 Review Article
Review Article
What are the Effects of the Cycle Ergometer on Critical Patients in the Intensive Care Unit? Systematic Review
Monizze Rocha de Souza1,2, Milena Santos Peixoto1,2,3, Gilson Rosa de Jesus1,2,3, Cynara Leticia Ferreira Sacramento1,2, Luisa Santos Mercês1,2, Maria Gabriela Da Silva Magalhães1,2, Raeli Sales Santana1,2 and Sidney de Souza Oliveira1,2,3,4*
1Academic League of Cardiorespiratory Physiotherapy (LAFCaR), Santo Antônio de Jesus, Brazil
2Faculty of Business Sciences (FACEMP), Santo Antônio de Jesus, Brazil
3Complete Rehabilitation Center (CMO), Santo Antônio de Jesus, Brazil
4Metropolitan Union of Education and Culture (UNIME), Lauro de Freitas, Brazil
Sidney de Souza Oliveira, Praça Renato Machado, Santo Antônio de Jesus, Bahia, Brazil.
Received Date:July 08, 2020; Published Date: July 27, 2020
Abstract
The cycle ergometer is a device that has been widely used in Intensive Care Units, its main feature is the ability to provide peripheral muscle strength gain, improve the circulatory system, assist in mobility and have great effects on the optimization of patients’ cardiorespiratory system that are in bed. This study aims to describe the benefits with the use of the cycle ergometer and the possibility of reducing the time of mechanical ventilation and the length of hospital stay. This is a systematic review of the literature carried out with randomized clinical trials, using the Medline, PubMed, BVS, SciELO and PEDro databases, using the descriptors intensive care unit, respiratory exercise, early ambulation, physical therapy, exercise physical exercise, ergometry and their correlates in English and Spanish. The results included only original articles published between 2009 and 2019. 08 manuscripts made up the discussion of this work. When evaluating the use of the cycle ergometer in the ICU, it could be highlighted that the training with early exercises in highly sick patients, had a significant improvement in the recovery of the functional exercise capacity, proprioception of the functional state, muscle strength after discharge, decreased the time of hospitalization, assisted in ventilatory weaning and optimized breathing.
Keywords: Intensive care unit; Respiratory exercise; Early walking; Physical therapy; Physical exercise; Ergometry
Introduction
Intensive Care Units (ICUs) have as main objective to provide life support to potentially serious patients [1]. There are several factors that contribute to the worsening of these patients, such as long period under Mechanical Ventilation (MV) and treatment with the use of drugs, evolving to a condition of muscle weakness and atrophy that can occur quickly and aggressively. In addition, patients on MV have a great loss of functional capacity, resulting from decreased oxidative capacity of skeletal muscles and decreased muscle perfusion, determining factors in strength and peripheral muscle function [2], being caused by a decrease in the supply of essential energy substrates for the functioning or alteration of skeletal muscle fiber, resulting in a decrease in strength and resistance [3,4]. Both osteoarticular and musculoskeletal and cardiorespiratory factors will be predisposing to increase MV time in these patients [5,6]. Among the severe functional deficiencies that affect these patients, immobility is the most common, causing several systemic changes and implying their recovery, increasing mortality or presenting several complications after their discharge from hospital. These deleterious consequences caused by immobilization can be reversed or improved with the performance of physiotherapy, aiming at the preservation of muscle mass and a better prognosis [7,8].
The early intervention of the physical therapist in critically ill patients is essential to avoid staying in the hospital and the respective risks caused by immobilization. The benefits presented by the therapeutic applicability have been evidenced in the literature, with the real need for assistance from the early kinesiotherapeutic resource, in order to avoid further dysfunctions and anticipate hospital discharge [9]. There are several techniques and devices described in the literature and being used early by the physiotherapist, in order to reverse or minimize the loss of muscle strength in these patients. New technologies have resulted in equipment for active or passive cycling of lower limbs while in bed, allowing early intervention in critically ill patients, improving functional status [10]. The cycle ergometer is described as a bedside ergometric bicycle [11] that is used to perform active, passive and resistance exercises, bringing benefits and can assist in the process of functional recovery and reduction in hospitalization time [12] and that seen being used in ICU in early mobilization increasing and improving functional capacity, muscle strength and decreasing hospital stay [13].
