 Research Article
Research Article
Hypotensive Potential of Desmodium Adscendens on Cardiovascular Functions
Seriki SA*
Department of Physiology, College of Medical Sciences, Edo University, Nigeria
Seriki SA, Department of Physiology, College of Medical Sciences, Edo University, Iyamho, Nigeria.
Received Date: March 16, 2020; Published Date: March 31, 2020
Abstract
Background: Desmodium adscendens is one of the medicinal herbs used in the management of some medical conditions in recent times. The current study investigates the effects of aqueous leave extract of Desmodium adscendens on the serum levels of Sodium, Chloride, Potassium, and Bicarbonate ions, and the implication on cardiovascular function in healthy wistar rats.
Method: Twenty-four (24) wistar rats grouped into four (n=6) were used for the research. Group 1 served as control, while Groups 2, 3, and 4 were treated orally with low, median and high doses of the extract of D adscendens for four weeks, after which blood was collected separately from each group and the serum level of the electrolyte determined by appropriate methods and comparison made with the control group and among the groups.
Results: There was significant decrease (P < 0.05) in serum concentration of Sodium, Chloride and Bicarbonate ions, and significant increase in Potassium ion concentration.
Conclusions: The significant decrease in serum concentration of Na+, Cl-, HCO3- and significant decrease indicate that the extract has the potential to lower blood pressure, and that may be attributed to the active phytochemical constituents present in the leave. Therefore D. adscendens leaf has beneficial hypotensive potential on cardiovascular functions.
Keywords: Serum electrolytes; Hypotensive potentials; Cardiovascular function; Desmodium adscendens
Introduction
Traditional Medicines have over time given alternatives to conventional and orthodox medicines in the treatment of many conditions at more affordable rates, especially to poor rural populace in developing nations. They are also readily available to the larger population living in the rural settlements [1].
Desmodium adscendens
Desmodium adscendens is one among such useful medicinal plants that have recently gained the attention of many researchers. Previous studies have revealed Desmodium adscendens that has been traditionally used by the locals in managing medical conditions such as: muscle cramp, tendon, spinal pain, bronchitis, epilepsy and some central nervous system disorders, among many others [2]. The herb is of the Family - “Fabaceae”; and genus - Desmodium. It is often described in English as Beggar- lice or Tick Clover [2].
Serum electrolytes
Serum electrolytes are salts and minerals, such as sodium, potassium, chloride calcium and bicarbonate, which are found in the blood, and conduct electrical impulses in the body. They could be higher or lower than the normal level it should be in the blood, sometimes resulting in serious health conditions. An electrolyte test can help determine whether or not an electrolyte imbalance exists in the body. It is among the most commonly used laboratory tests for assessment of a patient’s clinical conditions and disease states, because electrolyte balance in the body is essential for normal functioning of cells & organs [1]. Electrolyte disorders are common complications frequently seen in patients with heart failure, seizures, and coma. They occur when serum electrolytes concentrations are either too high or too low. Their concentrations need to be balanced for the body to function properly [3]. The imbalance may result from pathological alterations leading to stimulation of the renin-angiotensin-aldosterone system (RAAS), sympathoadrenergic stimulation, a neurohormonal activation, and sometimes, cardiac glycosides or ACE inhibitors [3].
Sodium ion
The most available cation in the extracellular fluid is sodium. It plays a very important role in regulating water balance in the body. Its normal serum level ranges from 130 to 145 mmol/L. Antidiuretic hormone (ADH), also known as arginine vasopressin is a non-peptide hormone that regulates renal handling of free water. Alteration of the amount of water reabsorbed by the kidney has an important effect on serum sodium concentration. ADH is secreted by the neurons in the supra-optic and paraventricular nuclei of the hypothalamus, and its release is stimulated by hypovolemia, thirst, increased serum osmolality, and angiotensin II [4]. In the renin-angiotensin-aldosterone system, renin from the juxtaglomerumar apparatus of the kidney catalyzes the conversion of angiotensinogen in the liver to angiotensin I, which is further converted to angiotensin II (in the lungs) by Angiotensin converting Enzyme (ACE) [5]. Angiotensin II, which is a vasoconstrictor enhances optimal perfusion pressure to end organs, especially when plasma volume is decreased. It also induces the release of aldosterone, ADH and cortisol. Aldosterone is a hormone released from the adrenal cortex of the kidneys with mineralocorticoidal actions, which affects the distal tubular reabsorption and retention of sodium rather than water [6].
