetics. Zirconia, a ceramic material known for its high strength and biocompatibility, has gained popularity in recent years for its applications in fixed dental prostheses [1-3]. Lithium disilicate, another ceramic material, offers excellent esthetics and mechanical properties, making it a preferred choice for anterior restorations [4-6]. Metal alloys, such as titanium metal, on the other hand, have long been used in dentistry for their durability and strength, particularly in implant and posterior restorations [7-8].
Durable and optimal bonding protocol has been established for zirconia, lithium disilicate and metal through chemically bonding. Airborne particle abrasion followed by a special phosphate monomer-containing primer provides long-term durable resin bonds to zirconia and metal [9-13]. Hydrofluoric acid etching followed by silane coupling agent provides a strong and reliable resin bonds to lithium disilicate [11, 14].
Contamination during try-in or handling by clinicians of these materials prior to bonding can significantly compromise the bond strength [15-18]. leading to clinical failures such as debonding, microleakage, and ultimately, restoration failure. Saliva protein or phosphate ion in blood chemically bonds to restoration surface, which occupy the reaction site for ceramic chemically bonding. Thus, degradation of ceramic chemically bonding occurs. Silicone oil deposits on the restoration surface, which change the wettability and surface free energy of the restorations, resulting in low bond strength. To mitigate the adverse effects of contamination, various cleansing methods have been studied and utilized in clinical practice [15-18].
According to the previous studies, air-abrasion, alkaline solution (such as sodium hydroxide or potassium hydroxide) cleaning, or sodium hypochlorite solution cleaning, was able to remove contamination and resulted in successful bond strength recovery\ between resin and zirconia [15-18]. The mechanism of cleansing is either using physical method (air-abrasion) to physically remove the contamination or using chemical method (alkaline solution or hypochlorite solution) to break down the chemical bond between contaminants and zirconia. However, previous studies mainly focus on saliva contamination of zirconia, few studies have been conducted on the contamination effects on bonding of lithium disilicate or metal, and few studies have been done on blood contamination effect on bond strength of dental restorations. Oil contamination of zirconia, metal and lithium disilicate has never been studied.
This research endeavors to investigate the effects of various cleansing methods on the bond strength of zirconia, lithium disilicate, and metal alloys, which are contaminated by saliva, blood, or oil. By elucidating the efficacy of different cleansing protocols, this study aims to contribute valuable insights to clinical practice, informing clinicians’ decisions in selecting the most effective cleansing methods to optimize bond strength and enhance the longevity of prosthetic restorations.
Materials and Methods
The composition of bonding agents and cleaning agent are listed in Table 1. Lithium disilicate ceramic blocks (IPS e.max, Ivoclar Vivadent, Schaan, Lichtenstein) and zirconia blocks (Upcera, shenzhen, China) were sintered according to manufacture’s instructions. The bonding surfaces of lithium disilicate, zirconia, and titanium blocks (Ruike, Baoji, China) were wet-polished with 400-grid SiC paper, cleaned with pressurized water and dried with oil-free air. Zirconia or titanium block were air-abraded with alumina particles (size 50μm) at distance of 10mm under 0.2 MPa for 20 seconds, rinsed with water, and air dried. Lithium disilicate blocks were etched with a hydrofluoric acid etchant DashEtch (DentDash Medical Technology, Changzhou, China) for 20 sec, rinsed with water, and air dried.
Table 1:The composition of materials using in the study.
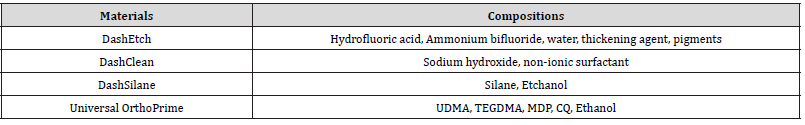
For each restoration material, the specimens were divided randomly into 10 groups (Table 2). Group 1 received no contamination (NC), and served as a positive control. The group 2-10 were contaminated with saliva (provided by tester), synthetic blood (Phygene Life Sciences, Fuzhou, China) or silicone oil (Azsmooth, Japan) for 1 minute. The contaminated specimens were cleaned with the following methods: ultrasonic cleaning in water for 1 minute (SW, BW, OW), ultrasonic cleaning in ethanol for 1 minute (SE, BE, OE), or cleaning with a cleaning agent DashClean for 20 seconds (SD, BD, OD). All of the specimens were then rinsed with water and dried with oil-free air. Zirconia and metal (titanium) groups were treated with an MDP-containing primer Universal OrthoPrime (DentDash Medical Technology), and gently air dried with oil-free air. Lithium disilicate group was treated with a silane primer DashSilane (DentDash Medical Technology), and gently air dried with oil-free air.