Several studies have investigated the applicability of this device in patients in the postoperative (PO) period of cardiac surgery, under MV and with several respiratory complications, they have demonstrated relevant benefits in the recovery of peripheral and cardiorespiratory muscle strength [14]. The main feature of this device is the ability to provide peripheral muscle strength gain, improve circulatory status, assist in mobility and improve the optimization of the cardiorespiratory system and can be used in the initial phase of functional and physical rehabilitation, aiming at restoring muscle strength, increased range of motion, clinical stability and improved cardiorespiratory function and conditioning [15]. The task force of the European Respiratory Society and the Europe Society of Intensive Care Medicine Task Force on Physiotherapy for Critically Ill Patients, stated about the effectiveness of this device at an early stage, as long as the cardiorespiratory evaluation for its use is judicious [6]. The subjective effort perception scale (PSE), also known as the Borg scale and the OMNI-Resistance Exercise Scale (OMNI-RES), were thoroughly authenticated to assess the exercise intensity, monitoring the effort exerted and clinical variables such as heart rate (HR), maximum tidal volume (CV), maximum oxygen consumption (VO2max), lactic acid concentration and ventilatory limits, the most used for exercise prescription on the cycle ergometer [16-18]. There is also the OMNI-Cycling Scale for Perception of Effort (OMNI-PE) which had its validity studied both in children and in adults, in carrying out different types of activities, such as: exercise on a cycle ergometer, exercise against resistance, walking and climbing stairs [19-21]. In view of the above, this study sought to evaluate the effects of the cycle ergometer in patients bedded in the ICU, on MV or with some respiratory complication, as well as in the significant improvement of the respiratory mechanics, the cardiovascular system, the gain of peripheral muscle strength, decreased time of admission and MV of these patients.
Methodology
Systematic literature review, carried out with original analytical and descriptive articles published between 2009 and 2019 in the Medline, PubMed, BVS, SciELO and PEDro databases using the descriptors intensive care unit, respiratory exercise, early ambulation, physical therapy, physical exercise, ergometry and its correlates in English and Spanish. The following inclusion criteria were used: a) randomized clinical studies; b) studies with participants who used the cycle ergometer in the ICU; c) studies that analyzed hemodynamic changes during application of the technique and as exclusion criteria: a) studies that did not meet the main objectives of the research; b) studies involving the use of the cycle ergometer outside the ICU; c) case series; d) pilot study that did not evaluate clinical intervention of the cycle ergometer in patients in the ICU; e) review articles, abstracts of dissertations and academic theses.
Result
Table 1: List of scores of selected articles, according to the PEDro scale.
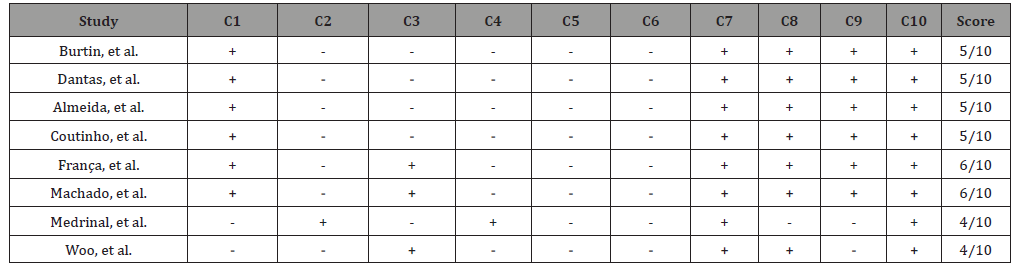
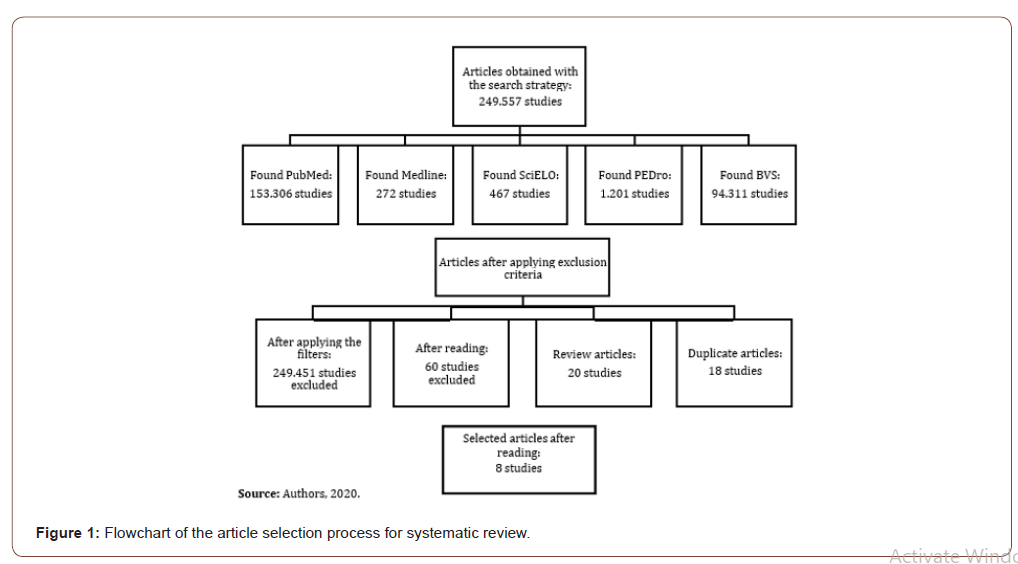
249,557 studies were found, of which 153,306 were in the Pubmed database, 272 articles in Medline, 467 in the SciELO database, 1,201 studies found in PEDro and 94,311 articles in the BVS database. In order to approximate the findings to what the research proposed, the following filters were used: randomized clinical studies, full text, year of publication (2009 to 2019). From the defined filters, 106 articles remained. Of these, abstracts were read, and 80 articles were excluded because they did not meet the eligibility criteria. The remaining 26 were obtained in full text, of which 18 were excluded because they were duplicates of another database. Thus totaling 8 articles, 4 in English and 4 in Portuguese, 3 found in the PubMed database, 4 in SciELO and 1 in the BVS, as shown in (Figure 1). The articles selected for this review were evaluated on the PEDro scale according to the quality indicators of the evidence presented and are shown in (Table 1). Participants: There were a total of 236 participants in the 8 studies, with an average age ranging between 31 and 82 years old, of both sexes, 144 men and 92 women. Interned in the hospital environment, with different clinical diagnoses, on mechanical ventilation, with stable hemodynamics and under the care of hospital physiotherapy.
