 Review Article
Review Article
Influence of Exercise or Physical Activity in the Angiogenesis Process: Integrative Review
Lucas Cecin de Deus Spirandelli1, Mateus Borges Soares1, Otávio Cortes Alves1, Vítor Brandão Veloso1, Pedro Teixeira Meireles1, Thiago Mantello Bianco2, Bruno Belmonte Martinelli Gomes2, Eduardo Elias Vieira de Carvalho3, Ana Karina Marques Salge4, George Kemil Abdalla5 and Douglas Reis Abdalla1,5*
1Medicine Course, University of Uberaba, Uberaba, MG, Brazil
2Biomedicine, Serrana State Hospital, Serrana, SP, Brazil
3Professor, Department of Applied Physical Therapy - Federal University of Triângulo Mineiro, Uberaba, MG, Brazil
4Professor, Faculty of Nursing, Federal University of Goiás, Goiânia, GO, Brazil
5Professor, Health Sciences, Faculty of Human Talents, Uberaba, MG, Brazil
Douglas Reis Abdalla, Department Health Sciences, Faculty of Human Talents, Uberaba, MG, Brazil.
Received Date: April 10, 2020; Published Date: April 23, 2020
Abstract
In order to understand the relationships of the influence of physical activity in the angiogenesis process, this review aims to recruit in the last ten years the evidence on this topic. The study data collection took place between February 20 and March 20, 2020. The electronic databases used to search the articles was PubMed (National Library of Medicine and National Institutes of Health). We used the keywords: angiogenesis, angiogenic effect, vascular endothelial growth factor (VEGF), physical activity, physical exercise, exercise and training, in the English languages, accompanied by the expression AND and selected through DeCS (Descriptors in Health Sciences). The performance of physical activity, (Figure 2), whether aerobic or resisted with load, promotes in the body an increase in pro-angiogenic factors such as: IL-6, Ang 1 and 2, VEGF, PDGF, FGF and stimulation of their receptors, being, respectively: IL -6Ra, TIE-2, VEGFR-1 and 2, PDGFR, FGFR. Higher levels of Adropine also encourage the expression of VEGFR-2. The activation of IL-6Ra, VEGFR-1 and 2 receptors elevates, together with increased expression of miR-126, a small fragment of non-coded RNA, the enzyme PI3k (Phosphoinositide 3-kinase). This increased enzyme induces the expression of protein Kinase B (Akt) which plays a fundamental role in cell metabolism via the mTOR pathway. We will then have the formation of MMP-2, MMP-9, VEGF, CD31 and HIF-1α, the latter being directly stimulated by the increase in NO. In this way, those responsible for proliferation, migration, survival and cell permeability will be present, necessary for improvements in the levels of angiogenesis to occur. It was possible to conclude that the physical activity induced in both experimental and human models favored the process of angiogenesis in organisms by increasing pro factors and decreasing anti-angiogenic factors, regardless of preexisting comorbidities and previous sedentary lifestyle.
Keywords: Physical activity; Exercise; Angiogenesis; Vascular Endothelial Growth Factor
Introduction
The word “angiogenesis” was derived from the Greek where “angio” means blood vessel and “genesis” means production or birth, together they refer to the generation of blood vessel within the body. Historically, the term angiogenesis was first used to describe the growth of endothelial shoots from pre-existing postcapillary veins. Over time, this term has been used to denote the process of growth and remodeling of the primitive network of a vascular complex [1]. The vascular system is responsible for the supply of nutrients and oxygen in an organism. New blood vessel formation or neovascularization is divided into two components like vasculogenesis and angiogenesis. The vasculogenesis process is the formation of blood vessels from hemangioblasts that differentiate into mature blood and endothelial cells [2]. Angiogenesis is the process of forming new blood vessels from a pre-existing vascular network, by capillary sprouting [3]. Vasculogenesis ascends the heart and the first primitive vascular plexus within the embryo and in the surrounding membranes, considering that angiogenesis is responsible for the remodeling and expansion of this network. During this process, mature endothelial cells are divided and incorporated into new capillaries. The signaling of vascular endothelial growth factors (VEGF) is necessary for the complete performance of vasculogenesis and angiogenesis [2,4].
