 Research Article
Research Article
Screening Echocardiography in Morbidly Obese Pregnant Women
Oluseyi K Ogunleye1*, Hongmei Zhang2, Stanette L Brown3, Paul C Browne4 and Anthony R Gregg1
1Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, University of Florida College of Medicine, USA
2Division of Epidemiology, Biostatistics and Environmental Health, University of Memphis, USA
3Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, University of South Carolina, USA
4Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, Medical College of Georgia, USA
Oluseyi K Ogunleye, Division of Maternal Fetal Medicine, Nationwide Children’s Hospital, Ohio State University, Columbus, Ohio, USA.
Received Date:September 01, 2022; Published Date:September 21, 2022
Summary
Objective: We sought to describe the echocardiographic findings of morbidly obese gravidas who had No history of underlying cardiac dysfunction.
Study design: We evaluated 27 morbidly obese pregnant patients between 16 and 35 weeks gestation. A single echocardiogram was obtained on each patient. Measurements of cardiac function and structure using echocardiogram data were compared across patient subgroups. Subgroups consisted of “healthy” obese patients (no diabetes or hypertension), those with pregnancy associated hypertension (gestational hypertension or preeclampsia), chronic hypertension, diabetes, or both (chronic hypertension and diabetes). Echocardiogram parameters were evaluated across each group and correlated with body mass index (BMI). Clinical outcomes were recorded. Nonparametric Spearman correlation tests were performed to evaluate these relationships.
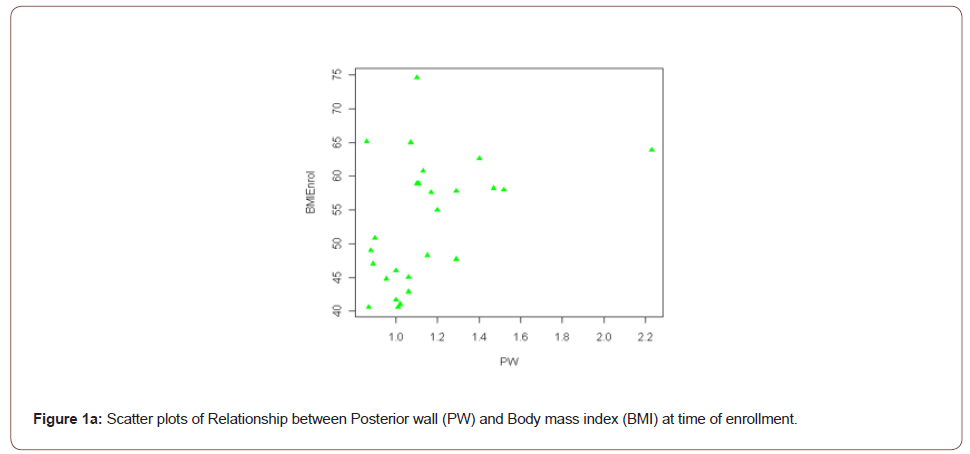
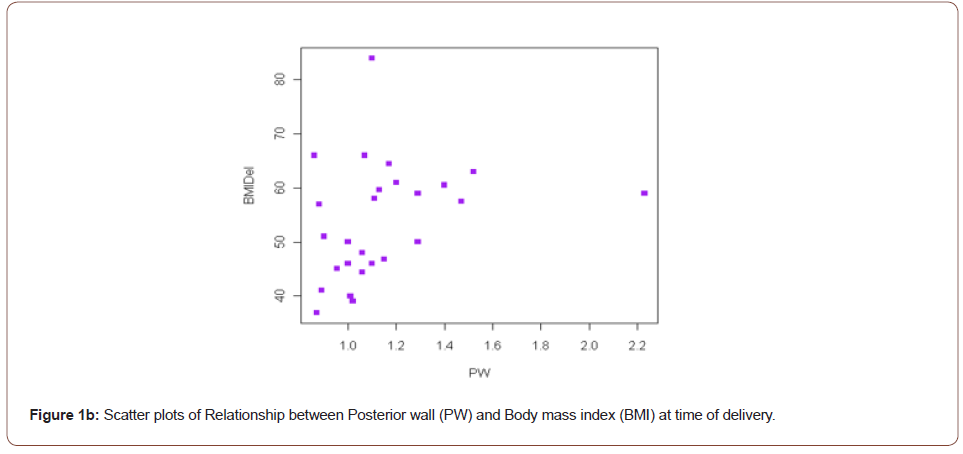
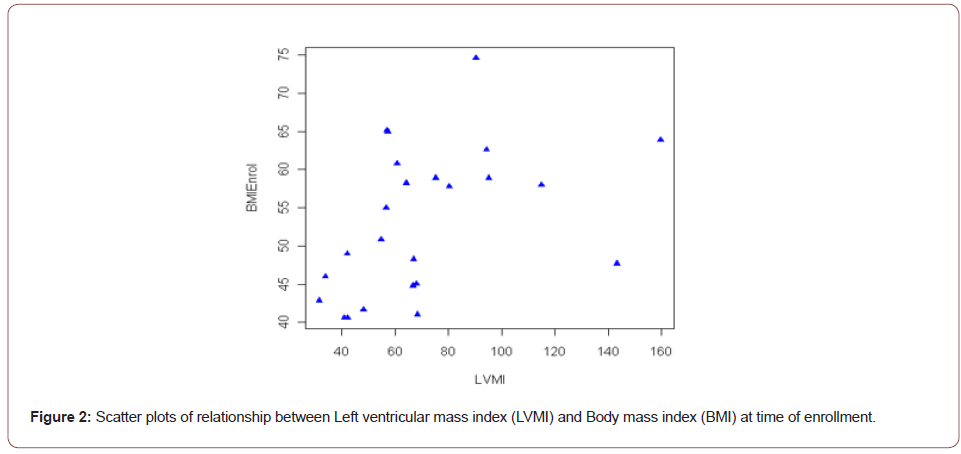
Result: Left Ventricular Mass Index (LVMI) was significantly associated with BMI at enrollment (r=0.48, p=0.02). The posterior wall thickness (PW) was also associated with BMI at enrollment and delivery (r=0.44, p=0.03; r=0.47, p=0.02 respectively). These relationships remained significant after controlling for a history of hypertension.
