 Case Report
Case Report
The Issue of Coronary Ostia Cannulation after Transcatheter Aortic Valve Implantation
Roberto Valvo, Giuliano Costa, Corrado Tamburino and Marco Barbanti*
Division of Cardiology, A.O.U. Policlinico “G. Rodolico-San Marco”, Italy
Marco Barbanti, Division of Cardiology, A.O.U. Policlinico “G. Rodolico - San Marco”, Catania, Italy.
Received Date:August 27, 2021; Published Date:November 02, 2021
Summary
Transcatheter aortic valve implantation is progressively expanding its indications towards low risk, younger patients. The consequent expansion towards younger patients poses the attention on long-term considerations, such as the chance of requirement of future coronary interventions after TAVI. In the present article, we will examine the conditions that can favor an easier coronary re-access after TAVI and show three different cases that explain the importance of a proper TAV selection in this regard.
Keywords:TAV1; TAVI2; Coronary Angiography3; PCI4
Abbreviations:TAVI: Transcatheter aortic valve implantation; AS: Aortic stenosis; CAD: Coronary artery disease; TAV: Transcatheter aortic valve; SoV: Sinus of Valsalva; LM: Left main; RCA: Right Coronary artery; BE: Balloon expandable; SE: Self-expanding; CA: Coronary angiography; PCI: Percutaneous coronary intervention; STJ: Sinotubular junction; CT: Computed tomography
Introduction
Transcatheter aortic valve replacement (TAVI) is progressively expanding its indications towards younger patients [1-3]. As a result, patients undergoing TAVI will have a longer life expectancy than before, and this shifts the focus on different long-term considerations. Among these, a particular interest is growing about the issue of coronary artery re-engagement after TAVI. Aortic stenosis (AS) and coronary artery disease (CAD) frequently coexist, due to similar risk factors and pathogenesis. It has been reported that up to 60-80% of patients with AS undergoing TAVI have concomitant CAD [4-6]. Furthermore, an analysis of 779 patient reported a rate of 10% post-TAVR acute coronary syndrome over two years [6]. In this paper, we look at the underlying conditions that could make coronary re-engagement less or more challenging, reporting three explanatory cases.
Factors Influencing Coronary Cannulation after Transcatheter Aortic Valve Implantation
The feasibility of coronary re-access after TAVI is intimately related to aortic root anatomy and its relationship with the transcatheter aortic valve (TAV), and it is affected by the choice of a proper cannulation strategy (Figure 1).
Aortic Root Anatomy and Transcatheter Aortic Valves Designs
The aortic root anatomy and TAV designs significantly impact on the feasibility of coronary re-access after TAVI, due to the spatial relationship between the aortic wall and the TAV stent, the final position of bioprostheses commissures in relation of the coronary ostia, and the bioprosthetic leaflets position. Recently, our group showed that a higher TAV implant depth, the oversizing of the TAV compared with sinuses of Valsalva (SoV), and the use of Evolut R/PRO TAVs are related to a higher risk of unsuccessful coronary cannulation after TAVI [7]. After evaluating the aortic root anatomy, the TAV choice is a crucial phase to make future coronary re-access feasible. The various devices differently impact on coronary re-access according to the specific design. As previously cited, the Evolut R/PRO valves showed a more challenging coronary re-access, due to the supra-annular structure, the high commissural posts, and the small cells of the stent frame. Besides, the misalignment of bioprosthetic commissures demonstrated to potentially play an important role in the feasibility of coronary reaccess after TAVI. Tang et al. have recently reported the different commissural alignment after TAVI according to the C-tab marker orientation on the delivery system. They showed that inserting the delivery system with the flush port at 3 o’clock position improved commissural alignment, lowering the incidence of severe coronary artery overlap with left main (LM), right coronary artery (RCA), both coronary arteries, and 1 or both coronary arteries (p < 0.001 for all) [8]. The ACURATE neo is designed to ensure easier access to coronary arteries due to its open cell frame design, and to reduce the risk of coronary obstruction in patients with low coronary artery height and small sinuses of Valsalva [7,9]. Similarly, balloonexpandable (BE) TAVs guarantee a less challenging coronary reengagement compared to the self-expanding (SE) counterpart [7]. This advantage is probably related to their intra-annular structure, to the lower frame height, and larger cell design. Finally, the possibility to tailor the device implantation depth in each anatomy in order to obtain an easier coronary re-access should be carefully considered during pre-procedural planning. Indeed, if on one hand, a lower TAV positioning could reduce the risk of coronary reaccess impairment, on the other hand, it showed to increase the risk of significant paravalvular regurgitation and new-onset high degree conduction disturbances requiring permanent pacemaker implantation [10,11]. Despite the limited number of studies, according to currently published data, it seems that Acurate neo and Sapien 3/ULTRA actually guarantee a less challenging coronary access after TAVI [7,12-16]. Future, larger studies involving recently approved devices (e.g., MyVal, Acurate neo2) will improve our current knowledge about this important issue.