The health benefits of regular physical activity are present in several chronic diseases, including cardiovascular disease, diabetes, hypertension and cancer [5-8]. However, physical inactivity is a risk factor for several pathological conditions, including obesity, hypertension, atherosclerosis and cancer [9-11]. Physical training is known to profoundly alter the morphology of blood vessels along the arterial tree [12-14]. Exercise provides increases related to the quantity (angiogenesis) and the diameter (arteriogenesis) of the arterial blood vessels in the skeletal muscle and in the myocardium. These changes in the architecture of the vascular tree are probably associated with functional changes and improved blood flow to the organ [15-18]. Changes in vascular morphology induced by physical exercise in healthy individuals [15,16] are extremely dependent on the size of the initial vessel. A greater number of vessels in response to training, angiogenesis, appears to occur on the level of very small capillaries and arterioles (<40 μm in diameter), but not in large arteries. The increase in capillary density occurs just after the beginning of the exercise and is transient. A similar pattern was observed in very small arterioles (<20 μm in diameter) and slightly in larger arterioles (20-40 μm in diameter) an increase in the number was also observed [19].
The molecular mechanisms underlying exercise-induced angiogenesis are not fully understood. It has been suggested that growth factors such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF) and angiopoietins (ANG) as well as their corresponding receptors are involved. In addition, the proteases necessary for the degradation of the capillary basement membrane such as matrix metalloproteinases (MMPs), urokinase, tissue plasminogen activator probably contribute to the mechanism of the emergence of angiogenesis [16,20,21]. Interestingly, some of these proteases appear to allow and/or facilitate the mobilization of endothelial progenitor cells (EPCs) from the bone marrow. It has become apparent that exercise can increase the number of circulating EPCs in animals and humans, and these cells are known to have a large capacity for neovascularization, a process that appears to be critically dependent on the protease cathepsin L [22,23]. In order to understand the relationships of the influence of physical activity in the angiogenesis process, this review aims to recruit in the last ten years the evidence on this topic.
Methodology
In the present study, an integrative review was conducted, which consists of research that allows the evaluation, synthesis and knowledge about a phenomenon from evidence, aiming to produce an overview of complex concepts, theories or relevant health problems from studies pre-existing, enabling the intervention proposal [24,25]. For the selection of articles, 6 methodological steps were carried out, namely: 1. elaboration of the guiding question or research hypothesis, that is, the problem was identified, the search engine and the keywords or keywords were presented; 2. establishment of the inclusion and exclusion criteria of the articles to be selected to compose the sample; 3. exploratory reading of the titles and abstracts of the articles for pre-selection; 4. analytical reading of the articles in order to compile, analyze and categorize the information; 5. interpretation of results. 6. synthesis followed by the presentation of the identified results, which permeate the guiding question [26].
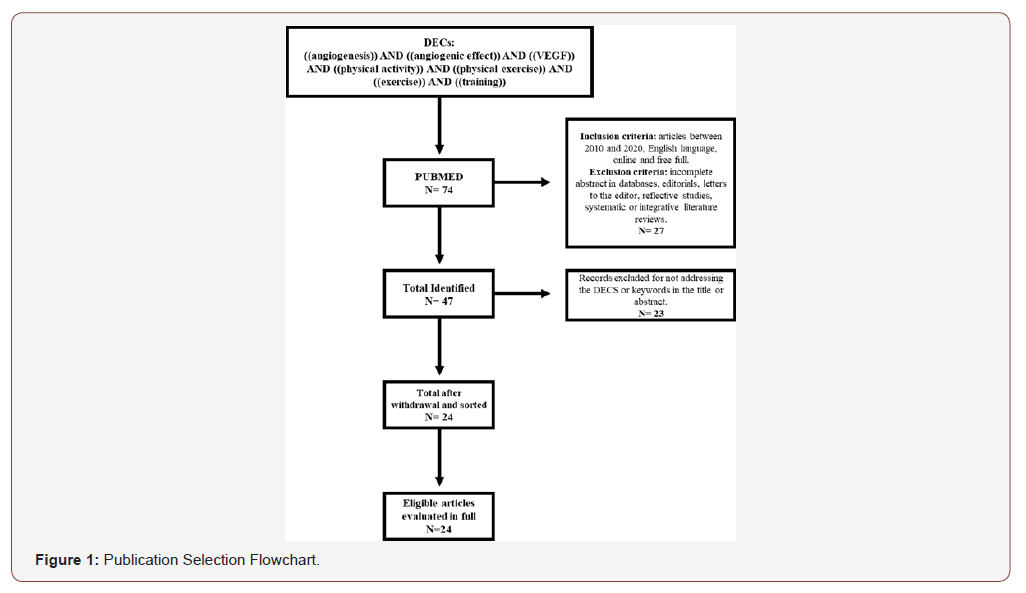
Therefore, in this study it was decided to search for the concepts: angiogenesis, angiogenic effect, vascular endothelial growth factor (VEGF), physical activity, physical exercise, exercise and training. From these concepts, the guiding question was defined: what is the scientific evidence available in the literature on the relationship between physical activity and events related to the angiogenesis process. After formulating the question to be researched, a bibliographic survey was carried out on the PubMed platform. The survey of the study took place between February 20 and March 20, 2020. And the selection of texts proceeded with searches on the platform, using the filters available for texts published between 2010 and 2020. For the selection of publications, the following were adopted inclusion criteria: scientific articles, published in the English language, between 2010 and 2020, available online and free of charge in full. Articles with no abstract in the database or incomplete, editorials, letters to the editor, reflective studies, systematic or integrative literature reviews were excluded. After defining the guiding question, location and selection of articles, 74 publications potentially eligible to be included in this review were identified. After applying the inclusion and exclusion criteria, the sample consisted of 47 publications, the abstracts of 24 records were analyzed to see if they would meet the eligibility criteria and answer the question that guides this review, thus excluding 23 records and only 24 were analyzed in full to confirm eligibility for quantitative synthesis and data analysis according to the selection flowchart (Figure 1).