Intervention
The early mobilization program in the studied patients included passive and active mobilization, changes from supine to sedation, functional electrical stimulation and use of the cycle ergometer, in addition to highlighting the importance of multiprofessional intervention for the insertion of intervention protocols. It was noted that most of the patients evaluated, reported high satisfaction after performing the cycle ergometer and described improvement in functional independence after the applicability of the device. It was also observed, a reduction in the hospitalization time after frequent early mobilizations, an increase in the strength of the peripheral muscles, an increase in the functional capacity, as described in (Table 2).
Discussion
The use of early physical exercise in critically ill patients for the prevention of atrophy and improvement of muscles with consequent improvement in the length of stay in the ICU and functional improvement has expanded rapidly in the past years. When evaluating the benefits of the cycle ergometer in the ICU, it could be highlighted that training with early exercises in highly ill patients, had a significant improvement, in addition to recovering the capacity for functional exercise, proprioception of the state of functionality and muscle strength after hospital discharge, contributes decreasing the length of hospital stay for patients, thus reducing higher costs for the hospital unit. It has the ability to cause cardiorespiratory repercussions, increased functional capacity, improved functional self-perception and quadriceps strength, with a high degree of acceptance and their preference to move their legs freely through the cycle ergometer.
A study carried out at the ICU of the University Hospital Gasthuisberg, Leuven, Belgium. It aimed to assess whether a daily exercise session, using the cycle ergometer, is a safe and effective procedure in preventing or reducing damage caused by immobilization and length of stay, divided into CG = 36 patients and IG = 31 patients, all in critical condition and use of MV with prolonged stay above 7 days in the ICU. The groups were submitted to respiratory physiotherapy with 01 daily session of active and passive kinesiotherapy in lower limbs and upper limbs. The is IG received, in addition to respiratory physiotherapy, 01 daily session of passive or active physical training, depending on individual conditions, of 20 minutes with the aid of a cycle ergometer in lower limbs. The CG performed only conventional physiotherapy, 05 times a week. The results at hospital discharge showed an increase in the distance covered in the 6-minute walk test, in the quadriceps isometric strength, recovery of functional exercise capacity and in the subjective feeling of functional well-being in the IG [22]. Corroborating the study above, other authors conducted a study aiming to evaluate the effects of an early mobilization protocol with the use of the cycle ergometer on the peripheral and respiratory muscles of critically ill patients admitted to the ICU and MV. The volunteers were divided into a conventional physical therapy group (CPTG = 14) receiving a daily service, 05 times a week, of passive mobilization in the lower and upper limbs, being optimized for active-assisted exercises according to the patient’s improvement and collaboration, and early mobilization group (EMG = 14) who received a systematized early mobilization protocol, 02 times a day, every day of the week which consisted in addition to passive mobilization of lower and upper limbs, the use of the cycle ergometer on lower limbs for 3, 5 and 10 minutes. They concluded that there was a gain in inspiratory and peripheral muscle strength in the group that performed early mobilization associated with the use of the cycle ergometer, however, they did not observe a significant increase in hospital discharge [23].