Table 2:the bonding protocols for each group.
The shear bond strength specimens were prepared according to ISO 29022 (Dentistry-Adhesion-Notched edge shear bond strength test). For each group, 10 resin composite specimens (Valus Plus, A2, 3M, Minnesota, USA) with size of 2.38 mm in diameter and less then 2mm high were fabricated on the bonding surface of zirconia, lithium disilicate or metal blocks, using an ultradent jig mold (Ultradent, Utah, USA). The resin composite specimens were lightcured with an LED light cure unit (CuringPen, Sifary, Changzhou, China) at 1000 mw/cm2 for 40 seconds.
The cured specimens were immersed in 37℃ water in an oven
for 24±1 hours prior to the shear bond strength test. The shear
bond strength test was performed with a universal testing machine
(FBS-500N, FURBS, Xiamen, China) with a cross-head speed of 1
mm/min. The shear force was applied to the bonding specimens
until fracture occurred. The data were analyzed by one-way ANOVA
and Tukey Test with Statistics Kingdom. All de-bonded specimens
were analyzed with a light microscope (RuiHoge, Nanjing, China)
using 20x magnification to grossly categorize the modes of failures
into 3 types as follows:
1) Adhesive: the failure occurred at the interface between
two different surfaces;
2) Cohesive: the failure occurred within the material;
3) Mixed failures: the failure occurred in both adhesive and
cohesive with at least 25% of either type.
Results
Table 3 summarizes the shear bond strength results. For zirconia bonding, SW (6.2 MPa), SE (8.7 MPa), BW (17.6 MPa), BE (18.0 MPa), OW (16.5 MPa) and OE (16.5 MPa) were significantly lower than NC (24.4 MPa), SD (24.8 MPa), BD (25.0 MPa), and OD (24.3 MPa) (p<0.05); for lithium disilicate bonding, SW (15.0 MPa) and OW (17.3 MPa) were significantly lower than NC (24.0 MPa), SD (24.5 MPa), BD (27.0 MPa), or OD (23.8 MPa) (p<0.05); for metal bonding, SW (18.0 MPa), SE (18.6 MPa), OW (18.3 MPa) and OE (19.4 MPa) were significantly lower than NC (25.4 MPa), SD (25.3 MPa), BD (24.1 MPa), or OD (24.7 MPa) (p<0.05). For each restoration material, the bond strength of DashClean group is not significantly different from the positive control (no contamination) (p>0.05).
Table 3:The mean shear bond strength (MPa) and standard deviation (in parentheses) of each group, n=10.
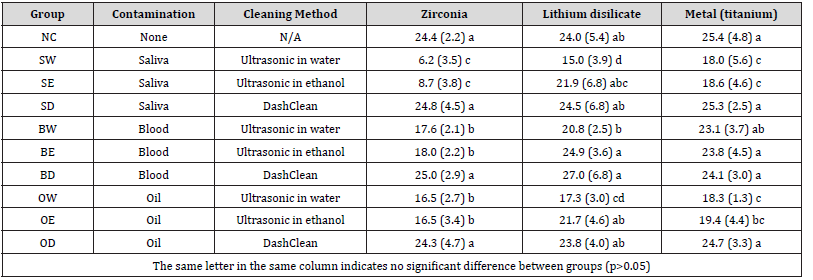
Abbreviations: No contamination (NC); saliva contamination followed by ultrasonic cleaning in water (SW), ultrasonic cleaning in ethanol (SE), or cleaning with DashClean (SD); blood contamination followed by ultrasonic cleaning in water (BW), ultrasonic cleaning in ethanol (BE), or cleaning with DashClean (BD); oil contamination followed by ultrasonic cleaning in water (OW), ultrasonic cleaning in ethanol (OE), or cleaning with DashClean (OD).
Table 4 summarized the type of failure modes of each shear bond strength test group. For zirconia bonding, NC, SD, BE, BD, OW, OD demonstrate the higher frequency of mixed failure when compared to adhesive failures. For lithium disilicate bonding, most of the groups except for OW show the higher frequency of mixed failures. For metal bonding, all of the groups show the higher frequency of mixed failures.
Table 4:Type of failure modes of each shear bond strength test group.
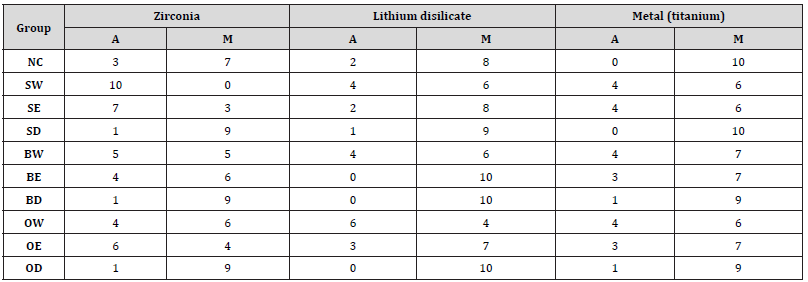
Abbreviations: Adhesive failure (A), Mixed failure (M).
