 Research Article
Research Article
Allergic Reaction: Etiology, Pathogenesis with Advanced Vision in Therapeutics Modalities
Shaymaa Hussein Rafat Kotb*
Al-Azhar university, Faculty of Dental Medicine, Department of Oral Medicine, Periodontology, Assuit branch, Ministry of Health & population, Egypt
Shaymaa Hussein Rafat Kotb, Al-Azhar university, Faculty of Dental Medicine, Department of Oral Medicine, Periodontology, Assuit branch, Ministry of Health & population, Egypt.
Received Date: November 14, 2022; Published Date:November 22, 2022
Abstract
Immunity is the cornerstone of the strenthening performance of the body health system. the immune system is the defense system of the body against any pathogens. Regulation of the immune system through the sympathetic and parasympathetic arms of The autonomic nervous system. Allergic reaction is an inappropriate immune response to antigen. Allergy is not a disease itself, but a mechanism leading to a disease. Allergic inflammation is a key contributor to several common diseases, including allergic asthma, allergic rhinosinusitis, atopic dermatitis and eosinophilic esophagitis, multiple sclerosis, rheumatoid arthritis, bullous pemphigoid and various types of cancer, which characterized by coordination between the innate and adaptive immune systems to promote type 2 (T2) inflammatory responses. Dietary habit and gut microbium considered the blamed factors contributing to this allergy.
Objective: to thorough the light on the correlation of daietry nutrition, gut microbiome, and intestinal immune systems, in the development of
allergy.
Conclusion: There is a sort of strong association between disbiosis in gut microbium, dietiary substances and resultant inflamatory allergic
disease which influence on defence mechanism of body and produce a cascede of events so modulate immune response. The cornerstone cells in this
reaction IgE and mast cell, where stimulate mediators such as histamine to produce urticaria and other immune response. There is a new approach
to use biological immune inhibitory therapy and antioxidant dietary nutrient to help modulate immune response.
Keywords:Immune system allergy; Anaphylaxis, Hypersensitivity reaction; IgE; Immunoglobulin; Mast cells; Innate immunity; Adaptive immunity; Dietary habits
Introduction
Allergy is an abnormal sensitivity to an ordinarily harmless substance, called “allergen” that produces a hypersensitivity reaction in response to the immune system’s detections of the substance’s presence. Hypersensitivity can be classified into four types; namely, type I (Immediate), type II (antibody-mediated), type III (immune complex-mediated), and type IV (cell-mediated or delayed-type) hypersensitivity. Type I hypersensitivity or allergy, the most common immune disorder, is mainly mediated immunoglobulin (Ig)E and mast cells. This reactions result in symptoms that manifest themselves within very short time periods may take hours to manifest after the immune stimulus. It may cause anaphylaxis, food allergy, angioedema and asthma, which consider as life threatening condition [1].
Diet has a significant role in the risk of allergic diseases. Dietary materials produces bioactive metabolites which influenced on gut microbium. The human gut harbours hundreds of microbial species. These metabolites influence host immune responses through the interplay with regulatory T (Treg) and dendritic cells. Therefore, the composition of the intestinal microbiota potentially links with the development of allergic diseases. Based on the microflora hypothesis, Prevotella species are the essential microorganisms for the development of immune system. The bundance of Prevotella in adults may serve as a surrogate marker for early life exposure to this microorganisms which are essential for immune system development. The development of allergies contributes to an unhealthy microbiota composition that is attributable to urbanisation or westernisation diet habits [2, 3].
the oral and gut microbiomes have a bidirectional interaction that mutually shape and/or reshape the microbial ecosystem of both habitats, finally modulating physiological and pathological processes in the GI system. Pathogenesis of oral microbium in gingivitis, periodontitis and dental caries reveals that oral microbiome possesses fascinating role affecting human systemic health beyond the oral cavity. Actually, a large variety of oral species can reach the intestinal microbiota through swallowing, these bacteria seems to colonize in the gut cause a dysbiosis. Anyhow, severe diseases and genetic susceptibility of the host may promote ectopic colonization of oral bacteria. The large amounts of swallowed dead bacteria from the mouth may stimulate several pathogens in the gut and create a new phenotype by upregulation of bacterial virulence genes and increased cytotoxicity [4].
