 Research Article
Research Article
How to Combat the Pandemic of Cardiovascular Disease? Vegetable Alpha-Linolenic Acid or Omega-3 “Fish Oil” EPA & DHA
Dr.Dr.Ir. Vincent van Ginneken1*, Elwin Verheij2, Evert de Vries1, Floris Schouten1 and Jan van der Greef3
1Bluegreentechnologies, Heelsum, Netherlands
2TNO-Pharma, Quality of Living, Zeist, Netherlands
3Sino-Dutch Center for Preventive and Personalized Medicine, Leiden University, Netherlands
Dr. Dr.Ir. Vincent van Ginneken (PhD-1, PhD-2, MSc), Bluegreen Technologies, Heelsum, Netherlands.
Received Date:June 11, 2020; Published Date: July 07, 2020
Abstract
There are currently two main directions with regard to nutritional supplements with PUFAs. The first research school recommends enriching the diet with the essential fatty acid (EFA) obtained from vegetable sources, the C18 lipid omega-3 molecule α-linolenic acid and then via the elongase/ desaturase activity naturally in omega-3 Eicosapentaenoic acid (EPA) and omega-3 Docosahexaenoic acid (DHA). The second research school says that the diet should be immediately enriched with EPA and DHA, obtained directly from fish oil. Here we want to investigate the controversies about the possible use of these FAs as preventive / curative instruments against the development of CVDs to combat the current pandemic of heart disease through nutritional intervention. We calculated from the [product] / [precursor] ration in the Cholesteryl (ChE) fraction the enzymatic activity of elongase/desaturase activity of the heart muscle of a juvenile high-fat-induced C57bl6 mouse model, which model we previously used in CVD studies. The main conclusion is that the omega-3 route from α- linolenic acid to EPA and DHA does not exist enzymatically in the heart and that the best strategy for preventing CVDs is direct diet enrichment with EPA and DHA. Because CVDs are currently the number one cause of death in the US and the WHO predicts that especially in the coming decades developing countries will be affected by this pandemic of CVDs. Research should focus on the underlying mechanism of omega-3 PUFA protection.
Keywords: Cardiovascular diseases; Heart; Metabolic syndrome; LC-MS; Lipidomics; Essential fatty acid (EFA); α-linolenic acid; Elongase/ desaturase activity; Eicosapentaenoic acid (EPA); Docosahexaenoic acid (DHA); Fish oil; C57bl6 mouse model
Introduction
Very recently, in the Lancet of October 7, 2019 [1], the WHO published projections about the pandemic of cardiovascular disease (CVDs) that currently plague the world population, resulting in 2030 with 22.2 million deaths a year [2]. In the past three decades, numerous epidemiological and observational studies have been published on the prevention of CVD and the benefits of diet enrichment with polyunsaturated fatty acids (PUFAs) [3]. There are currently two main directions with regard to nutritional supplements with PUFAs. The first research school recommends enriching the diet with the essential fatty acid (EFA) obtained from vegetable sources, the C18 lipid omega-3 molecule α-linolenic acid and then via the elongase/desaturase activity naturally in omega-3 Eicosapentaenoic acid (EPA) and omega-3 Docosahexaenoic acid (DHA) [4]. The second research school says that the diet should be immediately enriched with EPA and DHA, obtained directly from fish oil [5]. Here we want to investigate the controversies about the possible use of these FAs as preventive / curative instruments against the development of CVDs to combat the current pandemic of heart disease through nutritional intervention. We calculated from the [product] / [precursor] ration in the Cholesteryl (ChE) fraction the enzymatic activity of elongase/desaturase activity of the heart muscle of a juvenile high-fat-induced C57bl6 mouse model, which model we previously used in CVD studies [6,7]. The main conclusion is that the omega-3 route from α-linolenic acid to EPA and DHA does not exist enzymatically in the heart and that the best strategy for preventing CVDs is direct diet enrichment with EPA and DHA. Because CVDs are currently the number 1 cause of death in the US [8] and the WHO predicts that especially in the coming decades developing countries will be affected by this pandemic of CVDs [1]. Research should focus on the underlying mechanism of omega-3 PUFA protection.