Coronary Ostia Cannulation Strategy
Especially in the setting of acute coronary syndromes, it is mandatory to understand the technical implications and the potential challenges of coronary angiography (CA) and percutaneous coronary intervention (PCI) in patients who underwent TAVI, for a selective and quick coronary ostia cannulation. The coronary ostia cannulation strategy should be chosen after identifying the valve design. In the presence of a BE TAV, usually the access selection, catheter choice, and coronary ostia engagement technique did not have to be modified. The coronary ostia cannulation could be obtained in two different ways. In the case of the top of TAV stent faces the aortic wall above sinotubular junction (STJ), coronary engagement must be through the upper cells row of the stent. In this setting, if one of the commissural tabs faces the coronary ostium, it is necessary to obtain coronary engagement from a lateral open cell to the commissural tab. If only a nonselective angiogram is achieved, it is required to use a coronary wire to engage the artery with a railing technique. In rare cases, a downward-pointing catheter, such as a multipurpose 1 or an Amplatz right 2, may facilitate engagement from either a cell lies above and not properly aligned with the coronary ostia, or from the space between the valve frame and the STJ [17,18]. In the case of SoV height is higher than TAV stent, coronary artery engagement can be achieved from above the TAV. Coaxial engagement might be affected by the SoV width. If the SoV are narrow, there will be a relative lack of room to manipulate the catheter and an optimal coaxiality might be difficult to obtain. In the presence of an Evolut R/PRO valve, whose structure is supra-annular and stent frame extends up to ascending aorta with a closed cells design, the coronary cannulation strategy should be substantially modified. Femoral or left radial artery access is recommended for CA or PCI, particularly during an acute coronary syndrome. Selective catheter engagement might be particularly challenging. It should be performed in a coaxial manner through the diamond-shaped cell in front of the coronary ostia, but the supraannular position of valve leaflets and the position of commissural posts impair easy cannulation in a not negligible portion of patients. Otherwise, catheter engaging from a stent cell which does not match with coronary ostia, has been associated with kinking of the guide and the impossibility to remove it [19]. The engagement of LM usually requires a smaller catheter due to the narrow waist of the Evolut R/PRO valve. Downsizing catheter size by 0.5 cm may assist the coronary cannulation [7]. If data about catheter choice prior TAVI are not available, Judkins Left (JL) 3.5 and JL 3.0 catheters should be used as first choice catheters for femoral and left radial access, respectively. If the catheter is unable to enter into the coronary ostium due to the geometric interaction with one of the commissures, the coronary artery may be carefully engaged from an adjacent cell. If it is impossible to engage the coronary ostia with diagnostic catheters, a semi-selective angiogram should be performed. Then, a selective cannulation could be obtained by a railing technique with the use of a guiding catheter (e.g., extra backup (EBU) 3.0/3.5) and a coronary guidewire (0.014”). Regarding the engagement of right coronary artery (RCA), a standard strategy with a Judkins Right (JR) 4.0 should be adopted. An oversizing catheter size by 0.5 cm or an Amplatz Right 2 may be helpful with wide SoV. If a commissural post lies near the coronary ostium, a Multipurpose catheter or an Ikari right guide should be preferred [17]. In the case of PCI, the use of a guide extension catheter and/or a supporting balloon might be helpful to fully engage the coronary ostium and to obtain a higher backup [18,20]. Of note, the TAVIcathAID application has been recently released on both Apple store and Google play store. It is a mobile app with illustrated suggestions for a step-by-step guided approach to perform CA and PCI after TAVI [21].