Allergic diseases are the most common non-communicable diseases globally which mediated by the complex interplay between the innate and adaptive immunity. Allergens are initially recognized by the innate immune system, triggering downstream responses leading to production of type 2 effectors, before the initiation of adaptive immunity. This hypersensitivity reactions is a complex syndrome caused by set of intracellular signaling events triggered by a broad array of environmental allergens, including fungi, insects and mites or inhalation of a variety of antigens in susceptible, food and sensitized individuals. Climate change can invoke allergic diseases by a variety of mechanisms. Early life exposure to air pollution increases sensitization to aeroallergens and food allergens, Due to the effect of T -helper 2 (TH2)-promoting adjuvant which promotes eczema and worsens asthma [4, 5].
The innate immune system is the first line of defense against environmental agents. The epithelium layer constitutes a key component of this system, providing a physical and immunemodulatory barrier. Epithelium has a pleotropic role in promoting the development of allergic responses. After epithelium activation by environmental allergens it release a range of mediators promoting inflammation, barrier dysfunction and mucosal remodeling. Activation begin by stimulating release the ripoptosome, which is a protein complex that functions as a rheostat in cell death signaling. Also it secrete IL-33, an IL-1 superfamily alarmin cytokine which mediated activation of innate lymphoid type 2 (ILC2) cells, Th2 cells, neutrophils, mast cells and eosinophils [6].
IL-33 is a cytokine from the IL-1 family that is released by the epithelium and resides in the cell nucleus bound to chromatin. IL-33 functions as an epithelial alarmin that can be passively released in a full-length, precursor form (pIL-33) in response to cell necrosis or injury and coordinates the immune response after epithelial damage. IL-33 signals through its receptor, suppressor of tumorigenicity 2 (ST2), and coreceptor, IL-1 receptor accessory protein (IL-1RAcP), which are expressed by multiple key immune cells, including type 2 T helper cells, group 2 innate lymphoid cells, mast cells, eosinophils and macrophages, and stimulates a T2 inflammatory response by promoting the production of IL-4, IL-5 and IL-134 [7].
The Inflammatory Response stimulate huge numbers of macrophages and monocytes. Macrophages, monocytes and endothelia cells had the great regulatory function on fibroblasts. Fibroblasts show increase persistance at epithelial mesenchymal transition (EMT) signature. fibroblasts had significantly higher activity of adipogenesis and glycolysis than other cell types. fibroblasts exhibited greater activity of angiogenesis and Inflammatory Response signatures. Macrophages and fibroblasts exhibiting increase expression of chemokines and Cytokine signatures [8].
Responses of innate immune to microorganisms, elicite mast cells without prior experience of the infection. They directly activated through Toll-like receptors (TLRs)by pattern-recognition receptor (PRR) and release products that have direct bactericidal activity, enhance host defence mechanisms and kill pathogens. Tolllike receptor, can also activate the ripoptosome that is consider as intracellular molecular signaling platform triggering type 2 innate immune responses [9].
Myeloid cells is the cornerstone cells which express pattern recognition receptors (PRRs) that recognize both the pathogen and the damaged associated molecular patterns, which stimulate polarized T helper (Th) cell responses. Dendritic cell-expressed PRRs (for example, TLR) which implicated in recognizing allergen after the primary breach of the mucosal barrier. Myeloid cells sensing allergens respond secondary to effectors produced by mucosal epithelial cells, thus inducing an indirect response via PRR-dependent mechanisms [10].
Mast cells are tissue-resident haematopoietic cells which play a crucial role in host defence against certain parasites, bacteria and venoms. Mast cells are the major tissue source of histamine, which activates its primary receptor, the H1 receptor, to elicit a broad range of responses that cause the symptoms related to allergic and other inflammatory diseases, including urticaria. They can detect potential threats and respond to them due to several key features, due to their strategic location at barrier tissues, such as the skin and the mucosa of the gut and airways; their high level of heterogeneity and plasticity; their survival for months or years within the tissue; and their expression of a wide range of receptors and production of a broad array of mediators with different biological activities. The functions of mast cells and other type 2 immune cells, including eosinophils, are regulating cellular responses such as cell differentiation, growth and cytokine production [11].
Mast cells has a double end sword. “The light side “of mast cells that is contribute to adaptive immune responses by binding antibodies through Fc receptors. Mast cells can be ‘pre-armed’ with antibodies against venoms and bacteria, which enables them to respond rapidly to subsequent exposures to these challenges. The mast cells consider as the most potentially dangerous cells of the human body due to its capacity to mount an immediate response and to release a plethora of potent immune mediators. Activation of mast cells can lead to chronic inflammation and pathogenic tissue remodelling. However, pathological activation of mast cells can lead to rapid death via anaphylactic shock. When mast cell activities are not directed at protecting us from threats, they typically hurt us and make us sick. This is ‘the dark side’ of the mast cell and contributes to a wide range of diseases [12, 13].