Discussion
In recent years, the optimal bonding protocol has been established for zirconia, lithium disilicate and metal. Airborne particle abrasion followed by a special phosphate monomercontaining primer provides optimal resin bonds to zirconia and metal [9-13]. Studies show there is a chemical bond between phosphate monomers and zirconia [12]. Saliva contains phosphoprotein and phosphate ions which would chemically bond to zirconia, occupying the bonding site for phosphate-monomer containing-zirconia primer. This is why the bond strength of saliva contaminated zirconia is much lower than that of non-contaminated zirconia. This study shows ultrasonic cleaning in water or ethanol after saliva contamination, could not increase the bond strength back to that of non-contaminated zirconia, suggesting ultrasonic cleaning in water or ethanol was not able to break the chemical bond between saliva’s phosphate ion (or phosphoprotein) and zirconia. DashClean contains sodium hydroxide as one of the active ingredients. Sodium hydroxide solution was able to break the zirconia-phosphate chemical bond. Thus, it was effective in removing saliva contamination and enhance the resin-zirconia bond. Blood also contains small amount phosphate ions, which is probably the reason why blood contamination reduces the zirconia bond strength. In this study, after cleaning with DashClean, the bond strength of blood-contaminated zirconia is not significantly different from that of non-contaminated one, indicating that sodium hydroxide-based cleaning agent is able to remove the negative effect of blood contamination. For zirconia bonding, oil contamination also significantly reduces the zirconia bond strength. This is probably because oil deposits on the restoration surface, which change the wettability and surface free energy of the restorations, adversely affecting the reaction between zirconia primer and zirconia. This study shows ultrasonic cleaning in water or ethanol was not able to recover the bond strength completely, suggesting that it can’t clean off completely the residual oil on zirconia surface (OW, OD). DashClean is able to recover the bond strength, indicating it removes the oil contamination completely, probably because DashClean contains a surfactant, which helps to remove the oil contamination.
For lithium disilicate bonding, the result of this study shows that SW (saliva contamination followed by ultrasonic cleaning in water) has a statistically lower mean shear bond strength value compared to NC (non-contamination). The result obtained in this study is in agreement with the previous study [19]. Etching with hydrofluoric acid etchant results in microporosities of lithium disilicate surface, which has an increased surface roughness and a very high surface energy [20]. The various ions and proteins contained in saliva are trapped in the microporosities of etched lithium disilicate, which may not be completely removed by ultasonification in water. The residual ions and/or protein retained in the microporosities may decrease wettability and surface free energy of the restoration, which decreases the bond strength of lithium disilicate. Cleaning with DashClean (SD) recovered the bond strength, suggesting either sodium hydroxide or surfactant contained in DashClean helps to remove the contamination from the microporosities of lithium disilicate. Similarly, oil contamination followed by ultrasonication cleaning in water (OW) results in a lower mean shear bond strength than the positive control, indicating that ultrasonication in water could not completely remove the oil trapped in the microporosities of lithium disilicate. DashClean is able to remove it completely (OD), possibly due to its surfactant.
For the metal bonding, saliva contamination and oil contamination significantly decrease its bond strength. Ultrasonic cleaning in water or ethanol could not remove completely saliva contamination, probably because the phosphate ions or phosphoprotein are adhering or chemically bonding to metal surface, which is so stable that would not come off by simply water or ethanol cleaning. Cleaning with a sodium hydroxide-based agent DashClean (SD) results in a bond strength same as the positive control, meaning sodium hydroxide is able to break down the bonding between contaminants and metal. On the other hand, oil contamination followed by ultrasonication cleaning in water (OW) or ethanol (OE) results in a lower mean shear bond strength than the positive control, indicating that ultrasonication in water or ethanol could not completely remove the oil on the metal surface. Cleaning with DashClean results in a bond strength not significantly different from the positive control (OD), possibly because its surfactant helps to remove the oil contamination.
Conclusion
Within the limitations of this in vitro study, ultrasonic cleaning in water or ethanol were not able to remove the negative effects on resin bond strength of all of the contaminated zirconia, lithium disilicate or metal, while the sodium hydroxide based-cleaning solution DashClean was effective in removing saliva, blood, and oil contamination and enhancing the resin bond strength.
Acknowledgement
Thanks to DentDash Medical Technology for providing bonding materials and Sifary Medical Technology for providing the curing unit for using in the study.