Result and Discussion
The proposed review aimed to associate the practice of physical activity and angiogenic parameters, such as VEGF synthesis, expression of its receptors (VEGFR), as well as the expression of angiogenesis inducing molecules and involved in the quantification of microvascular density (CD31), as well as the synthesis of chemical mediators involved in this process. Thus, we divided the reviewed studies into two groups, namely: studies with experimental models and studies involving human beings.
Table 1 lists the studies with experimental models of physical activity and angiogenic study. Thus, Lee and Cols (2018) [27], report that the performance of voluntary wheel exercises performed by mice for 6 weeks resulted in an increase in angiogenic factors. As well as, moderate intensity exercises performed by mice, during 8 weeks, increased the gene expression of angiogenic factors and decreased insulin resistance [28]. In addition, in the performance of physical activity performed by elderly mice, for 10 weeks, an increase in VEGF, an increase in BFR was observed, occurring a cardiac physiological remodeling corresponding to the demand [29]. The treadmill practice performed by mice is correlated to the increased expression of angiogenic factors, both in healthy animals and in animals with previous comorbidities [30]. Likewise, there was an improvement in the levels of cardiac markers correlated with physical activity performed on a treadmill by male mice at moderate intensity, for 8 weeks [31]. The practice of aerobic exercises performed by male mice, for 15 minutes/day for 8 weeks, is directly related to an improvement in cardiac angiogenesis and in the intramuscular capillary density [32]. Still, the practice of AET performed by male mice, which consists of swimming sessions of 60 minutes, 5 times a week, for 10 weeks, proved to be effective in vascular remodeling, being an important therapeutic target for the treatment of several cardiovascular diseases [33]. Individuals who performed physical exercise in swimming mode, at different intensities, obtained an increase in angiogenic factors, some of them (VGEF-B, MEF-2, MMP-2) with greater proportional increases in intensity and, on the other hand, ANGPT-1 and HDAC4 showed more satisfactory results at moderate exercise intensity [34].
Table 1:List of articles that investigated the influence of physical activity on the angiogenesis process in experimental models.
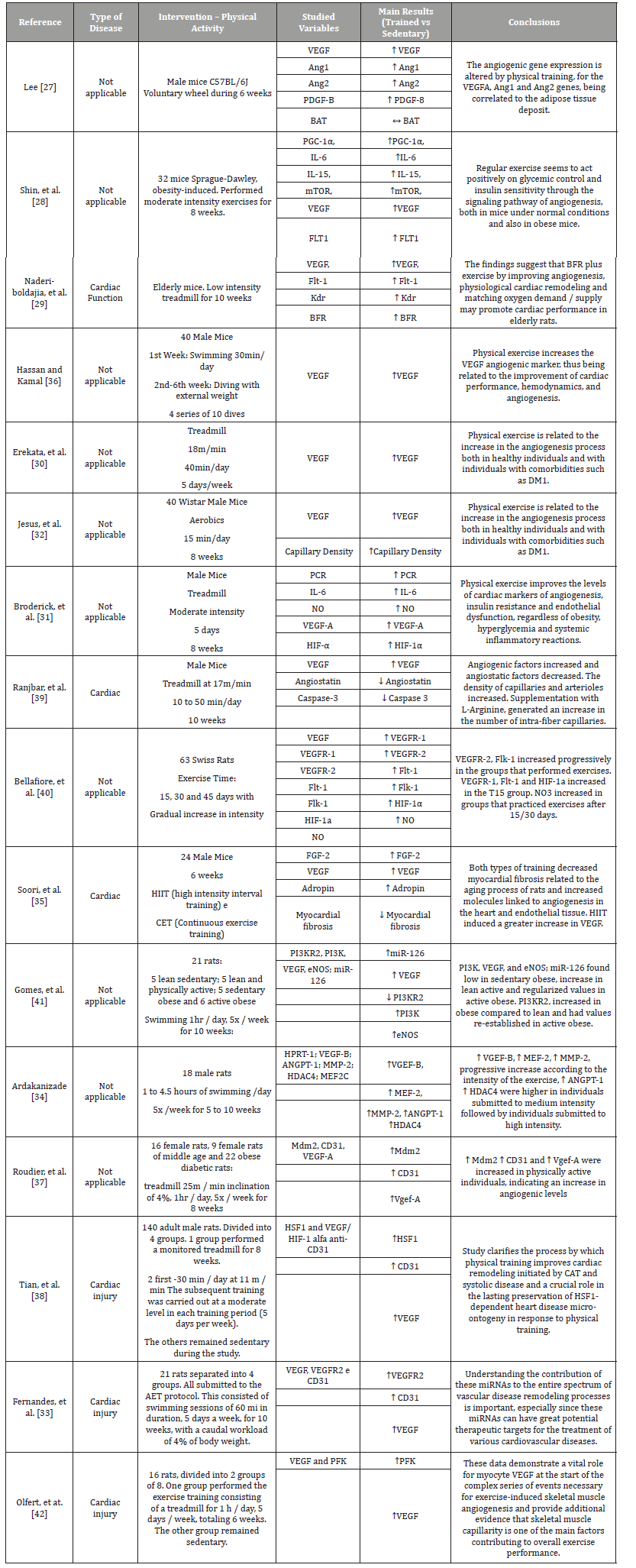