Conclusion: Our data suggest that echocardiography performed solely on the basis of a BMI ≥ 40 can identify echocardiogram abnormalities in structure that could be important for future follow-up.
Keywords:Echocardiogram; Left ventricular hypertrophy; Obesity; Pregnancy
Introduction
Approximately one third of United States women are obese (body mass index (BMI) ≥30). The prevalence of obesity has dramatically increased in the past 20 years and as a result more and more of this patient population is being seen in prenatal clinics. Morbidly obese pregnant patients (BMI ≥40) have increased lifetime cardiac morbidity and mortality [1]. Obese gravidas are more likely to have maternal complications such as hypertension, diabetes and throm bosis. Poor fetal and newborn outcomes are more common in obese women [2]. In an effort to ensure healthy maternal outcomes while optimizing perinatal outcomes a number of diagnostic challenges ensue [3]. Long standing neglected hypertension and diabetes are risk factors for abnormal cardiac function, furthermore pregnancy increases cardiac demands. These facts and prior clinical experience led to our practice of performing screening echocardiograms in this patient population. In this study we sought to describe the structural and functional changes of the heart identified during a screening echocardiogram in morbidly obese pregnant women.
Cardiac or ventricular remodeling refers to an alteration in the structure and/or function of the heart in response to hemodynamic changes or cardiac injury [4]. Left ventricular hypertrophy can be broadly divided into physiologic and pathologic and a RWT of >0.45 has often been used as a marker for pathologic left ventricular hypertrophy [5]. Utilizing the LVMI and RWT, four geometric patterns have been described: eccentric hypertrophy (elevated LVMI, normal RWT), concentric hypertrophy (elevated LVMI and RWT), concentric remodeling (normal LVMI and elevated RWT) and normal geometry (normal RWT, normal LVMI) [6,7]. Cardiac adaptation to obesity heralded by left ventricular dilatation and eccentric hypertrophy independent of an increased arterial blood pressure has been described in non-pregnant patients [8].