To assess the effects of performing passive exercises with a cycle ergometer, associated with conventional physiotherapy, on peripheral muscle strength, time on MV and length of hospital stay, 38 patients were selected, divided into CG = 16, who underwent conventional physiotherapy (respiratory and motor ), 02 times a day, for 30 min, 07 times a week and IG = 22 submitted to conventional physiotherapy and passive exercises on a lower limb cycle ergometer lasting 20 minutes, fixed cadence of 20 cycles / min, 05 times a week in supine. The Medical Research Council (MRC) scale was used on the lower and upper limbs to assess peripheral muscle strength before and after intervention. The results showed a significant increase in peripheral muscle strength (basal vs. final) in both groups, with the variation in strength increase being greater in the IG. There were no significant differences between groups in terms of time on mechanical ventilation and length of hospital stay [24]. In the postoperative period, several complications can arise, including reduced functional capacity, volume, the effectiveness of myocardial contraction and muscle mass, occurrence of atelectasis and joint contractures, dysfunction of the endothelial vascular system, increased insulin resistance, pressure ulcers and increased levels of anxiety and depression [25].
When it comes to the evaluation of hemodynamic variables using the cycle ergometer, some authors have evaluated the behavior of heart frequency (HF), respiratory frequency (RF), systolic blood pressure (SBP), diastolic blood pressure (DBP), peripheral oxygen saturation (SpO2) and peak expiratory flow (PEF), with all variables being assessed before and after the intervention. 30 patients divided into GA = 10 who underwent mobilization with a cycle ergometer with an intensity of 30 bfm, in 05 series of 3 minutes, with a 1-minute interval between series; GB = 10 performing active and passive mobilization in lower and upper limbs and changing from supine to sedestation with 02 sets of 10 repetitions in each exercise, with an interval of 1 minute between sets and GC = 10 that did not perform any motor activity, and used non-invasive ventilation (NIV) with an orofacial mask connected to the mechanical ventilator for 30 minutes in 03 series of 10 minutes each, with an interval of 2 minutes between sets. During the application of the protocols, the patients kept the bed at 45°. The results showed a significant increase in PEF values in all groups, a significant reduction in SBP in group A, an increase in HR and RF in group B. In the intergroup analysis, a reduction in DBP was observed in group C, with statistical significance [26].
Still comparing the variables, several authors carried out a study in order to compare hemodynamic and respiratory variables such as peak pressure (Ppeak), tidal volume (VT), positive pressure at the end of expiration (PEEP), inspired oxygen fraction (FIO2 ), respiratory frequency (RF), heart frequency (HF) and mean arterial pressure (MAP), as well as arterial blood gas analysis, lactate levels and C-reactive protein (PCr) .Randomized 25 critical patients under MV admitted to the ICU in GC = 11 who did a 30-minute physiotherapy session, consisting of diagonals of the Proprioceptive Neuromuscular Facilitation method (02 series of 10 repetitions each bilateral diagonal) of lower and upper limbs and bronchial hygiene techniques such as vibrocompression, manual hyperinflation and secretion aspiration, the IG = 14 patients used the passive cycle ergometer (20 cycles / min for 20 minutes) before a physiotherapy session equal to that performed by the GC. The patient’s position for applying the cycle ergometer was supine with the head elevated at 30º. The results showed that there were no cardiorespiratory or physiological variables changes in mechanically ventilated patients, reduction in the ICU and hospital stay when compared to the early mobilization protocol without its use. There was a significant decrease in peak pressure values, comparing pre and post-intervention, in the CG [27].