Mast cell receptors is a unique and crucial for IgE-independent mediator release which activated by a broad range of exogenous and endogenous substances. Immunoglobulin E (IgE) antibodies are the main mediators of allergic disorders but also provide protection against infections in both mice and humans. They also play a role in host defense against venoms, and cancer in mice. Serum IgE concentrations are tightly regulated in healthy individuals and elevated in various disease conditions, including autoimmunity, immuno-deficiency. IgE receptors stimulate B cells which rapidly class-switched into IgE isotype in the germinal centers and prone to differentiate into short-lived plasma cells. As IgE class-switching requires IL-4, CD8 T cells to be memory-like and the frequency of NKT2 cells is highest in the thymus [14].
Recent studies show that, Serum IgE levels are affected by the intestinal microbiome and dietary antigens, that thymusderived IgEs increase the numbers of mast cells (MCs) in the gut, so enhancing allergic immune responses. The level of homeostatic IgEs correlates with the number of MCs in the gut. Survival and activation of MCs are enhanced by IgE bound to FcεRI receptors on their surface, independent of its antigen reactivity. Thymusderived IgEs increase mast cells in the gut, which correlated with the severity of anaphylactic responses. The critical role of thymic Plasma Cells is maintaining homeostasis between serum IgE levels and mast cells [15, 16].
Mechanisms of systemic anaphylaxis is mediated by two ways: a classic pathway involving IgE and mast cells and an alternative pathway involving IgG and macrophages. This can be illustrated as, IgG blockage, switched to IgE, inhibited FA. A recent study showed that a topic skin release IL33 which increase in the gut and stimulate IL-4, which induces the proliferation of mast cells. In this report, expanded MCs promoted FA in an IgE-independent manner, suggesting the number of pre-existing MCs could be a critical risk factor of FA. Because IgE-deficient mice show a dramatic reduction of MCs in the gut, we speculated that thymic IgE would promote FA by increasing the number of MCs in the gut before antigenic challenge [17].
Mast cells release many mediators, Histamine is one of them which significantly contribute to urticaria, mastocytosis and allergic diseases via secretion of this mediator. Mast cells are a major source of PGD2, a lipid mediator that has been implicated in urticaria and allergy via its action on CRTh2, a chemoattractant receptor homologous molecule expressed on T-helper 2 cells (TH2 cells). Mast cells also a major source of IL-17 in patients with inflammatory diseases, including asthma, CSU and cancer. Thus, inhibition of the signs and symptoms of a disease by antihistamines suggests a role for mast cellsin allergic diseases. There is also evidence to support a role for mast cells in other chronic inflammatory diseases and cancer. For example, high numbers of mast cells and elevated levels of mast cell-derived mediators are seen in the tissues and blood of patients with multiple sclerosis, rheumatoid arthritis, bullous pemphigoid and various types of cancer and correlate with the intensity of inflammation in some of these diseases [18].
Drugs targeting inhibitory receptors on mast cells are powerful tools to help us understand their roles in disease. mast cell-targeted treatments can teach us about the role of mast cells in human disease and, specifically, where mast cells are critical disease drivers, where mast cells are pathogenically relevant but not critical, where they are bystanders and where they provide protection from getting sick or help control disease progression. These drug mechanism act on: Blockage of mast cell mediators, Inhibition of mast cell activation, Mast cell silencing and Mast cell depletion [19].
Blocking mast cell-activating receptors can help define the role of mast cells in diseases. For example, the anti-IgE antibodies omalizumab and ligelizumab and the BTK inhibitor fenebrutinib indicated the importance of the IgE-FcεRI pathway of mast cell activation in CSU. Omalizumab is the first highly effective anti-IgE mAb approved for the treatment of moderate and severe asthma and antihistaminedrugs. It is consider as monoclonal antibodies and small molecules that can specifically inhibit mast cell degranulation via key receptors (such as FcεRI), that block specific signal transduction pathways involved in mast cell activation that silence mast cells via inhibitory receptors or that reduce mast cell numbers and prevent their differentiation by acting on the mast/ stem cell growth factor receptor [20].
Antihistamines could be used to enhance immunotherapy responses in certain patients with cancer. patients who took antihistamines while receiving immunotherapy for cancer showed improved survival, with further indicating that histamine might limit antitumour immune responses through its effects on macrophage activation [21].