Case Report
Below, three cases of TAVI patients with similar aortic root anatomies and borderline coronary arteries height (~11 millimeters), who received different types of bioprostheses (Figure 2). Patient #1: 84-year-old female patient with hypertension, dyslipidemia, and no other significant comorbidities. At preprocedural planning, computed tomography (CT) showed annular perimeter and area of 6.3 cm and 3.1 cm2, respectively; SoV mean diameter of 24.8 mm, STJ mean diameter of 21.0 mm, and a coronary height of 11.7 mm and 11.2 mm for LM and RCA, respectively. Preprocedural CA was successfully performed using JL 4 and JR 4 catheters for LM and RCA, respectively, showing no coronary lesions. Our team decided to implant an Evolut PRO 26 mm TAV [22]. At post-TAVI CA with the same catheters, it was impossible to obtain a selective cannulation for both coronaries. A semi-selective cannulation was feasible for the LM, whereas the RCA was visualized only at the aortography.
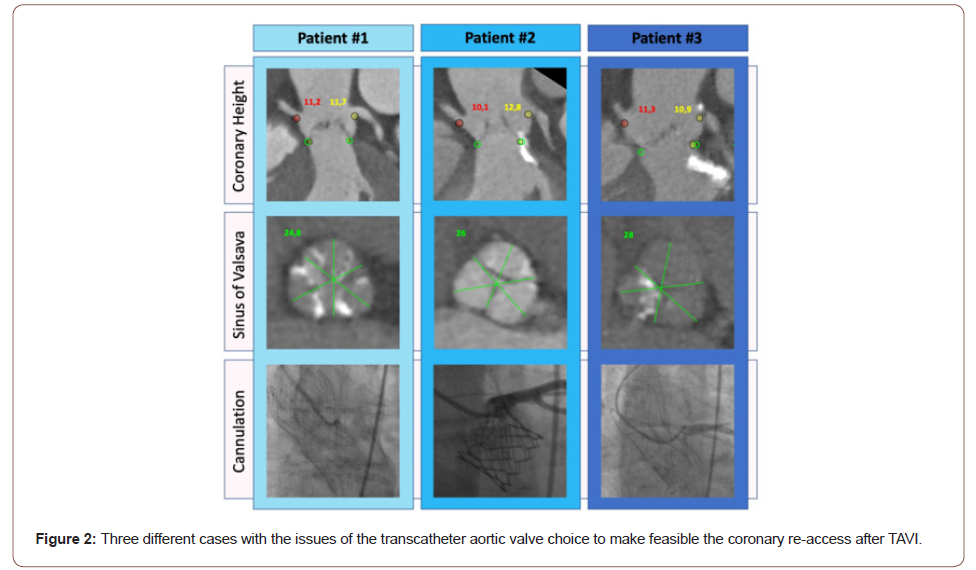
Patient No. 2: 80-year-old female patient with senile dementia, chronic obstructive pulmonary disease, and no other significant comorbidities. At pre-procedural planning, CT showed annular perimeter and area of 6.6 cm and 3.4 cm2, respectively, SoV mean diameter of 26.0 mm, STJ mean diameter of 25.0 mm, and a coronary height of 12.8 mm and 10.1 mm for LM and RCA, respectively. Pre-procedural CA was successfully performed using JL 4 and JR 4 catheters for LM and RCA, respectively, showing an isolated, moderate lesion on the middle tract of left anterior descending (LAD) artery. Due to the presence of CAD, we chose to implant a SAPIEN ULTRA 23 mm TAV, whose design ensure easy coronary access after TAVI. Indeed, at post-TAVI CA with the same catheters, a selective cannulation was obtained for both LM and RCA.