Conflict of Interest
No conflicts of interest regarding the publication of this manuscript.
References
- Sadowsky SJ (2020) Has zirconia made a material difference in implant prosthodontics? A review. Dent Mater 36(1): 1-8.
- Sulaiman TA, Suliman AA, Abdulmajeed AA, Zhang Y (2024) Zirconia restoration types, properties, tooth preparation design, and bonding. A narrative review. J Esthet Restor Dent 36(1): 78-84.
- Ban S (2023) Development and characterization of ultra-high translucent zirconia using new manufacturing technology. Dent Mater J 42(1): 1-10.
- Zarone F, Di Mauro MI, Ausiello P, Ruggiero G, Sorrentino R (2019) Current status on lithium disilicate and zirconia: a narrative review. BMC Oral Health 19(1): 134.
- Govare N, Contrepois M (2020) Endocrowns: A systematic review. J Prosthet Dent 123(3): 411-418.
- Chen Y, Yeung AWK, Pow EHN, Tsoi JKH (2021) Current status and research trends of lithium disilicate in dentistry: A bibliometric analysis. J Prosthet Dent 126(4): 512-522.
- Koizumi H, Takeuchi Y, Imai H, Kawai T, Yoneyama T (2019) Application of titanium and titanium alloys to fixed dental prostheses. J Prosthodont Res 63(3): 266-270.
- Hanawa T (2020) Zirconia versus titanium in dentistry: A review. Dent Mater J 39(1): 24-36.
- Alammar A, Blatz MB (2022) The resin bond to high-translucent zirconia-A systematic review. J Esthet Restor Dent 34(1): 117-135.
- Lima RBW, Silva AF, da Rosa WLO, Piva E, Duarte RM, et al. (2023) Bonding Efficacy of Universal Resin Adhesives to Zirconia Substrates: Systematic Review and Meta-Analysis. J Adhes Dent 25(1): 51-62.
- Chen L, Suh BI (2012) Bonding of resin material to all-ceramics: a review. Current Research in Dentistry 3 (1) 7-17.
- Chen L, Suh BI, Brown D (2012) Bonding investigation of primed zirconia ceramics for evidence of chemical bonding and improved bond strengths. Am J Dent 25(2): 103-108.
- Chen L, Suh BI, Kim JR, Tay FR (2011) Evaluation of silica-coating techniques for zirconia bonding. Am J Dent 24 (2): 79-84.
- Araújo Neto VG, Nobre CFA, Freitas MIM, Lima RBW, Sinhoreti MAC, et al. (2023) Effect of Hydrofluoric Acid Concentration on Bond Strength to Glass-Ceramics: A Systematic Review and Meta-Analysis of In-Vitro Studies. J Adhes Dent 25(1): 231-240.
- Feiz A, Rastghalam N, Swift EJ (2022) Effect of different cleansing methods on the artificially aged bond strength of resin to contaminated zirconia: A systematic review. J Prosthodont 31(9): e125-e137.
- Thammajaruk P, Guazzato M, Naorungroj S (2023) Cleaning methods of contaminated zirconia: A systematic review and meta-analysis. Dent Mater 39(3): 235-245.
- Silva NRD, Araújo GM, Vila Nova TEL, Bezerra MGPG, Calderon PDS, et al. (2022) Which Zirconia Surface-cleaning Strategy Improves Adhesion of Resin Composite Cement after Saliva Contamination? A Systematic Review and Meta-Analysis. J Adhes Dent 24(1): 175-186.
- Chen L, Gleave C, Fuessle G, Suh BI (2017) Removal of Zirconia’s Saliva Contamination with Z-Clean. CED-IADR, Austria.
- Nikolaus F, Wolkewitz M, Hahn P (2013) Bond strength of composite resin to glass ceramic after saliva contamination. Clin Oral Investig 17(3): 751-755.
- Puppin Rontani J, Sundfeld D, Costa AR, Correr AB, Puppin Rontani RM, et al. (2017) Effect of Hydrofluoric Acid Concentration and Etching Time on Bond Strength to Lithium Disilicate Glass Ceramic. Oper Dent 42(6): 606-615.
-
Liang Chen*, Chenye Zheng, Mengmeng Tao, Jialing Liu , Menglu He and Guohao Li. Effects of Various Cleansing Methods on Bond Strength of Contaminated Zirconia, Lithium Disilicate and Metals. On J Dent & Oral Health. 7(5): 2024. OJDOH.MS.ID.000675.
-
Restorative dentistry, Prosthetic restorations, Tooth structures, Modern dentistry, Cleansing protocols, Restoration material, Zirconia bonding, Metal bonding, Saliva contamination
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