Potassium ions
Potassium represents an important ion of the human body. About 98% of the body’s potassium pool is present in the intracellular compartment, leading to a steep potassium concentration gradient across cellular membranes, indicating why potassium is particularly important to maintain the cellular membrane potential. It regulates the heartbeat and function of muscles. Normal serum potassium level = 3.5-5.0 m Eq/L. Potassium, along with sodium is involved with regulation of water and acid-base balance in blood and tissue [7]. In mammals, the osmotic pressure and water distribution maintenance is the primary function of electrolytes like sodium and potassium. In addition, they play a role in maintenance of pH, in oxidation reduction reactions, in heart muscle functioning and as co-factors for enzymes [6]. The body has two mechanisms to restore potassium balance when the serum potassium level goes up: by shifting the plasma potassium into cells, and by renal elimination [8].
Chloride ion
The chloride ion is the principal extracellular anion in humans with a concentration of about 95-110 mmol/L. It is passively absorbed from the upper small intestine and primarily regulated by the renal proximal tubules, where it is exchanged for bicarbonate ions. and passively follows sodium and water through during renal tubular reabsorption by the nephron [8]. Homeostatic mechanisms indirectly regulate Chloride ion through changes in sodium and bicarbonate. Being an anion, Sodium will balance out positive charges in the extracellular fluid, and by passively following sodium, it helps to maintain extracellular osmolality.
Bicarbonate ion
Bicarbonate ion is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula HCO3-. It serves a crucial biochemical role in the physiological pH buffering system [8]. Bicarbonate (HCO−3) is a vital component of the pH buffering system [9] of the human body (maintaining acid– base homeostasis). 70%–75% of CO2 in the body is converted into carbonic acid (H2CO3CO), which is the conjugate acid of HCO−3 and can quickly turn into it.
With carbonic acid as the central intermediate species, bicarbonate, in conjunction with water, hydrogen ions, and carbon dioxide – forms the buffering system, which is maintained at the volatile equilibrium [9] required to provide prompt resistance to pH changes in both the acidic and basic directions. This is especially important for protecting tissues of the central nervous system, where pH changes too far outside of the normal range in either direction could prove disastrous. A higher serum bicarbonate concentration is associated with higher left ventricular mass, higher aortic pulse pressure and a higher risk of heart failure among nonusers of diuretics [10].
Methods
Phytochemical analysis
The ethanolic leaf extract of Desmodium adscendens was subjected to phytochemical analysis. 2g of the crude extract was weighed and dissolved in 20 ml of distilled. The solution was screened for the presence and absence of alkaloids, flavonoids, tannins, saponins, glycosides, reducing agents, polyphenols, anthraquinones, and phlobatanins following standard methods [11].
Test for alkaloids
Mayer’s test: 2 ml of the filtrate and control solutions were measured with pipette into two separate test tubes. To the test tubes were added 3 drops of Mayer’s reagent. The solutions were mixed and allowed to stand for 5 min and then observed for the presence of precipitate and colour change.
Wagner’s test: 2 ml of the filtrate and control solutions were pipetted into two separate test tubes. To the test tubes were added 3 drops of Wagner’s reagent. The solutions were mixed and allowed to stand for 5 min and then observed for the presence of precipitate and colour change.
Dragendorrf’s test: 2 ml of the filtrate and control solutions were pipetted into two separate test tubes. To the test tubes were added 3 drops of Dragendorff’s reagent. The solutions were mixed and allowed to stand for 5 min and then observed for presence of precipitate and colour change.
Test for tannins
1. 2 ml of the filtrate and control solution were pipetted into two separate test tubes. To the test tubes were added 3 drops of 10 % ferric chloride. The mixtures were observed for presence of precipitate and colour change.
2. 2 ml of the filtrate and control solution were pipetted into two separate test tubes. To the test tubes were added 3 drops of 10 % lead acetate. The mixtures were observed for presence of precipitate and colour change.