The cycle ergometer can prevent hypotrophy and improve peripheral muscle strength, causing a reduction in the ICU stay and functional improvement. This muscle weakness in critically ill patients is associated with an inflammatory dysregulation that seems to contribute to myopathy. The mechanism of muscle atrophy not due to immobility is not fully understood, however, two molecular interactions are involved: oxidative stress and selected pro-inflammatory cytokines. This synergy between oxidative stress, inflammatory cytokines and inactivity is believed to cause or accelerate muscle atrophy [28]. However, its effects on oxidative stress and immune system parameters remain unknown [29, 30]. Therefore, the study aimed at analyzing oxidative stress and the parameters of the immune system after the use of passive cycle ergometry in the lower limbs in critically ill patients. 19 patients of both sexes who were on MV and admitted to the ICU participated in this study. They were divided into CG = 10 who did not perform any type of therapeutic intervention and IG = 09 who underwent passive cycle ergometry on lower limbs, with a speed of 30 cycles / minutes, for 20 minutes on a cycle ergometer. The results showed that the passive cycle ergometry in the lower limbs was sufficient to reduce the levels of nitric oxide in the cells compared to the CG, that is, it was beneficial in reducing oxidative stress; in relation to inflammatory cytokines, the use of the passive cycle ergometer did not cause changes in the immune system [31].
Several technologies are suggested for the rehabilitation of critical patients admitted to the ICU [30]. Among these technologies, there is a growing interest both in the cycle ergometer and in functional electrical stimulation (FES), because both techniques do not require voluntary movements of patients [32, 33]. It is known that FES and early physical rehabilitation prevent weakness acquired in the ICU and preserve muscle mass, but there are no studies that investigate its effects on strength and muscle mass. Thus, in a study aimed at comparing the physiological effects of four common types of exercise on the bed of intubated and sedated patients, confined to the bed in the ICU and thus determining which intervention is more intense. They selected 19 patients who performed 04 consecutive sessions of 10 minutes of exercise in bed: 10 min of passive range of motion, 10 min of electrical stimulation of the quadriceps, a rectangular bidirectional current, intermittent and without ramp, with modulated intensity to obtain a palpable muscle contraction., 10 min of passive cycle ergometry with 20 cycles / minute and 10 min of FES cycling with 20 cycles / minute and with electrical stimulation synchronized with the knee extension. A rest period of 30 minutes was allowed between each intervention, with the order of interventions being randomized. The results showed that cycling with FES was the only exercise that increased cardiac output, with an average increase of 1 L / min (15%). There was a concomitant increase in muscle oxygen uptake, suggesting that muscle work occurred. No muscular or systemic effects were induced by passive techniques [34].
Still evaluating whether functional electrical stimulation and bed cycling have a positive effect on muscle mass in critically ill patients admitted to the ICU, using MV for at least 24 hours, at Severance Hospital, Seoul, Republic of Korea. They selected 10 patients where the muscular strength of both legs was measured using the Medical Research Council (MRC) scale before and after the intervention. After the passive range of motion exercise, bed cycling was applied for 20 minutes at the standard speed of 20 cycles / minutes, with 1 passive minute and 19 minutes active or active assisted, according to the level of participation. there was a 10-minute rest period and immediately after rest, patients were submitted to the application of functional electrical stimulation for 20 minutes on the left thigh, with the use of 04 electrodes placed on the lateral edge of the quadriceps. During a total treatment session of 20 minutes, electrical stimulation was performed at 35 Hz, a duty cycle of 10 seconds and 12 seconds off, and a pulse time of 250 seconds. The results showed that there was a significant increase in the circumference of the right femoral rectum. Thigh circumference was also increased and statistically significant. There was no difference between left and right in relation to the application of functional electrical stimulation. There was no significant change in muscle strength before and after the intervention [35].
Conclusion
Studies have shown that the use of the cycle ergometer, especially early in critical patients admitted to the ICU, improves hemodynamics, enhances cardiorespiratory, peripheral, circulatory mechanics and functional capacity, accelerates hospital discharge, assists in ventilatory weaning, optimizes breathing, promotes mobility in the bed in upper limbs and lower limbs aiming at a faster return of patients to activities of daily living, in addition to not having a negative impact on the hemodynamic system, bringing safety for professionals to apply the technique safely following the evaluation criteria. However, it is necessary to emphasize that this type of therapy must be performed in a complementary way to conventional motor physiotherapy in critical patients admitted to the ICU.
Limitations
This study had limitations regarding the number of articles relevant to the discussion. In the selection of articles and data extraction, there was a scarcity of research that portrayed cardiorespiratory analyzes during or after the use of the cycle ergometer in the ICU, requiring further studies that focus on this variable.
The study’s eligibility criteria also contributed to limiting the number of articles found in view of the fact that the research is not random and has a range of filters for choosing the studies to be stratified. Other limitations found were the scarcity of randomized studies found in this area, which is an essential factor in the composition of this study.