Finally, rats with heart disease undergoing physical training, high intensity interval (HIIT) and continuous rhythm (CET), presented a decrease in myocardial fibrosis related to the aging process, in addition to increasing the amount of molecules linked to angiogenesis in the heart and in the endothelial tissue. Being that HIIT training induced a greater increase in VEGF [35]. In this sense, aerobic and resistance exercise with load also promoted, in the study by Hassan and Kamal (2013) [36], improvement in cardiac performance, hemodynamics and angiogenesis, related to the VEGF marker. The practice of physical exercise on a 4% inclined treadmill, for 1 hour, 5 times a week, for 8 weeks, in female mice, being diabetic and obese, proved to be effective in increasing angiogenic levels [37]. With the practice of physical activity performed on a treadmill by male rats with varying intensity (from mild to moderate), an improvement in cardiac remodeling initiated by CAT is observed [38]. In addition, it was found that performing a 17m / min intensity mat in male rats, from 10 to 50 minutes / day, for 10 weeks, decreased angio-static factors and increased angiogenic factors [39]. In addition, the practice of exercise performed by mice with a gradual increase in intensity progressively increased the angiogenic factors VEGFR-2 (FLK-1) [40].
Likewise, obese and normal weight individuals, whether sedentary or not, were submitted to physical activity in the swimming modality. The results obtained by Gomes et al. (2017) [41], indicated an increase in pro-angiogenic factors in physically active individuals, this increase being higher in individuals with normal weight when compared to obese individuals. Analyzing the practice of physical exercises on a treadmill, performed by male rats to the detriment of sedentary mice, it can be concluded that those who exercised, obtained a significant increase in angiogenic factors when compared to those who did not practice [42].
Table 2 summarizes the studies involving human beings, in which the practice of physical activity with the use of an exercise bike, caused an increase in cytokines and angiogenesis and biogenesis factors in skeletal muscle cells [43]. Aerobic physical activity performed for 12 weeks promoted an increase in muscle capillarity, an increase in VEGF and VEGFR, an increase in HIF-α and α-tubulin. However, protein supplementation was not effective in changing these parameters, but the practice of physical activity [44].
Table 2:List of articles that investigated the influence of physical activity on the angiogenesis process in human trials.