Test for flavonoids
1) 2 ml of the filtrate and control solutions were pipetted into two separate test tubes. To the test tubes were added 3 drops of NaOH. The mixtures were allowed to stand for 2 min and then observed for presence of precipitate and colour change.
2) 2 ml of the filtrate and control solutions were pipetted into two separate test tubes. To the test tubes were added 3 drops of NaOH and 3 drops of 0.5 N HCl. The mixtures were observed for presence of precipitate and colour change.
Test for saponnins
Emulsifying test: 2 ml of the filtrate and control solution were pipetted into two separate test tubes. To the test tubes were added 3 drops olive oil and the mixture shaken vigorously. The mixtures were observed for presence of brown emulsion.
Frothing test: 1 ml of the filtrate and control solution were pipetted into two separate test tubes. To the test tubes were added 4 ml distilled water. The mixture was shaken vigorously and then observed for presence of frothing.
Test for anthraquinone
0.1 g of the crude extract was dissolved in 10 ml concentrated chloroform. The solution was filtered and used for this test. To 5 ml of filtrate and control solution in separate test tubes was added 5 ml ammonia solution. The mixtures were shaken vigorously. The mixtures were observed for presence of precipitate and colour change.
Test for glycoside
To 2 ml of filtrate and control solutions in separate test tubes were added 2 ml of Fehling I and Fehling’s II solutions. The solutions were mixed thoroughly and boiled in a water bath for 2 min. The mixture was observed for the presence of precipitate and colour change.
Test for terpenes
0.1 g of the crude extract was dissolved in 10 ml concentrated chloroform. The solution was filtered and used for this test. To 1 ml of filtrate and control solutions in separate test tubes were added 1 ml acetic anhydride. The solutions were mixed thoroughly with a glass rod. The test tubes were then placed in slanting positions and 1 ml H2SO4 was added to the side of each test tube into the mixture. The junction of the two liquid layers was observed for the presence of colour change.
Experimental Animals
A total of forty 24 adult male albino Wistar rats weighing between 120-160g were used for this experiment. The animals were obtained from the faculty of Basic medical science animal house, University of Calabar. The rats were maintained on standard rat feed (growers feed) and tap water available all through the period of experiment. The animals were maintained at an ambient temperature between 28 - 300C, humidity of 55 ± 5%, and standard (natural) photoperiod of approximately 12 hours of light (06:30 hour – 18:30 hour) alternating with approximately 12 hours of darkness (18:30 hour - 06:30 hour). The rats were allowed to get familiarized with the environment for a period of 7 days before treatments commenced.
Preparation of Extract
Two (2) grams of the aqueous leaves extract of Desmodium adscendens was dissolved in 10ml of distilled water as follows; 2 g = 10 ml of water (200 mg = 1 ml). If 200 mg = 1 ml, therefore, 300 mg = 1.5 ml, 450mg = 2.25 ml, and 600 mg = 3 ml of water. Volume per animal was determined as follows; for the low dose treated group, 300mg of extract was dissolved in 1.5 ml of water. A rat in the low dose group with a body weight 120g received 36mg of the extract. If 300 mg of extract was dissolved in 1.5 ml of water, 36 mg of the extract will be dissolved in 0.18 ml of water. Therefore, an animal in the low dose group with a body weight of 120g will receive 0.18 ml of the extract daily all through the treatment period. Same was applicable to all the experimental animals all the extract treated groups. Extract administration was done orally with the aid of an orogastric cannula and treatment lasted for four (4) weeks.
Experimental design
At the end of the acclimatization period, the animals were randomly assigned into four (4) groups, n=6, as follows:
1. Control (Received normal rat chow and tap water)
2. Low dose treated group (Received low dose of extract (300mg/kg)
3. Median dose treated group (Received middle dose of extract (450mg/kg)
4. High dose treated group (Received high dose of extract (600mg/kg)
Treatments lasted for a period of four (4) weeks, all animals had free access to fed and water ad libitum.
Collection of blood samples
At the end of treatment period, animals from all the experimental groups were sedated and made unconscious using chloroform anaesthesia. Blood samples from each rat was collected via cardiac puncture [11] into EDTA and plain sample bottles for the estimation of haematological and biochemical parameters [12].