Acknowledgement
Souza MR de, Jesus GR de, Oliveira S de S conceived the study and research design. Souza MR de, Mercês LS, Magalhães MG da S, Santana RS,Sacramento CLF and Peixoto MS analyzed and interpreted the data. Souza MR de, Peixoto MS, Jesus GR de, Mercês LS, Magalhães MG da S, Santana RS, Sacramento CLF and Oliveira S de S wrote the manuscript. Souza MR de, Oliveira SS did a critical review of the manuscript for important intellectual content.
Conflict of Interest
No potential conflicts of interest relevant to this article have been reported.

Table 1: Systolic blood pressure and variation of people’s interest for watching cricket.

References
- Evelyn E Hill, Paul Herijgers, Piet Claus, Steven Vanderschueren, Marie-Christine Herregods, et al. (2006) Infective endocarditis: changing epidemiology and predictors of 6-month mortality: a prospective cohort study. Eur Heart J 28(2): 196-203.
- Bruno Hoen, François Alla, Christine Selton-Suty, Isabelle Béguinot, Anne Bouvet, et al. (2002) Changing Profile of Infective Endocarditis: Results of a 1-Year Survey in France. JAMA 288(1): 75-81.
- C Loupa, N Mavroidi, I Boutsikakis, O Paniara, O Deligarou, et al. (2004) Infective endocarditis in Greece: a changing profile. Epidemiological, microbiological and therapeutic data. Clin Microbiol Infect 10(6): 556-561.
- Christopher H Cabell, James G Jollis, Gail E Peterson, G Ralph Corey, Deverick J Anderson, et al. (2002) Changing Patient Characteristics and the Effect on Mortality in Endocarditis. JAMA INT MED 162(1): 90-94.
- Shi-Min Yuan (2014) Right-sided infective endocarditis: recent epidemiologic changes Shi-Min Yuan. Int J Clin Exp Med 7(1): 199-218.
- Rob Moss, Brad Munt (2003) Injection drug use and right sided endocarditis. Heart 89(5): 577-581.
- Susan R Hecht, Marvin Berger (1992) Right-sided Endocarditis in Intravenous Drug Users: Prognostic Features in 102 Episodes. Ann Intern Med 117(7): 560-566.
- Eeva Ruotsalainen, Kari Sammalkorpi, Janne Laine, Kaisa Huotari, Seppo Sarna, et al. (2006) Clinical manifestations and outcome in Staphylococcus aureus endocarditis among injection drug users and nonaddicts: a prospective study of 74 patients. Randomized Controlled Trial 6: 137.
- Lucy E Wilson, David L Thomas, Jacqueline Astemborski, Terri L Freedman, David Vlahov (2002) Prospective Study of Infective Endocarditis among Injection Drug Users. J Infect Dis 185(12): 1761-1766.
- Tom Kai Ming Wang, Timothy Oh, Jamie Voss, James Pemberton (2014) Characteristics and outcomes for right heart endocarditis: six-year cohort study. Clinical Trial 23(7): 625-627.
- Musci M, Siniawski H, Pasic M, Grauhan O, Weng Y, et al. (2007) Surgical treatment of right-sided active infective endocarditis with or without involvement of the left heart: 20-year single center experience. Eur J Cardiothorac Surg 32: 118-125.
- Pilar Martín-Dávila, Enrique Navas, Jesús Fortún, Jose Luis Moya, Javier Cobo, Vicente Pintado, et al. (2005) Analysis of mortality and risk factors associated with native valve endocarditis in drug users: the importance of vegetation size. Am Heart J 150(5): 1099-1106.
- J Remadi, G Habib, G Nadji, Amel Brahim, Franck Thuny, et al. (2007) Predictors of Death and Impact of Surgery in Staphylococcus aureus Infective Endocarditis. Annals of thoracic surgery 83(4): 1295-1302.
- Murdoch DR, Corey GR, Hoen B, José M Miró, Vance G Fowler Jr, et al. (2009) Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 169(5): 463-473.
- Cabell CH, Jollis JG, Peterson GE, G Ralph Corey, Deverick J Anderson, et al. (2002) Changing Patient Characteristics and the Effect on Mortality in Endocarditis. Arch Intern Med 162(1): 90-94.
- Jiang SL, Li BJ, Zhang T (2011) Surgical treatment of isolated right-sided infective endocarditis. Tex Heart Inst J 38(6): 639-642.