Material and Method
The Institutional Review Board of Palmetto Health Richland Hospital approved this study (PH IRB 2007-19). The study was a prospective, non-randomized cohort study which examined the echocardiogram findings of obese patients with a Body Mass Index (BMI) ≥ 40. BMI was recorded at the first prenatal care visit and patients were invited to enroll when a BMI of ≥ 40 was identified. Patients with a history of cardiac dysfunction were excluded. Patients were classified into 5 clinical categories. These consisted of “healthy” obese patients (no diabetes or hypertension), those with pregnancy associated hypertension (gestational hypertension or preeclampsia), chronic hypertension, diabetes, or both (chronic hypertension and diabetes). BMI at the time of delivery, blood pressure at enrollment and the first blood pressure at the time of admission for delivery were recorded. Mean Arterial Pressure (MAP) was calculated from blood pressure measurements (Systolic blood pressure +2(diastolic blood pressure))/3. Hypertension associated conditions and diabetes were defined as per the American College of Obstetricians and Gynecologists [9, 10].
A two dimensional echocardiogram, was performed once after the first trimester of pregnancy by board certified echocardiographers. We targeted a time period after the first trimester in order to gain insight into cardiac function with at least minimal impact by pregnancy. All images were reviewed and calculations were determined in collaboration with these cardiologists. In order to mirror clinical practice, echocardiogram reports were extracted for data. Left ventricular internal dimension in diastole (LVIDd), posterior wall thickness (PW) and inter- ventricular septum diameter in diastole (IVS) were available on every report. We calculated the left ventricular mass (LWM) using the Troy formula: ([LVIDD + PWTD + IVSTD]3- [LVIDD]3) 1.05 [11,12]. Although the best way to standardize LVM is still uncertain, we indexed for height to create the LVM index (LVMI) as follows: LVMI = LVM (grams) / height (meters)2.7. Indexing for height in LVMI is helpful in reducing variability in LVM amongst individual patients [11-13]. Relative wall thickness (RWT) was defined as: PW / LVIDd. Ejection fraction (EF) and pulmonary arterial pressure (PAP) were both calculated by the cardiologist reading the study. We defined subgroups of ventricular hypertrophy according to accepted norms 1) normal ventricular geometry: LVMI <51 g/ m2.7 and RWT <0.45 2) eccentric hypertrophy was defined: LVMI> 51 g/ m2.7 and RWT < 0.45 3) concentric hypertrophy: LVMI>51 g/m2.7 and RWT>0.45 4) concentric LV remodeling: LVMI <51 g/ m2.7 and RWT >0.4513. Medical complications (e.g. diabetes and hypertension) were managed according to institutional practice patterns. Pregnancy outcomes for all participants were recorded. This includes gestational age at delivery, birth weight, Apgar scores at 1 and 5 minutes of life, admission to the Special Care Nursery/Neonatal Intensive Care Unit and neonatal length of hospital stay (LOS).
Statistical methods
Non-parametric Spearman correlations were calculated based on the ranks to evaluate associations between recorded variables. In the spearman correlation tests, the significance level is set at α = 0.05. Statistical differences between group means were evaluated using ANOVA.
Result
There were twenty-seven patients included in the final analysis. The demographics of patients involved in the study including age, parity are summarized (Table 1). The gestational age at entry into perinatal care was 8- 32 weeks gestation. A single echocardiogram was obtained on each patient between 16 and 35 weeks gestation. Patient demographic for each clinical category is shown (Table 1). The measurements obtained during the echocardiogram and those calculated are shown (Table 2). Echocardiogram measurements and indices were evaluated across each category. There were no statistically significant differences across any clinical group (Table 3).
Table 1: Demographic distribution of patient subgroups*.