Patient No. 3: 81-year-old female patient with hypertension, family history for heart disease, and no other significant comorbidities. At pre-procedural planning, CT showed annular perimeter and area of 7.3 cm and 4.1 cm2, SoV mean diameter of 28.0 mm, STJ mean diameter of 28.0 mm, and coronary height of 10.9 mm and 11.3 mm for LM and RCA, respectively. Preprocedural CA was successfully performed using JL 4 and JR 4 catheters for LM and RCA, respectively, showing no coronary lesions. Due to the low cut-off of both coronaries, we decided to implant an Acurate neo size M, whose design and deployment allow to push down native leaflets, away from the coronary ostia, and whose open cells guarantee a frame-free coronary re-access. As a matter of fact, at post-TAVI CA with the same catheters, selective cannulation was feasible for both LM and RCA.
Conclusion
To date, it is challenging to estimate the real feasibility and success rates of CA and PCI after TAVI due to the limited number of studies currently available. Nevertheless, it seems evident that valve design matters in terms of re-access, being supra-annular, closed stent frame cells, SE TAVs associated with greater challenges in coronary re-access. Therefore, particular attention should be paid to the choice of a specific TAV for each anatomy and clinical setting (i.e., extensive CAD), to adapt the implantation depth according to the selected device characteristics, as well as to the possibility to orientate bioprosthetic commissures in order to avoid a severe overlap with coronary ostia. Moreover, it is required to modify the standard technique of coronary cannulation according to the characteristics of each valve design. In summary, the issue of coronary re-access after TAVI requires further observations and experience. The future iterations of currently used TAV are expected to tackle this issue, minimizing the risk of unfeasible access to coronaries after TAVI.
Acknowledgement
None.
Conflict of Interest
Marco Barbanti is consultant for Edwards Lifesciences, Boston Scientific and Medtronic. Corrado Tamburino is consultant for Medtronic. All other authors have no conflicts of interests to declare.
References
- Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, et al. (2019) Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med 380: 1706-1715.
- Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, et al. (2019) Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med 380: 1695-1705.
- Tamburino C, Valvo R, Crioscione E, Reddavid C, Picci A, et al. (2020) The path of transcatheter aortic valve implantation: from compassionate to low-risk cases. Eur Heart J Suppl 22: L140-L145.
- Czer LSC, Gray RJ, Stewart ME, Robertis M De, Chaux A, et al. (1988) Reduction in sudden late death by concomitant revascularization with aortic valve replacement. J Thorac Cardiovasc Surg 95: 390-401.
- Madhavan MV, Gersh BJ, Alexander KP, Granger CB, Stone GW (2018) Coronary Artery Disease in Patients ≥80 Years of Age. J Am Coll Cardiol 71: 2015-2040.
- Vilalta V, Asmarats L, Ferreira-Neto AN, Maes F, de Freitas Campos Guimarães L, et al. (2018) Incidence, Clinical Characteristics, and Impact of Acute Coronary Syndrome Following Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv 11: 2523-2533.
- Barbanti M, Costa G, Picci A, Criscione E, Reddavid C, et al. (2020) Coronary Cannulation After Transcatheter Aortic Valve Replacement: The RE-ACCESS Study. JACC Cardiovasc Interv 13(21): 2542-2555.