Epidemiology Versus Lipidomics Enzymatic Conversion
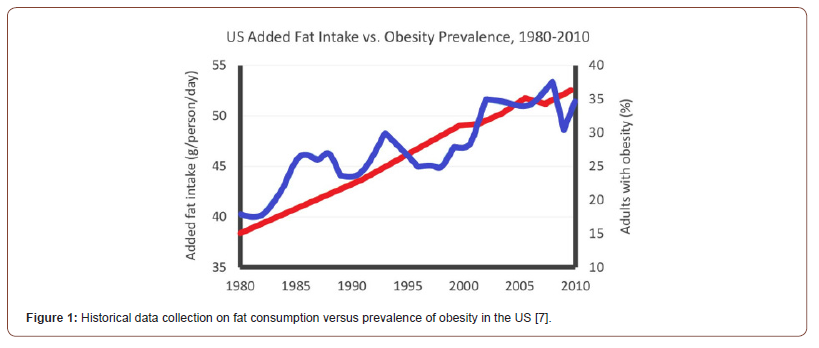
Currently, more than 1/3 of the world’s population is obese (Body Mass Index, BMI> 30) [9]. As a result, cardiovascular disease and stroke are currently the largest killer in the US. Every year more than 2 million Americans suffer from a heart attack or stroke and more than 800,000 die. CVDs are the leading cause of death in the United States and the biggest cause of lower life expectancy among black African Americans [10]. The confluence of many westernizing factors has led to a worldwide increase in fat consumption in the US, which is partly due to an increased consumption of fast food. Total added fat intake increased from 57 to 66 pounds / person from 1980 to 1997 [11]. Thus, there is a close link between obesity morbidity and fat consumption as shown in (Figure 1) for the US.
We recently conducted a study to systematically identify the cause of cardiovascular disease (CVD) related to the high-fat diet (HFD) in a juvenile insulin resistant (IR) C57bl6 mouse model [7] according to a system biology [12] Lipidomics-based approach [13]. We have used LC-MS techniques to determine the 7 most important lipid classes: the lyso-phosphatidylcholines (LPC), phosphatidylcholines (PC), Sphingomyelins (SPM), Diacylglycerols (DG), phosphatidylethanolamines (PE), Triacylglycerols (TG) and Cholesteryl -esters (ChE) as previously performed [6,7]. The HFD had an extremely high TG content of 633.4% increase based on lard (P <0.0001***). Effects of the high-fat diet can be seen on the heart muscle, where the TG level increased by 278% (P≤ 0.029*) compared to the control chow diet, resulting in lipo-toxicity related to hypoxic disorders. So, our main conclusion in that recent study was that lipo-toxicity due to excessive TGs accumulation, resulting in hypoxic disorders, was the leading cause of CVD [7]. From various studies there are indications that increasing the amount of polyunsaturated fatty acids (PUFAs) that we eat can lower our cholesterol levels in the blood and give us less chance of cardiovascular disease, especially if PUFAs are eaten instead of saturated fats, e.g. fats from animal dairy sources such as meat and cheese [14,15]. From such epidemiological studies, hard statements such as: “Replacing 5% of energy intake from dairy fat with equivalent energy intake from polyunsaturated fatty acids (PUFA) has been linked to a 24% lower risk of cardiovascular disease (CVD)”, have been made [16].
But it is our perception, interpretation, and major disadvantage of such epidemiological studies - based primarily on systematic review and meta-analysis of prospective cohort studies - that there are many confusing factors that can ‘fail’ the outcomes and conclusions of such a study. Secondly, PUFAs is a large hub of fatty acids (FAs) (Figure 2), starting with the two essential fatty acids (EFAs) LA and ALA which then end up with a complex enzyme conversion pattern in the elongase-desaturase array important ‘fish oils’ EPA and DHA. LA and ALA are of vegetable origin, while EPA and DHA are obtained from fish oil or fish capsules. The most evidence for the benefits of PUFAs is obtained from Eicosapentaenoic acid (C20: 5, ω-3; EPA) and Docosahexaenoic acid (C22: 6, ω-3; DHA), the ‘fish oil’ such as fat with long chain acids (FAs) in this family. However, there is some epidemiological support for an advantage of the EFA α-linolenic acid (C18: 3, ω-3; ALA), the plant-based precursor of EPA for CVDs [17]. The American Heart Association (AHA) has currently approved the use of ω-3 PUFAs in a dose of approximately 1 g / day of combined DHA and EPA, either in the form of fatty fish or fish oil supplements (in capsules or liquid form) in patients with documented coronary artery disease (CHD) [5]. In the past three decades, numerous epidemiological and observational studies have been published on the benefits of CVDs from omega-3 PUFAs, to mention a few studies: [18-20]. Because cardiovascular diseases and strokes are number 1, it is important to have clear guidelines at the population level with regard to supplementing the diet with PUFAs.