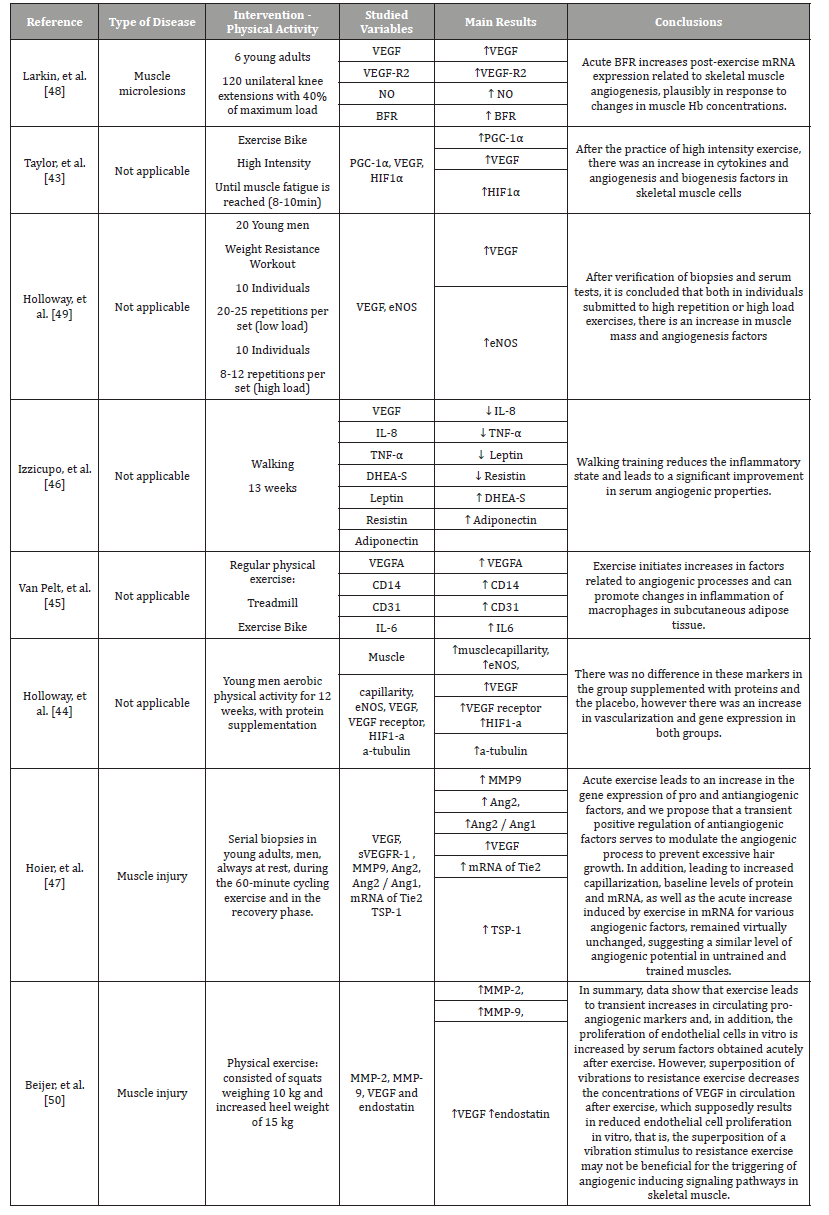
Positive results regarding the induction of angiogenesis mediators were reported in the study by Van Pelt, et al. (2017) [45], in which regular physical exercises, including: physical maintenance on a treadmill and exercise bike, promoted changes in inflammation of macrophages in the subcutaneous tissue and increased levels of VEGFA, CD31, CD14 and IL-6. Walking as the physical activity of choice for 13 weeks reduces the inflammatory state and significantly improves serum angiogenic properties. A reduction in IL-8, TNF-α, Leptin, Resistin, Adiponectin and an increase in DHEA-S were evidenced [46].
On the other hand, when performing serial biopsies in young adults at rest, after cycling for 60 minutes, Hoier and Cols (2012) [47], evidenced despite the slight increase in TIE-2, TSP-1, Ang2, MMP-9 and VEGF, which would practically not influence the angiogenic potential of trained muscles. Regarding the practice of resistance exercises, Larkin and Cols (2012) [48], show that the acute restriction of blood flow in patients with muscle microlesions, who performed knee extensions with load showed an increase in mRNA related to skeletal muscle angiogenesis in response to changes in hemoglobin concentration in skeletal muscle. In addition, resisted physical activity with weight, either with high load and little repetition, or with little load and high repetition, promoted an increase in muscle mass and also in angiogenic factors [49]. In individuals undergoing resistance physical exercise, there is a transient increase in pro-angiogenic markers and an increase in cell proliferation in vitro. However, the overlapping of vibrations to that same exercise results in decreased proliferation of cells by decreasing the circulating VEGF, and may therefore not be beneficial for triggering angiogenesis-inducing signaling pathways [50].