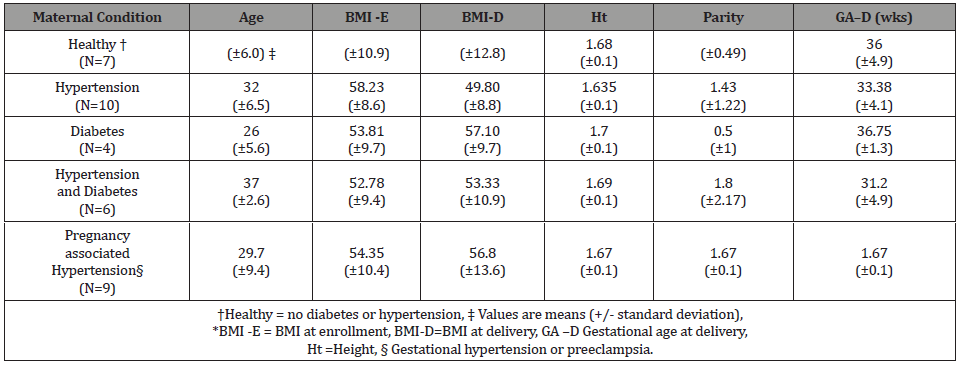
Table 2: Echocardiogram reported (LVIDd, IVS, PW) and calculated (RWT, and LVMI) measures*.
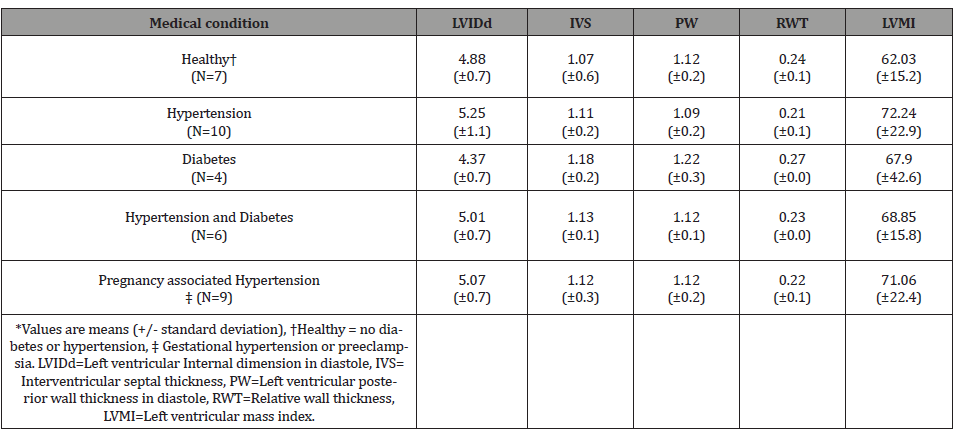
Table 3: Echocardiogram reported (LVIDd, IVS, PW) and calculated (RWT, and LVMI) measures*.
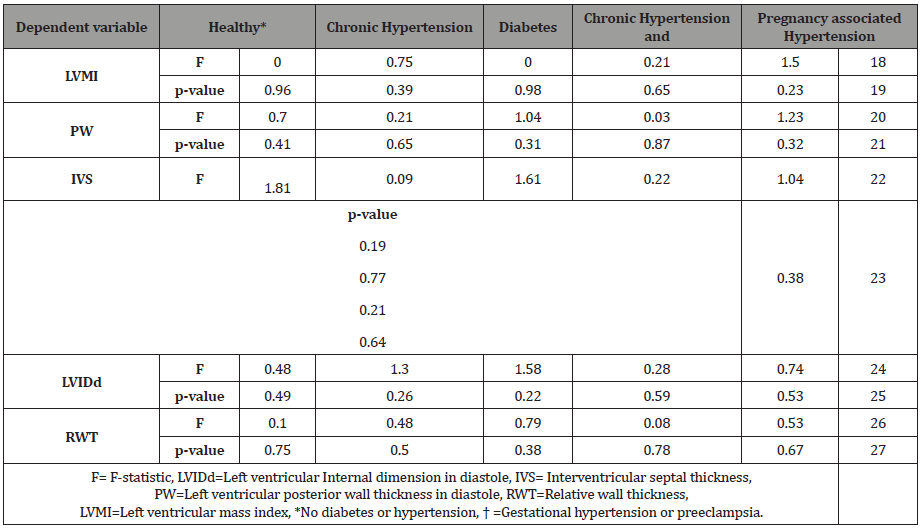
Table 4: Association between BMI and echocardiogram parameters*.