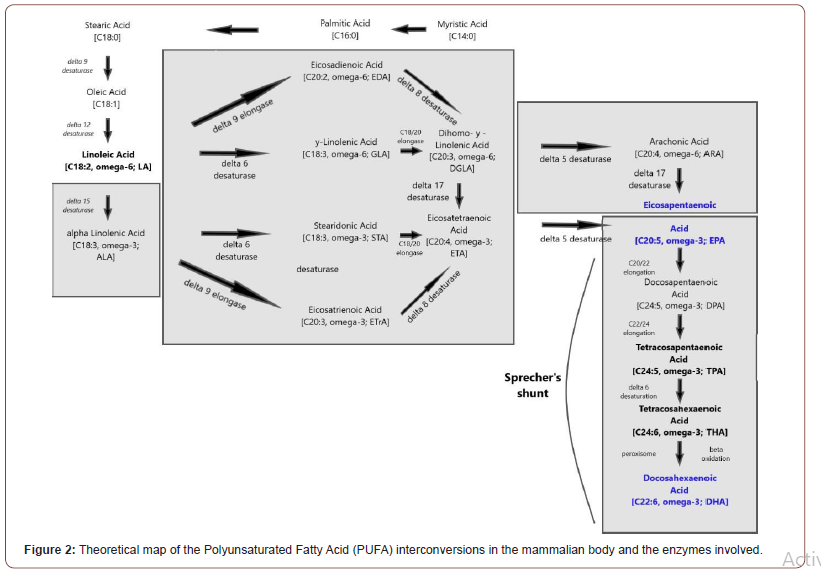
There are currently two main directions: the first research school recommends enriching the diet with the essential fatty acid (EFA) obtained from vegetable sources, the C18 lipid molecule α-linolenic acid (C18: 3, omega-3; ALA) and then via the elongase -desaturase activity (with sufficient enzymatic activity) naturally ends up in the EPA and DHA. The second research school says the diet is directly enriched with EPA and DHA, obtained directly from fish oil or via fish oil capsules. With a stronger data base, the nutrition community will be better placed to follow the dietary recommendation for α-linolenic acid (C18: 3, omega-3; ALA) for CVD risk reduction [4] or through direct supplementation from EPA & DHA via fish oil or fish oil capsules [5].
Result and Discussion
Before statements can be made about whether the diet should be supplied with the “fish oil” EPA & DHA versus the option to enrich the diet with vegetable α-linolenic acid (C18: 3, omega-3; ALA), we believe that we must first acquire fundamental knowledge about enzymatic PUFA conversions in the heart muscle. Here we present our recommendations regarding nutritional intervention via α-Linolenic Acid (C18: 3, omega-3; ALA) or via direct supplementation of EPA & DHA via fish oil or fish oil capsules for CVD risk reduction based on indirect lipidomics based on LCMS- measurements of PUFA content in the heart muscle of a C57bl6 mouse model. The innovative aspect of this study is that we use PUFA enzyme conversions - based on product / precursor ratios - to elucidate the PUFA interconversions via the elongasedesaturase array [8] in the heart muscle. This is an entirely new, exciting approach at the molecular biochemical level of the heart muscle instead of these endless discussions after epidemiological studies due to the complexity of interpreting the results due to so many confounding factors. We hope in the end to be able to make a statement as to whether the diet should be enriched with vegetable ALA or with the omega-3 “fish oils” EPA & DHA via fish oil supplementation. To the best of our knowledge, no comparative LCMS studies have been conducted into enzymatic conversion patterns in the heart muscle. That is why it is difficult to set an initial zero hypothesis which is that the diet should directly be supplemented with the fish oils EPA and DHA because the heart muscle of vertebrates doesn’t has the ‘enzymatic machinery’ to convert them from plant resources like α-Linolenic Acid (C18: 3, omega-3; ALA).