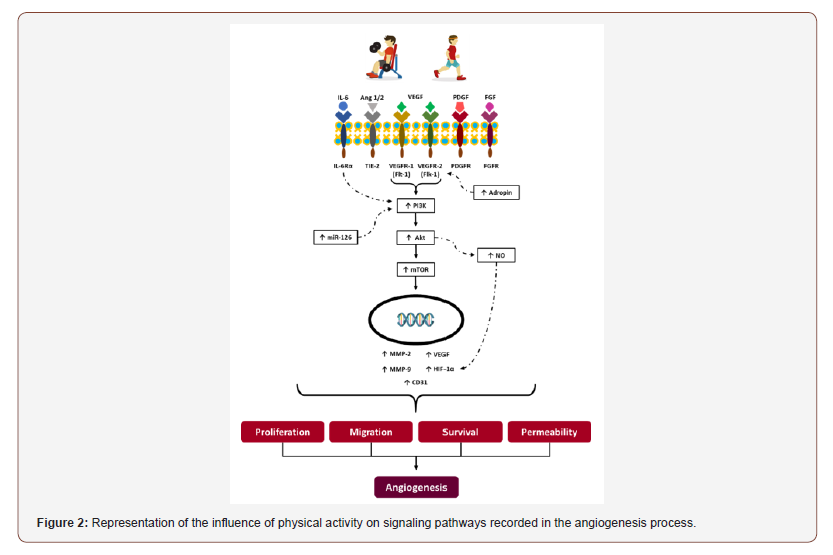
The performance of physical activity, (Figure 2), whether aerobic or resisted with load, promotes in the body an increase in pro-angiogenic factors such as: IL-6, Ang 1 and 2, VEGF, PDGF, FGF and stimulation of their receptors, being, respectively: IL -6Ra, TIE-2, VEGFR-1 and 2, PDGFR, FGFR. Higher levels of Adropine also encourage the expression of VEGFR-2. The activation of IL- 6Ra, VEGFR-1 and 2 receptors elevates, together with increased expression of miR-126, a small fragment of non-coded RNA, the enzyme PI3k (Phosphoinositide 3-kinase). This increased enzyme induces the expression of protein Kinase B (Akt) which plays a fundamental role in cell metabolism via the mTOR pathway. We will then have the formation of MMP-2, MMP-9, VEGF, CD31 and HIF-1α, the latter being directly stimulated by the increase in NO. In this way, those responsible for proliferation, migration, survival and cell permeability will be present, necessary for improvements in the levels of angiogenesis to occur.
Conclusion
Physical activity was of fundamental importance in inducing angiogenesis. Through these studies, it was possible to notice that both in experimental models and in human beings, foods or comorbidities, the practice of physical exercises, either resistant to loads or aerobics, considerably improved the levels of vascular evaluation, clinical factors, expression enzyme and its receptors. In addition, anti-angiogenic factors apply at the highest levels in those who remain sedentary, corroborating the idea that the practice of physical activity is of paramount importance for neovascular formation, or that it may possibly cause a determining factor in the quality of life and health of individuals.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Carmeliet P (2000) Mechanisms of angiogenesis and arteriogenesis. Nat Med 6: 389-395.
- Roskoski R (2007) Vascular endothelial growth factor (VEGF) signaling in tumor progression. Crit Rev Oncol Hematol 62: 179-213.
- Abdalla D, Simoens C, Bogers J-P, Murta EFC, Michelin M (2015) Angiogenesis Markers in Gynecological Tumors and Patents for Anti- Angiogenic Approach: Review. Recent Patents on Anti-Cancer Drug Discovery 10(3): 298-307.
- Hall AP, Westwood FR, Wadsworth PF (2006) Review of the effects of anti-angiogenic compounds on the epiphyseal growth plate. Toxicol Pathol 34: 131-147.
- Abdalla DR, Murta EFC, Michelin MA (2013) The influence of physical activity on the profile of immune response cells and cytokine synthesis in mice with experimental breast tumors induced by 7,12-dimethylbenzanthracene. European Journal of Cancer Prevention 22(3): 251-258.
- Abdalla DR, Rocha Aleixo AA, Murta EFC, Michelin MA (2014) Innate immune response adaptation in mice subjected to administration of DMBA and physical activity. Oncology Letters 7(3): 886-890.
- Abdalla DR, Gomes BBM, Murta EFC, Michelin MA (2017) Bone marrow-derived dendritic cells under influence of experimental breast cancer and physical activity. Oncology Letters 13(3): 1406-1410.
- Bianco TM, Abdalla DR, Desidério CS, Thys S, Simoens C, et al. (2017) The influence of physical activity in the anti-tumor immune response in experimental breast tumor. Immunology Letters 190: 148-158.
- Booth FW, Gordon SE, Carlson CJ, Hamilton MT (2000) Waging war on modern chronic diseases: Primary prevention through exercise biology. J Appl Physiol 88: 774-787.
- Lai YC, Chen CY, Chen CC, Kuo CH (2009) Exercise and type 2 diabetes. Adapt Med 1: 1-16.
- Thune I, Furberg AS (2001) Physical activity and cancer risk: Doseresponse and cancer, all sites and site-specific. Med Sci Sports Exerc 33: S530-S550.