Analysis of serum
Serum from the different groups was analyzed for the following Antioxidant Enzymes; Super oxide dismutase (SOD), Catalase (CAT), Glutathione peroxidase (GPx), and marker of Lipid peroxidation (MDA).
Results
Comparison of serum electrolytes in the different experimental groups
Sodium ion concentration (Na+)
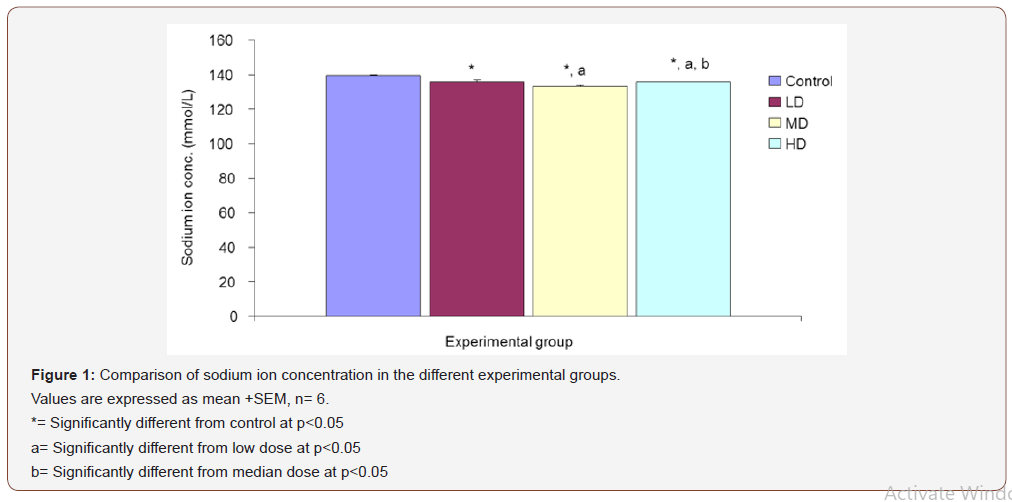
Desmodium adscendens extract caused significant (p<0.05) decrease in Na+ concentration in the three groups given low, median and high doses of the extract when compared with the control (139.67 ±0.48 mmol/L) group. Values for LD, MD and HD were 136.00 ±0.97, 133.17 ±0.48 and 135.67 ±0.33 mmol/L respectively (Figure 1).
Potassium ion concentration (K+)
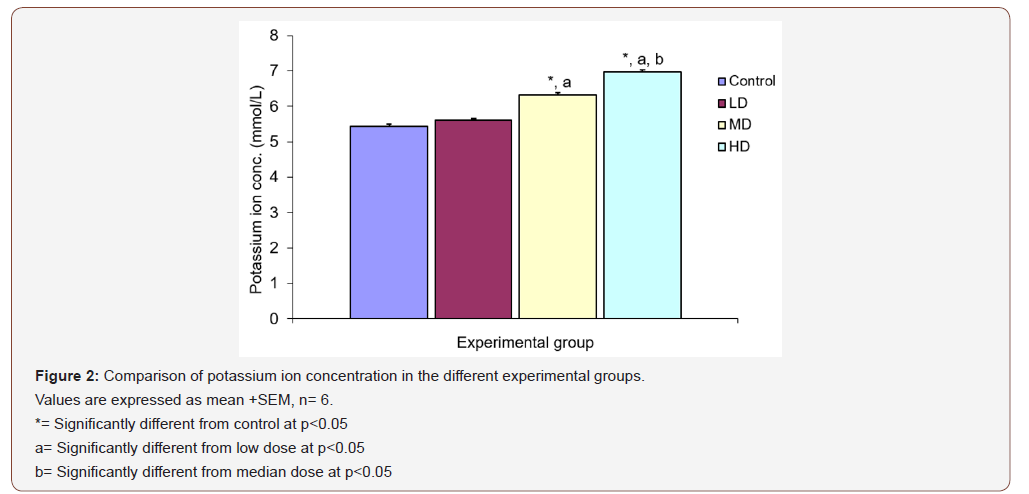
There was significant increase in potassium ion concentration in the groups given medium and high doses of extract when compared with the control (5.43 ±0.08 mmol/L) and low dose (5.60 ±0.06 mmol/L) groups. The group treated with high dose of the extract also showed significant increase in K+ concentration (6.97 ±0.07 mmol/L) over the group given median dose. There was however no significant different in potassium ion concentration in the group given low dose of extract when compared with the control group (Figure 2).
