 Mini Review
Mini Review
Optimizing Cardiovascular and Renal Outcomes with Canagliflozin- A Focus on CANVAS and CRENDENCE Trials
Tarek Samy Abdelaziz* and Salwa Ibrahim
Department of Renal medicine, Cairo university hospitals, Egypt
Tarek Samy Abdelaziz, Department of Renal medicine, Cairo university hospitals, Egypt.
Received Date: October 26, 2019; Published Date: November 01, 2019
Abstract
Canagliflozin is one of the sodium glucose cotransporters 2 (SLGT2) inhibitors. In addition to its glucose lowering effect, it has cardiovascular and renal protective mechanisms, through decreasing blood pressure, body weight, diuresis and natriuresis. This has translated into beneficial cardiovascular and renal outcome as compared to placebo in two recent large trials. The most pronounced effects occur in patients with moderate and severe albuminuria.
Introduction
Patients with type 2 diabetes mellitus have an accelerated risk of developing cardiovascular morbidity and mortality. The presence of chronic kidney disease constitutes a significant cardiovascular risk. Certainly, there is a thriving need to mitigate cardiovascular risk without causing insult to the kidneys, especially in patients with type 2 diabetes mellitus where both cardiovascular and renal morbidities exist. Angiotensin converting enzyme inhibitors (ACEI) and angiotensin II receptor blockers (ARBs) are key medications in primary and secondary prevention of cardiovascular diseases. They act to slow the progression of albuminuria in patients with diabetic nephropathy. ACEI and ARBs delay progression to dialysis and decrease mortality in patients in the predialysis stage [1,2]. However, their use in advanced stages of diabetic nephropathy carries the risk of hyperkalaemia, necessitating cautious and judicious titration of doses especially with accelerated progression of nephropathy [1,2]. Sodium glucose co-transporter inhibitors (SLGT2) are evolving agents that have been recently used for the treatment of type 2 diabetes mellitus. Interestingly, they have proved to exert beneficial cardiovascular effects. The exact mechanisms remain to be fully understood.
Cardiovascular effects of SLGT inhibitors in preclinical studies and clinical studies
Cardiac protection of SLGT2 inhibitors may be attributed to the class effects. The beneficial effects could not be explained solely by the improvement in glycaemic control. Empagliflozin was shown to achieve the most pronounced reduction in cardiovascular morbidity and mortality. In the EMPAREG-OUTCOME trial, a 35 %relative risk reduction of hospitalization due to heart failure and 38% reduction of death due to cardiovascular cause [3]. This relative risk reduction is the higher than cardiovascular risk reduction in the CANVAS study of canagliflozin. This may suggest that empagliflozin has a better cardio-protective profile than other SLGT2 inhibitors. However, since there is no head to head comparison of more than one agent of SLGT2 inhibitors family, this hypothesis lacks robust evidence. Several mechanisms have been proposed to explain the SLGT2 inhibitors effects in reducing cardiovascular morbidity and mortality in patients with type 2 diabetes mellitus [4]. The experimental evidence led to several theories that might explain this striking beneficial effect [5]. Diuresis is one of the most important mechanisms that lead to unloading of the left ventricle. SLGT2 inhibitors can induce diuresis by natriuretic and osmotic effects. Diuresis induced by SLGT2 does not lead to reflex sympathetic activation, unlike loop or thiazide diuretics. Diuresis leads to reduction in systolic blood pressure [4]. Another important theory is fuel switching of the cardiac myocytes from utilizing fatty acid oxidation as the main fuel to utilizing ketone bodies (B hydroxybutyric acid) especially with empagliflozin. Through different mechanisms, SLG2 inhibitors can lower the blood pressure.
Cardiovascular outcomes CANVAS and CREDENCE
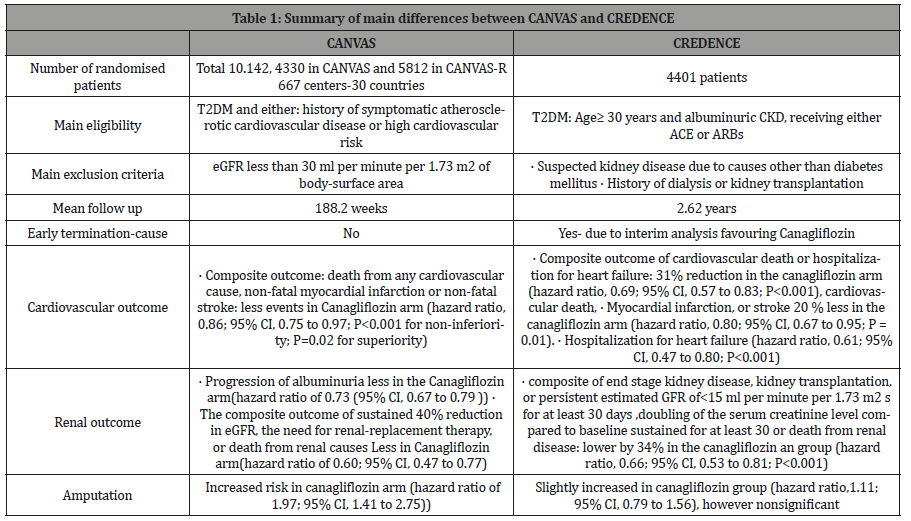
Two large studies, assessing cardiovascular outcomes of canagliflozin, have been recently published. The CANVAS (Canagliflozin Cardiovascular Assessment Study) and its extension the CANVAS-R (Renal) assessed cardiovascular events as a composite primary outcome, while CREDENCE (Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy) study assessed composite cardiovascular outcome as a composite secondary outcome [6]. Both studies have demonstrated lower rates of cardiovascular events in patients receiving canagliflozin. CANVAS has assured FDA regarding cardiovascular safety of the use of Canagliflozin. Interestingly, a pronounced cardiovascular benefit was observed in patients who received canagliflozin as compared to placebo.