- Laughlin MH, McAllister RM (1992) Exercise training-induced coronary vascular adaptation. J Appl Physiol 73: 2209-2225.
- Skalak TC, Price RJ, Zeller PJ (1998) Where do new arterioles come from? Mechanical forces and microvessel adaptation. Microcirculation 5: 91-94.
- Brown MD, Hudlicka O (2003) Modulation of physiological angiogenesis in skeletal muscle by mechanical forces: involvement of VEGF and metalloproteinases. Angiogenesis 6: 1-14.
- Brown MD (2003) Exercise and coronary vascular remodelling in the healthy heart. Exp Physiol 88: 645-658.
- Prior BM, Yang HT, Terjung RL (2004) What makes vessels grow with exercise training. J Appl Physiol 97: 1119-1128.
- Heil M, Schaper W (2004) Influence of mechanical, cellular, and molecular factors on collateral artery growth (arteriogenesis). Circ Res 95: 449-458.
- De Oliveira LFL, Thackeray JT, Neto JAM, Dias Romano MM, De Carvalho EEV, et al. (2018) Regional myocardial perfusion disturbance in experimental chronic chagas cardiomyopathy. Journal of Nuclear Medicine 59(9): 1430-1436.
- White FC, Bloor CM, McKirnan MD, Carroll SM (1998) Exercise training in swine promotes growth of arteriolar bed and capillary angiogenesis in heart. J Appl Physiol 85: 1160-1168.
- Roy S, Khanna S, Sen CK (2008) Redox regulation of the VEGF signaling path and tissue vascularization: Hydrogen peroxide, the common link between physical exercise and cutaneous wound healing. Free Rad Biol Med 44: 180-192.
- Yang AL, Su CT, Lin KL, Lee SD (2008) Enhancement of vascular function mediated by insulin and insulin-like growth factor-1 following single exercise session. Chinese J Physiol 51: 71-77.
- Laufs U, Werner N, Link A, Endres M, Wassmann S, et al. (2004) Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation 109: 220-226.
- Urbich C, Dimmeler S (2004) Endothelial progenitor cells: characterization and role in vascular biology. Circ Res 95: 343-353.
- Galvão CM, Sawada NO, Trevizan MA (2004) Systematic review: a resource that allows the incorporation of evidence into nursing practice. Rev Latino-am Enfermagem 17(4): 758-764.
- Whittemore R, Knafl K (2005) The integrative review: updated methodology. J Adv Nurs. England 52(5): 546-553.
- De Sousa LD, Lunardi Filho WD, Lunardi VL, Santos SS, Dos Santos CP (2011) The nursing scientific production about the clinic: an integrative review. Rev Esc Enferm USP (SP) 45(2): 494-500.
- Lee HJ (2018) Exercise training regulates angiogenic gene expression in white adipose tissue. J Exerc Rehabil 14(1): 16-23.
- Shin KO, Bae JY, Woo J, Jang KS, Kim KS, et al. (2015) The effect of exercise on expression of myokine and angiogenesis mRNA in skeletal muscle of high fat diet induced obese rat. J Exerc Nutrition Biochem 19(2): 91-98.
- Naderi-Boldaji V, Joukar S, Noorafshan A, Raji-Amirhasani A, Naderi-Boldaji S, et al. (2018) The effect of blood flow restriction along with low-intensity exercise on cardiac structure and function in aging rat: Role of angiogenesis. Life Sci 209: 202-209.
- Erekat NS, Al-Jarrah MD, Al Khatib AJ (2014) Treadmill Exercise Training Improves Vascular Endothelial Growth Factor Expression in the Cardiac Muscle of Type I Diabetic Rats. Cardiol Resn 5(1): 23-29.
- Broderick TL, Sennott JM, Gutkowska J, Jankowski M (2019) Anti-inflammatory and angiogenic effects of exercise training in cardiac muscle of diabetic mice. Diabetes Metab Syndr Obes 12: 565-573.
- Jesus I, Herrera NA, Andreo JC, Santos CF, Amaral SL (2020) Training counteracts DEX-induced microvascular rarefaction by improving the balance between apoptotic and angiogenic proteins. Steroids 156: 108573.
- Fernandes T, Casaes L, Soci Ú, Silveira A, Gomes J, et al. (2018) Exercise Training Restores the Cardiac Microrna-16 Levels Preventing Microvascular Rarefaction in Obese Zucker Rats. Obes Facts 11(1): 15-24.
- Ardakanizade M (2018) The effects of mid- and long-term endurance exercise on heart angiogenesis and oxidative stress. Iran J Basic Med Sci 21: 800-805.