However, both the PW and LVMI were correlated with BMI at enrollment and delivery (Table 4). These relationships are shown graphically for PW (Figure 1) and LVMI (Figure 2). The PW was significantly associated with increasing BMI at enrollment and at delivery (p= .02 and .03 respectively) whereas, LVMI was significantly correlated with BMI at enrollment only (p=.02). We evaluated the impact of mean arterial blood pressure (MAP) at enrollment and delivery on echocardiogram measures and found the MAP at enrollment and at delivery failed to show any significant correlation with any measured or calculated echocardiogram measurement. Twenty- two of twenty-seven patients had a LVMI greater than 51 g/ m2.7 (the upper limit of normal). All twenty-seven patients had an ejection fraction of > 50%. Cardiac geometry determined by LVMI and RWT revealed normal geometry in 6 (22.2%) patients and eccentric hypertrophy in 21 (77.8%). There were no patients with concentric hypertrophy.
Comments
In this study, we performed screening maternal echocardiography in our morbidly obese pregnant patients due to concerns surrounding occult cardiac dysfunction in this population with a history of neglected health care and increased risk of cardiac derived morbidity and mortality. We found few studies in the literature that specifically examined echocardiographic changes or cardiac remodeling in morbidly obese pregnant patients. Where descriptive studies exist comparisons are limited by variation in the calculation of composite variables involved in LVM and LVMI as well as different indexing methods. Furthermore, definitions of cardiac dysfunction and hypertrophic conditions are not uniform across studies [2,6,11,14-16].
Pregnancy is associated with significant physiologic changes in the cardiovascular system. Cardiac output is increased 40- 50% in pregnancy [17, 18] maximizes by 30 -32 wks of gestational age [19]. This increase is in response primarily to increased volume loading that is a normal physiologic response during pregnancy. A single echocardiographic study was done in each of our patients; most were performed close to this time of maximal cardiac demand. Obesity is associated with a further increase in volume loading beyond that seen in normal weight patients, consequently there is a propensity for an even greater cardiac demand [7,8]. Since obesity has been associated with increased left ventricular mass [6, 20] we were not surprised to find that BMI was related to LVMI in our study. Others evaluated morbidly obese pregnant patients with a single echocardiogram and found an increased PW thickness and left ventricular mass [14]. A 30% increase in PW thickness and a 24.6% increase in LVMI was also reported among 34 non-obese pregnant women who were evaluated serially during pregnancy [21]. A similar relationship was found in another study of severely obese non-pregnant population [15]. Our study provides further evidence of enhanced regional specific remodeling, the PW and left ventricular mass, and establishes a correlation with increasing BMI in morbidly obese pregnant patients. The study of non-obese pregnant across pregnancy is evidence that pregnancy physiology impacts the same parameters in the same direction. Since we performed only one echocardiogram we cannot answer whether the PW and LVMI continues to increase as pregnancy progresses in morbidly obese pregnant patients. It is possible that there is a threshold over which cardiac function is impaired. It is also possible that except under unusual circumstances these changes plateau without a detrimental effect on cardiac function.
Although an impaired nocturnal oxygen saturation and systolic blood pressure are strong predictors of left ventricular hypertrophy [15] we explored the relationship between the BMI and LVMI in our severely obese pregnant patients. A measure of the left ventricular mass is particularly important as a higher than usual LVM may predispose a patient to later cardiovascular morbidity and mortality [22,23]. In the current study, LVMI was strongly correlated with BMI at enrollment (p < 0.02) indicating a greater risk of post-pregnancy morbidity and mortality in this sub-group of patients. Twenty two of the twenty-seven patients we evaluated had a LVMI greater than 51 g/ m2.7, the accepted upper limit of normal [6]. The threshold of 51 g/ m2.7 has been validated for black and white populations [24]. This suggests that 76 percent of our evaluated patients may benefit from follow-up aimed at early identification and intervention for abnormal cardiac function and/or cardiovascular disease. Cardiac or ventricular remodeling refers to an alteration in the structure and/or function of the heart in response to hemodynamic changes or cardiac injury [4]. Left ventricular hypertrophy can be broadly divided into physiologic and pathologic and a RWT of >0.45 has often been used as a marker for pathologic left ventricular hypertrophy [5]. Utilizing the LVMI and RWT, four geometric patterns have been described: eccentric hypertrophy (elevated LVMI, normal RWT), concentric hypertrophy (elevated LVMI and RWT), concentric remodeling (normal LVMI and elevated RWT) and normal geometry (normal RWT, normal LVMI) [6,7].