- Tang GHL, Zaid S, Fuchs A, Yamabe T, Yazdchi F, et al. (2020) Alignment of Transcatheter Aortic-Valve Neo-Commissures (ALIGN TAVR): Impact on Final Valve Orientation and Coronary Artery Overlap. JACC Cardiovasc Interv 13: 1030-1042.
- Okuno T, Lanz J, Pilgrim T (2020) ACURATE neo: How Is This TAVR Valve Doing to Fit into an Increasingly Crowded Field? Curr Cardiol Rep 22(10): 107.
- Hahn RT, Kodali S, Généreux P, Leon M (2014) Paravalvular Regurgitation Following Transcutaneous Aortic Valve Replacement: Predictors and Clinical Significance. Curr Cardiol Rep 16: 475.
- Nazif TM, Dizon JM, Hahn RT, Xu K, Babaliaros V, et al. (2015) Predictors and Clinical Outcomes of Permanent Pacemaker Implantation After Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv 8: 60-69.
- Chetcuti S, Kleiman N, Matthews R, Popma JJ, Moore J (2016) TCT-743 Percutaneous Coronary Intervention after Self-Expanding Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 68: B300-B301.
- Zivelonghi C, Pesarini G, Scarsini R, Lunardi M, Piccoli A, et al. (2017) Coronary Catheterization and Percutaneous Interventions After Transcatheter Aortic Valve Implantation. Am J Cardiol 120: 625-631.
- Ferreira-Neto AN, Puri R, Asmarats L, Vilalta V, Guimaraes L, et al. (2019) Clinical and Technical Characteristics of Coronary Angiography and Percutaneous Coronary Interventions Performed before and after Transcatheter Aortic Valve Replacement with a Balloon-Expandable Valve. J Interv Cardiol 2019: 3579671.
- Tanaka A, Jabbour RJ, Testa L, Agnifili M, Ettori F, et al. (2019) Incidence, Technical Safety, and Feasibility of Coronary Angiography and Intervention Following Self-expanding Transcatheter Aortic Valve Replacement. Cardiovasc Revascularization Med 20: 371-375.
- Ochiai T, Chakravarty T, Yoon SH, Kaewkes D, Flint N, et al. (2020) Coronary Access After TAVR. JACC Cardiovasc Interv 13: 693-705.
- Yudi MB, Sharma SK, Tang GHL, Kini A (2018) Coronary Angiography and Percutaneous Coronary Intervention After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 71: 1360-1378.
- Jackson M, Williams PD (2018) Coronary access following TAVI – Selective coronary engagement using balloon-assisted tracking of a guide catheter extension. Cardiovasc Revascularization Med 19: 384-389.
- Harhash A, Ansari J, Mandel L, Kipperman R (2016) STEMI After TAVR. JACC Cardiovasc Interv 9: 1412-1413.
- Bharadwaj AS, Bhatheja S, Sharma SK, Kini AS (2018) Utility of the guideliner catheter for percutaneous coronary interventions in patients with prior transcatheter aortic valve replacement. Catheter Cardiovasc Interv 91: 271-276.
- Bhatheja S, Tang G, Khan M, Khan A, Sharma S, Kini A (2019) Tavrcathaid: An Educational Mobile Application to Learn Coronary Access after Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 73: 3041.
- Barbanti M, Immè S, Ohno Y, Gulino S, Todaro D, et al. (2016) Prosthesis choice for transcatheter aortic valve replacement: Improved outcomes with the adoption of a patient-specific transcatheter heart valve selection algorithm. Int J Cardiol 203: 1009-1010.
-
Roberto Valvo, Giuliano Costa, Corrado Tamburino Marco Barbanti. The Issue of Coronary Ostia Cannulation after Transcatheter Aortic Valve Implantation. On J Cardio Res & Rep. 6(1): 2021. OJCRR.MS.ID.000629.
-
Coronary angiography, Aortic stenosis, Coronary artery disease, Heart disease, Self-expanding, Balloon expandable, Computed tomography, TAV1; TAVI2
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