- Soori R, Amini AA, Choobineh S, Eskandari A, Behjat A, et al. (2019) Exercise attenuates myocardial fibrosis and increases angiogenesis-related molecules in the myocardium of aged rats. Arch Physiol Biochem 2: 1-6.
- Hassan AF, Kamal MM (2013) Effect of exercise training and anabolic androgenic steroids on hemodynamics, glycogen content, angiogenesis and apoptosis of cardiac muscle in adult male rats. Int J Health Sci (Qassim) 7(1): 47-60.
- Roudier E, Forn P, Perry ME, Birot O (2012) Murine double minute-2 expression is required for capillary maintenance and exercise-induced angiogenesis in skeletal muscle. FASEB J 26(11): 4530-4539.
- Tian X, Zhou N, Yuan J, Lu L, Zhang Q, et al. (2020) Heat shock transcription factor 1 regulates exercise‐induced myocardial angiogenesis after pressure overload via HIF‐1α/VEGF pathway. J Cell Mol Med 24: 2178-2188.
- Ranjbar K, Nazem F, Nazari A, Gholami M, Nezami AR, et al. (2015) Synergistic effects of nitric oxide and exercise on revascularisation in the infarcted ventricle in a murine model of myocardial infarction. EXCLI J 14: 1104-1115.
- Bellafiore M, Battaglia G, Bianco A, Palma A (2019) Expression Pattern of Angiogenic Factors in Healthy Heart in Response to Physical Exercise Intensity. Front Physiol 10: 238.
- Gomes JL, Fernandes T, Soci UP, Silveira AC, Barretti DL, et al. (2017) Obesity Downregulates MicroRNA-126 Inducing Capillary Rarefaction in Skeletal Muscle: Effects of Aerobic Exercise Training. Oxid Med Cell Longev pp. 2415246.
- Olfert IM, Howlett RA, Wagner PD, Breen EC (2010) Myocyte vascular endothelial growth factor is required for exercise-induced skeletal muscle angiogenesis. Am J Physiol Regul Integr Comp Physiol 299(4): R1059-R1067.
- Taylor CW, Ingham SA, Hunt JE, Martin NR, Pringle JS, et al. (2016) Exercise duration-matched interval and continuous sprint cycling induce similar increases in AMPK phosphorylation, PGC-1α and VEGF mRNA expression in trained individuals. Eur J Appl Physiol 116(8): 1445-1454.
- Holloway TM, Snijders T, VAN Kranenburg J, VAN Loon LJC, Verdijk LB (2018) Temporal Response of Angiogenesis and Hypertrophy to Resistance Training in Young Men. Med Sci Sports Exerc 50(1): 36-45.
- Van Pelt DW, Guth LM, Horowitz JF (2007) Aerobic exercise elevates markers of angiogenesis and macrophage IL-6 gene expression in the subcutaneous adipose tissue of overweight-to-obese adults. J Appl Physiol (1985) 123(5): 1150-1159.
- Izzicupo P, D’Amico MA, Di Blasio A, Napolitano G, Nakamura FY, et al. (2017) Aerobic Training Improves Angiogenic Potential Independently of Vascular Endothelial Growth Factor Modifications in Postmenopausal Women. Front Endocrinol (Lausanne) 8: 363.
- Hoier B, Nordsborg N, Andersen S, Jensen L, Nybo L, et al. (2012) Pro- and anti-angiogenic factors in human skeletal muscle in response to acute exercise and training. J Physiol 590(Pt 3): 595-606.
- Larkin KA, Macneil RG, Dirain M, Sandesara B, Manini TM, et al. (2012) Blood flow restriction enhances post-resistance exercise angiogenic gene expression. Med Sci Sports Exerc 44(11): 2077-2083.
- Holloway TM, Morton RW, Oikawa SY, McKellar S, Baker SK, et al. (2018) Microvascular adaptations to resistance training are independent of load in resistance-trained young men. Am J Physiol Regul Integr Comp Physiol 315(2): R267-R273.
- Beijer Å, Rosenberger A, Bölck B, Suhr F, Rittweger J, et al. (2013) Whole-body vibrations do not elevate the angiogenic stimulus when applied during resistance exercise. PLoS One 8(11): e80143.
-
Lucas Cecin de DS, Mateus BS, Otávio Cortes A, VB Veloso, D Reis Abdalla, et al., Influence of Exercise or Physical Activity in the Angiogenesis Process: Integrative Review. On J Cardio Res & Rep. 3(5): 2020. OJCRR.MS.ID.000574.
-
Physical activity, Exercise, Angiogenesis, Vascular Endothelial Growth Factor, Blood vessel, Neovascularization, Vasculogenesis, Hypertension, Atherosclerosis, Myocardial fibrosis, Heart disease, Cardiovascular disease
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