Dietary antioxidants may considered adjunctive therapy that play a key role in the prevention of allergic diseases. Since the systemic oxidative stress enhances infammatory responses relevant to allergy. The fruits and vegetables that contain several antioxidants protect against allergic diseases. Vitamin A and carotenoids are the most effective antioxidants. Vitamin A is essential for multiple functions in the human body, including embryonal development, good vision, epithelial diferentiation, and maintenance of immune function, especialy in the diferentiation of naïve T cells. Without retinoic acid, a metabolite of vitamin A, transforming growth factor beta (TGF-β) promotes the diferentiation of naïve T cells into T helper 17 (T17) cells, which are involved in infammation, autoimmunity, and allergic disorders. Copper, is another essential trace metal, that is involved in iron metabolism, antioxidant activity, and immune function. Copper is essential for humans but toxic at high levels; and therefore, both copper deficiency and excess can produce adverse health effects. Copper deficiency influences the oxidant defence system, resulting in increased oxidative damage to lipids, DNA, and proteins. Similarly, the toxic effects of copper at high concentrations are related to the generation of oxygen free radicals, and excess copper enhances lipid peroxidation and DNA damage [22, 23] (Figures 1-3).
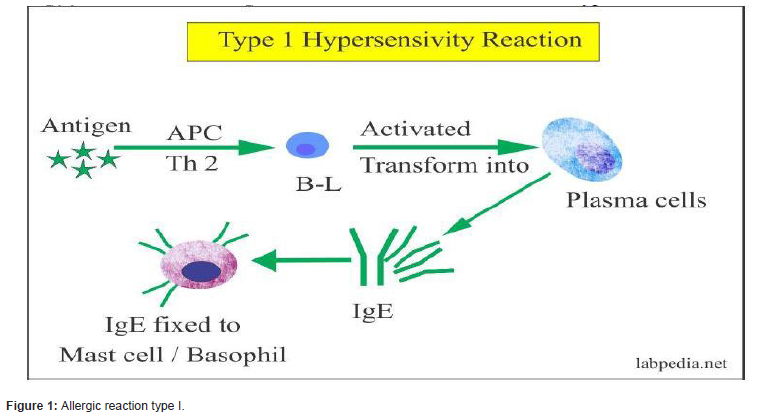
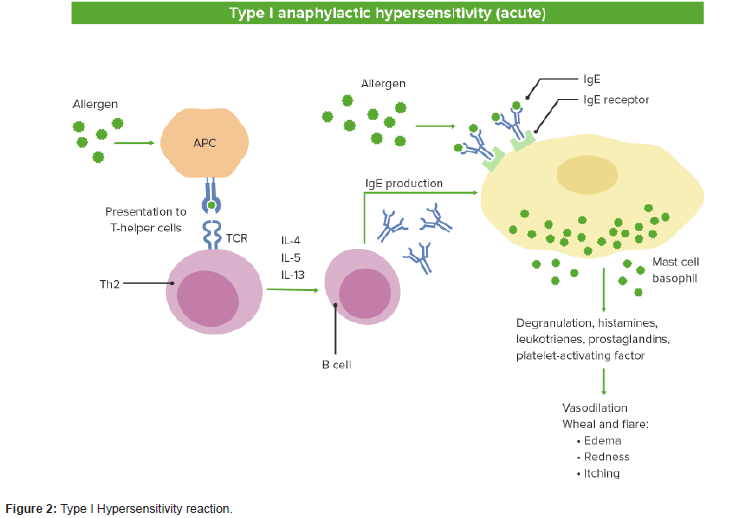
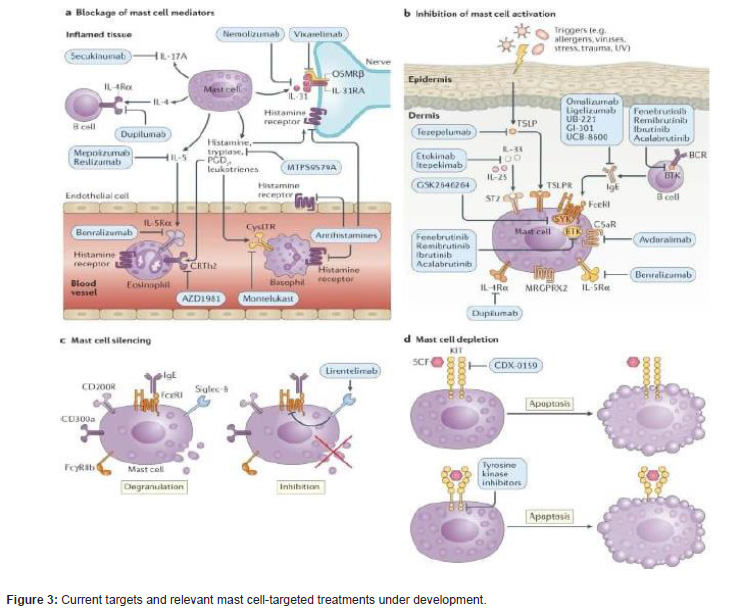
Box 1 | Bona fide mast cell-driven diseases: Urticaria
Urticaria presents with itchy wheals or angioedema, or both. It is considered a mast cell-driven disease with secondary pathogenic contributions of other cells, such as eosinophils and basophils. Chronic urticaria persists longer than 6 weeks and is classified as chronic spontaneous urticaria (CSU) and chronic inducible urticaria (CIndU) 54. In the latter disease, symptoms are triggered by a specific and definite factor, for example cold (in cold urticaria), pressure (in delayed pressure urticaria) or physical exercise (in cholinergic urticaria).