Chloride ion concentration (Cl-)
The comparison of Chloride ion concentration in the different experimental groups reveals a significant (p<0.05) decrease in chloride ion concentration in the groups given low, median and high doses of the extract when compared with the control group (100.83 ±0.40 mmol/L). Values for LD, MD and HD groups were 96.00 ±0.58, 97.00 ±0.84 and 95.00 ±0.42 mmol/L respectively (Figure 3).

Bicarbonate ion concentration (HCO3-)
There was significant (p<0.05) decrease in bicarbonate ion concentration in the groups given median and high doses of extract when compared with control (21.17 ±0.40 mmol/L) and low dose (21.33 ±0.49 mmol/L) groups. The group given high dose (17.00 ±0.37 mmol/L) extract also showed significant (p<0.05) decrease in bicarbonate ion concentration when compared with the group given median dose (18.17 ±0.31 mmol/L) of the extract (Figure 4).

Discussion
Extract of Desmodium adscendens caused significant decrease in plasma sodium, bicarbonate and chloride ions concentration. Serum sodium concentration contributes substantially to blood pressure regulation. The mechanisms by which salt raises blood pressure are not fully understood. However, there is increasing evidence that small changes in plasma sodium may directly affect the hypothalamus, the local renin-angiotensin system, and the heart and vasculature, all of which may play a role in changing blood pressure independent of and additive to that which occurs with the tendency for the changes in extracellular volume. In other words, small increases in plasma sodium may be in part directly responsible for the elevated blood pressure, and vice versa [13]. So, Desmodium adscendens, which causes decrease in serum sodium concentration, will cause a drop in blood pressure. Evidence from monogenic syndromes, dietary and animal studies on renal Cl− balance, and Cl− transporters in vascular tissues point to a critical role for Cl− in mechanisms that contribute to blood pressure regulation. Monogenic syndromes associated with Cl− transporters manifest high and low blood pressure phenotypes. In Gordon’s syndrome, hypertension occurs as a consequence of increased Cl− reabsorption in the thiazide-sensitive segment of the distal renal tubule [14]. Therefore, any agent that reduces serum concentration of Chloride ion will reduce blood pressure.
Another likely mechanism may relate to non-cardiac and nonrenal roles for Cl−. Cl− channels are present in the surface and transverse tubular membranes of mammalian skeletal muscle and Cl− moves into muscle during t-tubular action potentials or with K+-induced depolarization of the sarcolemma. Extracellular Cl− has been shown to be protective against fatigue, with implications for survival and cardiovascular risk, involving high-intensity contractions in both fast- and slow-twitch mammalian muscle possibly by preventing excessive depolarisation with exerciseinduced decline in trans-sarcolemmal K+ gradient [15]. The treated groups had significant increase in serum potassium ion levels. This is beneficial, as high potassium helps to lower the arterial blood pressure by stabilizing the resting membrane potential. Potassium helps lower blood pressure by balancing out the negative effects of salt. The kidneys use a delicate balance of sodium and potassium to pull fluid across a wall of cell from the blood stream for excretion [16]. Serum bicarbonate (HCO3-) level was significantly decreased in the extract-treated groups in this study. The decrease may imply that the pH of the blood went up. However, this is not to pose any serious problem as the serum chloride concentration also went down. Cl− is the major extracellular strong ion and it’s key to maintenance of acid-base homeostasis. Cl− levels are inversely related to bicarbonate, which acts as the major acid-base buffer in humans. Cl− was identified as the primary factor influencing the occurrence of metabolic alkalosis and non-anion gap metabolic acidosis in critical illness. This decrease in HCO3 could be attributed to increased renal excretion of bicarbonate [17, 18].