Cardiac adaptation to obesity heralded by left ventricular dilatation and eccentric hypertrophy independent of an increased arterial blood pressure has been described in non-pregnant patients [8]. We were able to show in our study using ANOVA that the echocardiographic findings we observed were independent of the patient’s co-morbidities (Table 3). Controversy still exists over the predominant type of remodeling observed in obese patients. The current study found eccentric cardiac hypertrophy to be the most common form (78%). While we found no patients with concentric hypertrophy, this was the predominant finding in some other studies although eccentric hypertrophy was also commonly seen [6,15]. Mild eccentric hypertrophy has been described as a physiologic response to volume loading in normal pregnancy [25]. This remodeling is consistent with the physiologic response seen in athletes [26]. It is our belief that cardiac remodeling whether in pregnant or non-pregnant severely obese patients serves an important role in preserving ventricular function.
We found a normal ejection fraction, >50% [27], in all patients whether they were examined early or late in pregnancy. Thus, occult cardiac dysfunction was not identified in our patients who reported no history or no symptoms suggestive of underlying cardiac disease. Although obesity is a risk factor for peripartum cardiomyopathy, no patients in the current study developed this complication. Our study has important limitations; these include a small sample size, the absence of a control group and echocardiograms performed a single point in time. Furthermore, some have suggested that 3- dimensional rather than 2- dimensional echocardiogram should be considered the gold standard for determination of LV mass [28]. Serial echocardiograms (2-dimensional and 3-dimensional) early and late in pregnancy followed by an exam during the post-partum period would have been the much more revealing of the impact of BMI, pregnancy physiology, and medical conditions on cardiac function and remodeling. Limited resources prevented us from carrying out such a study.
Our data suggest a screening echocardiogram in morbidly obese patients may help identify a subgroup of patients with increased LVMI who are at-risk for subsequent cardiovascular disease. These findings may be useful in designing medical strategies for follow-up care and motivating patients towards a healthier lifestyle that includes weight reduction.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- GM Cooper, JH McClure (2008) Anesthesia chapter from saving Mothers' Lives; Reviewing maternal deaths to make pregnancy safer: British Journal of Anesthesia 100(1): 17-22.
- Cedergren Marie I (2004) Maternal Morbid Obesity and the Risk of Adverse Pregnancy Outcome. Obstet Gynecol 103(2): 219-224.
- American College of Obstetricians and Gynecologists (2013) ACOG Committee opinion no. 549: obesity in pregnancy. Obstet Gynecol 121(1): 213-217.
- Cohn JN, Colucci WS, Yeon SB (2016) Cardiac Remodeling.
- Feigenbaum H, Armstrong W, Ryan T (2007) Feigenbaum’s Echocardiography 6th
- Angela J Woodiwiss, Carlos D Libhaber, Olebogeng H I Majane, Elena Libhaber, Muzi Maseko, et al. (2008) Obesity promotes left ventricular concentric rather than eccentric Geometric Remodelling and hypertrophy independent of blood pressure. Am J Hypertens 21(10): 1144-1151.
- DeDivitiis O, Fazio S, Petitto M, Maddalena G, Contaldo F, et al. (1981) Obesity and cardiac function. Circulation 64: 477-482.
- FH Messerli, K Sundgaard-Riise, ED Reisin, GR Dreslinski, HO Ventura,et al. (1983) Dimorphic cardiac adaptation to obesity and arterial hypertension. Ann Intern Med 99: 757-761.
- (2013) Practice Bulletin No. 137: Gestational diabetes mellitus. Obstet Gynecol 122(2 Pt 1): 406-416.
- ACOG Committee on Practice Bulletins—Obstetrics (2002) ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol 99(1): 159-167.
- Murilo Foppa, Bruce B Duncan, Luis E P Rohde (2005) Echocardiography-based left ventricular mass estimation. How should we define hypertrophy? Cardiovascular Ultrasound 3: 17.