Mastocytosis
Mastocytosis is characterized by increased numbers and accumulation of neoplastic mast cells in different organs with symptoms of mast cell mediator release, for example pruritus and anaphylaxis. Mastocytosis is classified as cutaneous mastocytosis, systemic mastocytosis and mast cell sarcoma 152. KIT mutations, mostly D816V, are the key drivers of the proliferation and maturation of mast cell progenitors in the bone marrow. In indolent systemic mastocytosis (ISM), mast cell accumulation is usually limited to the sites of
physiological mast cell presence-that is, bone marrow, skin and gastrointestinal tract-and organ function is not affected. Advanced forms of systemic mastocytosis-for example, mast cell leukaemia-are associated with secondary and tertiary mutations, for example in TET2 and JAK2, in addition to KIT mutations. Mast cells accumulate in organ systems where they are usually absent or minor-for example, lymph nodes, bones, spleen and liver-leading to organ dysfunction and poor prognosis.
Mast cell activation syndrome
Mast cell activation syndrome is a debilitating heterogeneous chronic mast cell-mediated disorder. It is diagnosed in patients who present with multiple mast cell mediator-induced symptoms but in whom differential diagnosis including systemic mastocytosis has been excluded. The reasons for mast cell activation and the exact pathomechanisms of mast cell activation syndrome are still poorly investigated
Box 2 | Major advances in understanding of human mast cells
functions in disease we have learned from mast cell-targeted
treatments:
•Antihistamines: mast cells are the major source of histamine
in tissues and significantly contribute to urticaria, mastocytosis
and allergic diseases via secretion of this mediator.
•Anti-IgE therapies: the IgE-FcεRI pathway of mast cell activation
is crucial in urticaria and allergic diseases. FcεRI-mediated
mast cell activation is associated with IgE autoantibodies in a
subpopulation of patients with chronic spontaneous urticaria
(CSU).
•BTK inhibition: FcεRI signalling associated with BTK activation
in mast cells is linked to the development of symptoms of CSU.
•Silencing therapies: IgE-independent pathways of mast cell
activation can be important in a subpopulation of patients
with CSU refractory to omalizumab and responsive to antisialic
acid-binding immunoglobulin-like lectin 8 (anti-Siglec-8)
mAb treatment. Mast cells might be involved in eosinophilic
gastrointestinal diseases. The beneficial effects of anti-Siglec-8
treatment may relate to its combined effects on eosinophils and
mast cells.
•Mast cell depletion therapies: mast cells are the main players
in mastocytosis and urticaria. These therapies may also help
decipher the contribution of mast cells to other diseases [24].
Conclusion
Immune system is the defence mechanism of our body against any pathogens. Allergy consider as dysfunction in immune system. There is a recent approach suggested that dietary habits and gut-microbium and intestinal mucosal contribute to this allergic inflamatory diseaes. This hypothesis based on oral-gut microbium affected by dietary nutrients that made bioactive metaboliate which affect on intestinal mucosa and begin a sequence of reactions precepitate allergic inflamatory disorders which influence host immune responses. The prime cells in this allergic reaction IgE and mast cells as they drive the diseases. There is a new treatment approach to use the drugs targeting immune inhibitory receptors on mast cells to block mast cell mediators and Inhibit mast cell activation. Another adjunctive way to use dietary antioxidant to empower immune system response against this allergic reaction.