Conclusion
Desmodium adscendens extract has the potential to reduce high blood pressure in normotensive and hypertensive patients by increasing serum level of potassium ion and also reducing serum level of sodium, chloride and bicarbonate ions. This potential may be attributed to its phytochemical constituents of flavonoid, alkaloids, tannins, saponins, glycosides, reducing sugar, polyphenols, anthraquinones, and phlobatanins.
Acknowledgements
None.
Conflicts of Interest
No conflict of interest.
References
- Seriki Samuel Adinoyi, Omolaso Blessing Oluwagbamila, Adegbite Olutunde Ademola (2017) Role of Moringa oleifera on Electrolytes Levels and Cardiovascular Function in Human. Therapeutic Advances in Cardiology 1(3): 80-86.
- Seriki SA and Nyoro KI (2019) Asian Hematology Research Journal 2(2): 1-15.
- Endemann DH, Pu Q, De Ciuceis C, Carmine Savoia, Agostino Virdis, et al. (2004) Persistent remodeling of resistance arteries in type 2 diabetic patients on antihypertensive treatment. Hypertension 43: 399-404.
- Huang A, Luethi N, Martensson J, Bellomo R, Cioccari L (2017) Pharmacodynamics of intravenous frusemide bolus in critically ill patients. Crit Care Resusc 19(2): 142-149.
- Luke RG, Galla JH (2012) It is chloride depletion alkalosis, not contraction alkalosis. J Am Soc Nephrol 23(2): 204-207.
- Haque SK, Ariceta G, Batlle D (2012) Proximal renal tubular acidosis: a not so rare disorder of multiple etiologies. Nephrol Dial Transplant 27(12): 4273-4287.
- Asano S, Kato E, Yamauchi M, Ozawa Y, Iwasa M (1966) The mechanism of acidosis caused by infusion of saline solution. Lancet 1(7449): 1245-1246.
- Shires GT, Holman J (1948) Dilution acidosis. Ann Intern Med 28(3): 557-559.
- Vaduganathan M, Pallais JC, Fenves AZ, Butler J, Gheorghiade M (2016) Serum chloride in heart failure: a salty prognosis. Eur J Heart Fail 18(6): 669-671.
- Jessica Kendrick, Leila Zelnick, Michel Chonchol, David Siscovick (2017) Serum Bicarbonate is Associated with Heart Failure in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Nephrol 45(2): 118-126.
- Trease GE, Evans WC (1984) Pharmacognosy 12th Ed, Bailliere Tindal, London, pp. 622.
- Ohwada K (1986) Improvement of cardiac puncture in mice. Jikken Dodutsu 35(3): 353-355.
- He FJ, Markandu ND, Sagnella GA, de Wardener HE, MacGregor GA (2005) Plasma sodium: ignored and underestimated. Hypertension 45: 98-102.
- Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, et al. (2009) Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med 15(5): 545-552.
- McCallum L, Lip S, Padmanabhan S (2015) The hidden hand of Chloride in hypertension. Pflügers Archiv 467(3): 595-603.
- Xi L, Hao YC, Liu J, Wang W, Wang M, et al. (2015) Associations between serum potassium and sodium levels and risk of hypertension: a community-based cohort study. J Geriatr Cardiol 12(2): 119-126.
- Shirley DG, Capasso G, Unwin RJ (2003) Renal physiology. In: R Johnson and J Feehally (eds.), Principles of clinical nephrology (2nd edn.), Mosby International, Philadelphia.
- Fisher EW (1969) Hydrogen ion concentration- Anion-cation (acid-base) balance. In: Textbook of Veterinary Clinical Pathology, Medway W, Prier JE and Wilkinson JS (eds.), The Williams and Wilkins Co, Baltimore.
-
Seriki SA. Hypotensive Potential of Desmodium Adscendens on Cardiovascular Functions. On J Cardio Res & Rep. 3(4): 2020. OJCRR. MS.ID.000570.
-
Serum electrolytes, Hypotensive potentials, Cardiovascular function, Desmodium adscendens, Heart failure, Seizures, Coma, Hypovolemia, Aldosterone, Heartbeat, Osmotic pressure, Cardiac puncture
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