Renal outcomes in CANVAS and CREDENCE
The CANVAS and CANVAS R showed improvement in a composite renal outcome due to use of canagliflozin as noted in (Table 1). Participants with very low eGFR (less than 30 ml per minute per 1.73 m2 of body-surface area) were excluded from this study, unlike CREDENCE. Moreover, the levels of albuminuria in the participants of CANVAS were not as high as CREDENCE participants. A post-hoc analysis of the CANVAS data revealed an interesting finding [7]. The effect of canagliflozin was more pronounced in patients with moderate and severe albuminuria; delay in loss of renal function was greater in patients with severe albuminuria [7]. This effect was not due to the mere improvement in glycaemic control, other mechanisms contribute to SLG2 inhibitors action on the kidney and the heart [6,7]. The CREDENCE was a large multicentre study designed to examine the effect of canagliflozin on renal outcomes in patients with diabetes and albuminuria chronic kidney disease [6]. This is one of the limitations of the study, as now it is known that other phenotypes of diabetic kidney disease exist. Non-albuminuria diabetic kidney disease is currently a recognized phenotype in patients with type 2 diabetes, where progression to end stage renal disease occurs regardless of progression of proteinuria [8]. Patients with non- albuminuric kidney disease were not included in this study. Patients were required to be on stable dose of either ACEI or ARB. Potential renal protective mechanisms of SLGT2 inhibitors, other than improved glycaemic control are decompression of the glomerulus and reduction of blood pressure [1,9].
Table 1: Summary of main differences between CANVAS and CREDENCE.
Risk of lower limb amputation
A major concern in CANVAS was the higher rate of amputation. The main risk factor was history of previous amputation, the highest level of amputation was at the level of toes or metatarsals. In CREDENCE, however amputation rate was not significantly higher in patients receiving canagliflozin [6]. The reason for this discrepancy is not exactly clear, but may be explained by the difference in population, as CANVAS participants had established cardiovascular morbidity or high cardiovascular risk as entry criteria [6,7].
Conclusion
Canagliflozin has been shown to reduce adverse cardiovascular and renal outcomes in patients with T2DM. This was clear from both CANVAS and CREDENCE trials. The most pronounced cardiovascular benefit was reduction of mortality from cardiovascular causes. Renal benefits include regression of proteinuria, slowing proteinuria progression and slowing progression to dialysis. Canagliflozin cardiovascular and renal benefits are more obvious in patients with marked albuminuria and when added on top of ACE or ARBs.
Acknowledgement
None.
Conflict of Interest Statement
No conflict of interest.
References
- Hsu FY, Lin FJ, Ou HT, Huang SH, Wang CC, et al. (2017) Renoprotective Effect of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers in Diabetic Patients with Proteinuria. Kidney Blood Press Res 42(2): 358-368.
- Hsu TW, Liu JS, Hung SC, Kuo KL, Chang YK, et al. (2014) Renoprotective effect of renin-angiotensin-aldosterone system blockade in patients with predialysis advanced chronic kidney disease, hypertension, and anemia. JAMA Intern Med 3: 347-354.
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, et al. (2015) Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 22: 2117-2128.
- Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ, et al. (2016) Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus. Circulation 10: 752-772.
- Bertero E, Prates Roma L, Ameri P, Maack C (2018) Cardiac effects of SGLT2 inhibitors: the sodium hypothesis. Cardiovasc Res 1: 12-18.
- Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, et al. (2019) Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med 24: 2295-2306.
- Neuen BL, Ohkuma T, Neal B, Matthews DR, de Zeeuw D et al. (2019) Effect of Canagliflozin on Renal and Cardiovascular Outcomes across Different Levels of Albuminuria: Data from the CANVAS Program. J Am Soc Nephrol ASN.2019010064.
- Pugliese G (2014) Updating the natural history of diabetic nephropathy. Acta Diabetol 6: 905-915.
- Fioretto P, Zambon A, Rossato M, Busetto L, Vettor R, et al. (2016) SGLT2 Inhibitors and the Diabetic Kidney. Diabetes Care 2: S165-S171.
-
Tarek Samy Abdelaziz, Salwa Ibrahim. Optimizing Cardiovascular and Renal Outcomes with Canagliflozin- A Focus on CANVAS and CRENDENCE Trials. On J Cardio Res & Rep. 3(1): 2019. OJCRR.MS.ID.000552.
-
Blood pressure, Cardiovascular, Kidney disease, Nephropathy, Albuminuria, Proteinuria, Cardiac myocytes, Heart failure, Hyperkalemia
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