Recommendation
Eating healthy diet, enrich with vitamins, fibers and protiens that help in enhancement the immune system, Avoid soft drinks, Drink plenty water, Adequate sleep time, Avoid junk food, Avoid spicy food, Avoid smoking, finally recommendation of healthy lifestyle.
Competing Interests
The authors declare that they have no competing interests.
Ethical Considerations
Not applicable.
References
- Trompette A (2009) Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature 457: 585-588.
- Grizotte Lake M (2018) Commensals suppress intestinal epithelial cell retinoic acid synthesis to regulate interleukin-22 activity and prevent microbial dysbiosis. Immunity 49: 1103-1115.e6.
- Chiu CY (2019) Gut microbial dysbiosis is associated with allergen-specific IgE responses in young children with airway allergies. World Allergy Organ J 12: 100021.
- Rodriguez Herrero E, Boon N, Pauwels M (2017) Necrotrophic growth of periodontopathogens isa novel virulence factor in oral biofilms. Sci Rep 7 (1): 1107.
- Costabel U, Miyazaki Y, Pardo A, Koschel D, Bonella F, et al. (2020) Hypersensitivity pneumonitis. Nat Rev Dis Prim 6: 65.
- Jain A (2020) T cells instruct myeloid cells to produce infammasome independent IL-1β and cause autoimmunity. Nat Immunol 21: 65-74.
- Lambrecht BN, Hammad H (2012) The airway epithelium in asthma. Nat Med 18: 684-692.
- Brusilovsky M (2021) Nat Immunol.
- Simpson E L (2016) Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 375: 2335-2348.
- Zhang L, Wang Y, Wu G, Xiong W, Gu W, et al. (2018) Macrophages: friend or foe in idiopathic pulmonary fibrosis? Respir Res 19: 170.
- Wills Karp M (2012) Trefoil factor 2 rapidly induces interleukin 33 to promote type 2 immunity during allergic asthma and hookworm infection. J Exp Med 209: 607-622.
- Wernersson S, Pejler G (2014) Mast cell secretory granules: armed for battle. Nat Rev Immunol 14: 478-494.
- Starkl P (2020) IgE effector mechanisms, in concert with mast cells, contribute to acquired host defense against staphylococcus aureus. Immunity 53: 793-804.
- Galli SJ, Tsai M (2012) IgE and mast cells in allergic disease. Nat Med 18: 693-704.
- McCoy KD (2006) Natural IgE production in the absence of MHC Class II cognate help. Immunity 24: 329-339.
- Perera J (2016) Self-antigen-driven thymic B cell class switching promotes T cell central tolerance. Cell Rep 17: 387-398.
- Bryce P J (2004) Immune sensitization in the skin is enhanced by antigen independent effects of IgE. Immunity 20: 381-392.
- Min HS (2011) MHC class II-restricted interaction between thymocytes plays an essential role in the production of innate CD8(+) T cells. J Immunol 186: 5749-5757.
- Tu JF (2016) Mast cells comprise the major of interleukin 17-producing cells and predict a poor prognosis in hepatocellular carcinoma. Medicine 95: e3220.
- Kerr SC (2020) An anti-Siglec-8 antibody depletes sputum eosinophils from asthmatic subjects and inhibits lung mast cells. Clin Exp Allergy 50: 904-914.
- Okayama Y (2020) Roles of omalizumab in various allergic diseases. Allergol Int 69: 167-177.
- Maurer M (2013) Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med 368: 924-935.
- Nurmatov U, Devereux G, Sheikh A (2011) Nutrients and foods for the primary prevention of asthma and allergy: Systematic review and meta-analysis. J Allergy Clin Immunol 127: 724-733.
- Bost M (2016) Dietary copper and human health: Current evidence and unresolved issues. J Trace Elem Med Biol 35: 107-115.
-
Shaymaa Hussein Rafat Kotb*. Allergic Reaction: Etiology, Pathogenesis with Advanced Vision in Therapeutics Modalities. On J Dent & Oral Health. 6(3): 2022. OJDOH.MS.ID.000639
-
Oral cavity, Dental caries, Oral bacteria, Food allergens, Mucosal barrier, Junk food, Immune system, Barrier tissues, Pathogenic tissue remodelling, Immunoglobulin E.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