- Levy D, Savage DD, Garrison RJ, Anderson KM, Kannel WB, et al. (1987) Echocardiographic criteria for left ventricular hypertrophy: the Framingham Heart Study. Am J Cardiol 59: 956-960.
- De Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, et al. (1992) Left ventricular mass and body size in normotensive children and adults assessment of allometric relations and impact of overweight. J Am Col Cardiol 20(5): 1251-1260.
- Veille, Jean-Claude, Hanson, Regina (1994) Obesity, pregnancy and left ventricular Functioning during the third Trimester. Am J Obstet Gynecol 171(4): 980-983.
- Erick Avelar, Tom V Cloward, James M Walker, Robert J Farney, Michael Strong, et al. (2007) Left ventricular Hypertrophy in severe obesity.Hypertension 49(1): 34-39.
- (2014) Practice bulletin no. 145: antepartum fetal surveillance. Obstet Gynecol 124(1): 182-192.
- M Vázquez Blanco, O Grosso, C A Bellido, O R Iavícoli, C S Berensztein, et al. (2000) Left ventricular Geometry in pregnancy –induced Hypertension. Am J Hypertens 13(3): 226-230.
- Elkayam U, Gleicher N (1998) Haemodynamic and Cardiac Function During Normal Pregnancy. In: Cardiac Problems in Pregnancy, New York, USA. Pp. 3-20.
- Gabbe SG, Niebyl JR, Simpson LJ, Galan H, Jauniaux RM, et al. (2012) Obstetrics: Normal and problem pregnancies 6th
- Lauer MS, Anderson KM, Kannel WB, Levy D (1991) The impact of obesity on left ventricular mass and geometry. The Framingham Heart study.JAMA 266: 231-236.
- Geva T, Mauer MB, Striker L, Kirshon B, Pivarnik JM (1997) Effects of physiologic load of pregnancy on left ventricular contractility and remodeling. Am Heart J 133(1): 53-59.
- Nunez E, Arnett DK, Benjamin EJ, Liebson PR, Skelton TN, et al. (2005) Andrew M.Optimal threshold value for left ventricular hypertrophy in blacks: The Atherosclerosis Risk in communities Study. Hypertension 45(1): 58-63.
- Vakili BA, O kin PM, Devereux RB (2001) Prognostic implications of left ventricular hypertrophy. Am Heart J 141: 334-341.
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP (1990) Prognostic implications of echocardiographically determined left ventricular mass in the Framingham heart study. N England J Med 322: 1561-1566.
- Nunez E, Arnett DK, Benjamin EJ, Liebson PR, Skelton TN, et al. (2005) Optimal threshold value for left ventricular hypertrophy in blacks. The Atherosclerosis Risk in communities Study. Hypertension 45: 58-63.
- Mabie WC, DiSessa TG, Crocker LG, Sibai BM, Arheart KL (1994) A longitudinal study of cardiac output in normal human pregnancy. AM J obstet Gynecol 170: 849-856.
- Lusiani L, Ronsisvalle G, Bonanome E, Visona A, Castellani V, et al. (1986) Echocardiographic evaluation of the dimensions and systolic properties of the left ventricle in freshmen athletes during physical training. Eur Heart J 7: 196-203.
- U Elkayam, PP Tummala, K Rao, MW Akhter, IS Karaalp, et al. (2001) Maternal and Fetal Outcomes of Subsequent Pregnancies in Women with Peripartum Cardiomyopathy. N Engl J Med 344(21): 1567-1571.
- Isbell DC, Kramer CM (2005) Cardiovascular magnetic resonance: structure, function, perfusion, and viability. J Nucl Cardiol 12(3): 324-336.
-
Oluseyi K Ogunleye*, Hongmei Zhang, Stanette L Brown, Paul C Browne and Anthony R Gregg. Screening Echocardiography in Morbidly Obese Pregnant Women. On J Cardio Res & Rep. 6(5): 2022. OJCRR.MS.ID.000647.
-
Echocardiogram, Left ventricular hypertrophy, Obesity, Pregnancy, Cardiac dysfunction, Gestational hypertension, Preeclampsia, Arterial blood pressure, Echocardiography, Cardiac morbidity, Mortality
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