- Martín-Dávila P, Navas E, Fortún J, Jose Luis Moya, Javier Cobo, et al. (2005) Analysis of mortality and risk factors associated with native valve endocarditis in drug users: the importance of vegetation size. Am Heart J 150(5): 1099-1106.
- Pingkwan Chan, J David Ogilby, Bernard Segal (1989) Tricuspid valve endocarditis. Am Heart J 117 (5): 1140-1146.
- Leitman M, Dreznik Y, Tyomkin V, Fuchs T, Krakover R, et al. (2012) Vegetation size in patients with infective endocarditis. Eur Heart J Cardiovasc Imaging 13(4): 330-338.
- Robbins MJ, Frater RVM, Soeiro R, WH Frishman, JA Strom (1986) Influence of vegetation size on clinical outcome of right-sided infective endocarditis. Am J Med 80: 165-171.
- Bayer AS, Blomquist IK, Bello E, Chiu Cy, Ward JI, et al. (1988) Tricuspid valve endocarditis due to Staphylococcus aureus. Chest 93: 247-253.
- Arbulu A, Holmes RJ, Asfaw I (1993) Surgical treatment of intractable right-sided infective endocarditis in drug addicts: 25 years experience. J Heart Valve Dis 2: 129-137.
- Hust MH, Metzler B, Ebermann F, Heinemann M, Ziemer G (1997) Tricuspid valvulectomy in antibiotic-refractory right-heart endocarditis. Dtsch Med Wochenschr 122(4): 80-85.
- P Nihoyannopoulos (2001) Tricuspid valvectomy following tricuspid valve endocarditis on an intravenous drug addict. Heart 86(2): 144.
- Ali TA, Fatimi SH, Naeem SS, Rawasia WF (2015) Successful tricuspid valvectomy in a septic patient with tricuspid valve endocarditis. J Coll Physicians Surg Pak 25 Suppl 1: S8-S9.
- Jeffrey G Gaca, Shubin Sheng, Mani Daneshmand, J Scott Rankin, Matthew L Williams, et al. (2013) Current Outcomes for Tricuspid Valve Infective Endocarditis Surgery in North America. Ann Thorac Surg 96 (4): 1374-1381.
- (2015) Guidelines on the prevention, diagnosis, and treatment of infective endocarditis Eur Heart J.
- A S Bayer, I K Blomquist, E Bello, C Y Chiu, J I Ward, et al. (1988) Tricuspid valve endocarditis due to Staphylococcus aureus: correlation of two-dimensional echocardiography with clinical outcome. Chest 93: 247-253.
- MD John, PL Hibberd, AW Karchmer, LA Sleeper, SB Calderwood (1998) Staphylococcus aureus prosthetic valve endocarditis: optimal management and risk factors for death. Clin Infect Dis 26: 1302-1309.
- C Chirouze, CH Cabell, VG Fowler Jr, N Khayat, L Olaison, et al. (2004) Prognostic factors in 61 cases of Staphylococcus aureus prosthetic valve infective endocarditis from the International Collaboration on Endocarditis merged database. Clin Infect Dis 38: 1323-1327.
- Moreillon P, Que YA (2004) Infective endocarditis. Lancet 363(9403): 139-149.
- Lowes JA, Hamer J, Williams G, Houang E, Tabaqchali S, et al. (1980) 10 Years of infective endocarditis at St. Bartholomew's Hospital: analysis of clinical features and treatment in relation to prognosis and mortality. Lancet 1: 133-136.
- Cooper JP, Jayawickreme SR, Swanton RH (1995) Permanent pacing in patients with tricuspid valve replacements. Br Heart J 73: 169-172.
- T Ohata, I Kigawa, E Tohda, Y Wanibuchi (2001) Comparison of durability of bioprostheses in tricuspid and mitral positions. Ann Thorac Surg 71: S240-S243.
- GJ Van Nooten, F Caes, Y Taeymans, Y Van Belleghem, K François, et al. (1995) Tricuspid valve replacement: postoperative and long-term results. J Thorac Cardiovasc Surg 110: 672-679.
- Rizzoli G, Vendramin I, Nesseris G, Tomaso Bottio, Cosimo Guglielmi, et al. (2004) Biological or mechanical prostheses in tricuspid position? A metaanalysis of intra-institutional results. Ann Thorac Surg 77: 1607-1614.
- Anselmi A, Ruggieri VG, Harmouche M, Flécher E, Corbineau H, (2005) Appraisal of Long-Term Outcomes of Tricuspid Valve Replacement in the Current Perspective. Ann Thorac Surg 1(3): 863-871.
- Ho Young Hwang, Kyung-Hwan Kim, Ki-Bong Kim, Hyuk Ahn (2012) Mechanical Tricuspid Valve Replacement Is Not Superior in Patients Younger Than 65 Years Who Need Long-Term Anticoagulation. Ann Thorac Surg 93: 1154-1161.
- Scully HE, Armstrong CS (1995) Tricuspid valve replacement. Fifteen years of experience with mechanical prostheses and bioprostheses. J Thorac Cardiovasc Surg 109: 1035-1041.
- Kaplan M, Kut MS, Demirtas MM, Cimen S, Ozler A (2002) Prosthetic replacement of tricuspid valve(bioprosthetic or mechanical). Ann Thorac Surg 73: 467-473.
- Carrier M, Hébert Y, Pellerin M, Bouchard D, Perrault LP, et al. (2003) Tricuspid valve replacement: an analysis of 25 years of experience at a single center. Ann Thorac Surg 75(1): 47-50.
- Ratnatunga CP, Edwards MB, Dore CJ, Taylor KM (1998) Tricuspid valve replacement: UK Heart Valve Registry mid-term results comparing mechanical and biological prostheses. Ann Thorac Surg 66(6): 1940-1947.
- Altaani H, Jaber S (2013) Tricuspid Valve Replacement, Mechanical vs Biological Valve, Which is Better? Int Cardiovasc Res J 7(2): 71-74.
- Chang B, Lim S, Yi G, Hong Y, Lee S, et al. (2006) Long-Term Clinical Results of Tricuspid Valve Replacement. Ann Thorac Surg 81: 1317-1324.
- Farzan Filsoufi, Ani C Anyanwu, Sacha P Salzberg, Tim Frankel, Lawrence H, et al. (2005) Long-Term Outcomes of Tricuspid Valve Replacement in the Current Era. Ann Thorac Surg 80: 845-850.
- Grover FL, Cohen DJ, Oprian C, Henderson WG, Sethi G, et al. (1994) Determinants of the occurrence of and survival from prosthetic valve endocarditis. Experience of the Veterans Affairs Cooperative Study on Valvular Heart Disease. J Thorac Cardiovasc Surg 108: 207-214.
- Robin E, Thomas NW, Arbulu A, Ganguly SN, Magnisalis K (1975) Hemodynamic consequences of total removal of the tricuspid valve without prosthetic replacement. Am J Cardiol 35: 481-486.
- Carozza A, Renzulli A, Feo MD, Ismeno G, Corte AD, et al. (2001) Tricuspid Repair for Infective Endocarditis. Clinical and Echocardiographic Results. Tex Heart Inst J 28(2): 96-101.
- Singh SK, Tang GH, Maganti MD, Armstrong S, Williams WG, et al. (2006) Midterm outcomes of tricuspid valve repair versus replacement for organic tricuspid disease. Ann Thorac Surg 82(5): 1735-1741.
- Moon MR, Miller DC, Moore KA, Oyer PE, Mitchell RS, et al. (2001) Treatment of endocarditis with valve replacement: the question of tissue versus mechanical prosthesis. Ann Thorac Surg 71(4): 1164-1171.
- Kang CH, Ahn H, Kim KH, Kim KB (2005) Long-term result of 1144 CarboMedics mechanical valve implantations. Ann Thorac Surg 79: 1939-1944.
- Weymann A, Borst T, Popov AF, Sabashnikov A, Bowles C, et al. (2014) Surgical treatment of infective endocarditis in active intravenous drug users: a justified procedure? J Cardiothorac Surg 9: 58.
- Roman Gottardi, Jan Bialy, Elena Devyatko, Heinz Tschernich, Martin Czerny, et al. (2007) Midterm Follow-Up of Tricuspid Valve Reconstruction Due to Active Infective Endocarditis. Annals of Thoracic Surgery 84(6): 1943-1948.
-
Simran Kaur Matta. Right Sided Endocarditis. On J Cardio Res & Rep. 4(3): 2020. OJCRR.MS.ID.000590.
-
Cardiorespiratory system, Breathing, Intensive care unit, Respiratory exercise, Early walking, Physical therapy, Physical exercise, Ergometry, Osteoarticular, Musculoskeletal, Cardiorespiratory factors, Myocardial contraction
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