At first, we compared the composition of Hindlimb – with heart muscle for the 7 major lipid fractions: LPCs, PCs, SPMs, DGs, PEs, TGs and ChEs as previously conducted [6,7]. The very first major difference between Hindlimb muscle and heart muscle is the fact that the Cholesteryl lipids are missing in the Hindlimb muscle while they are clearly present in the heart muscle (Figure 3). All major lipid fractions in the Hindlimb muscle (Table 1a) measured with LCMS such as the Lysophosphatidylcholines (LPCs), Phosphatidylcholines (PC), Sphingomyelines (SPMs) and Triacylglycerols (TGs) show a decrease in the High-Fat Diet group of which those of the SPMs, is a significant decrease with (P <0.036*). While the heart muscle (Table 1b) for all major measured lipid fractions LPCs, PCs, SPMs, Ches, TGs shows a measured increase for all said lipid fractions, of which that of the ChEs is significant increase (P <0.024*) by approximately 340%. This ChE lipid fraction is first and foremost a reflection of the PUFA composition of the heart muscle (Table 2a), but also shows the important PUFA enzyme conversions (Figure 2) and the enzyme activities (Table 2b) based on product / precursor ratio.
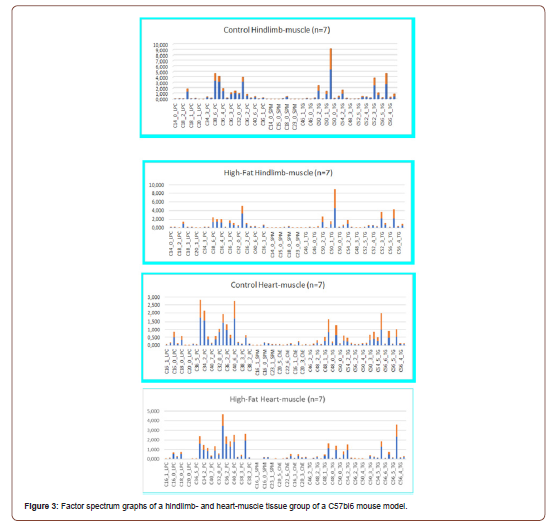
Table 1: (Top: Hindlimb-muscle): Hindlimb- muscle composition in a Control-Chow diet and a High-Fat diet C57bl6 mouse group raised for 40 days on a High-fat diet with 22.0% Bovine lard (≈Triacylglycerols) and 0.25% Cholesterol for the four by LCMS techniques [7,15] measured major lipid classes (Lyso-phosphatidylcholines (LPC), Phosphatidylcholines (PC), Sphingomyelins (SPM) hosphatidylethanolamines (PE), PC- plasmalogens, PE-plasmalogens, Cholesteryl-esters (ChE) and Triacylglycerols (TG) and Total-Sum determined by LCMS techniques.
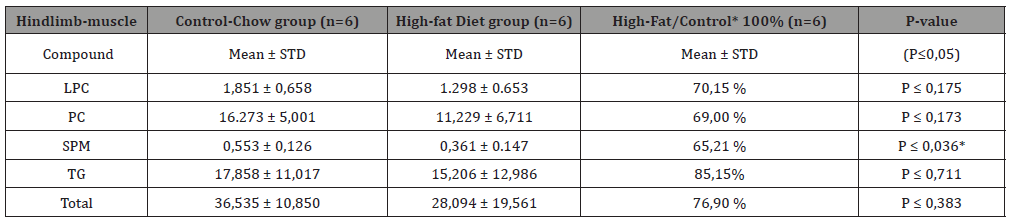
Table 2: (Bottom: Heart-muscle): Similar LCMS measurements but for Heart-muscle in a Control-Chow diet and a High-Fat diet C57bl6 mouse group. Notify that in heart-muscle besides the 4 earlier entioned major lipid classes the important Cholesteryl-fraction is measured from which based onproduct- precursor ratio’s ‘enzymatic activity’ can be measured.
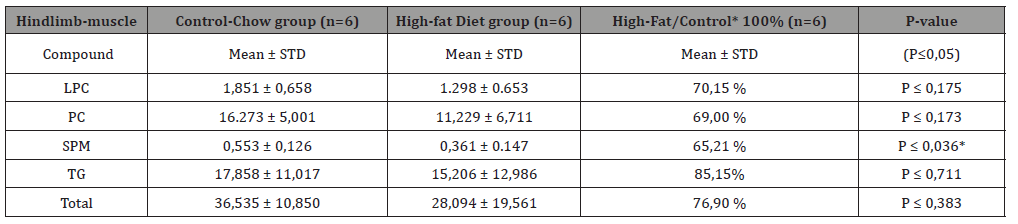
Table 3: Comparison between Control Chow- and a High-fat diet group for the Cholesteryl-ester monounsaturated fatty acid (MUFA) and polyunsaturated fatty acid (PUFA) fractions in heart homogenate muscle tissue and for a C57bl6 High-Fat diet induced Insulin Resistant (IR) obese mouse model.
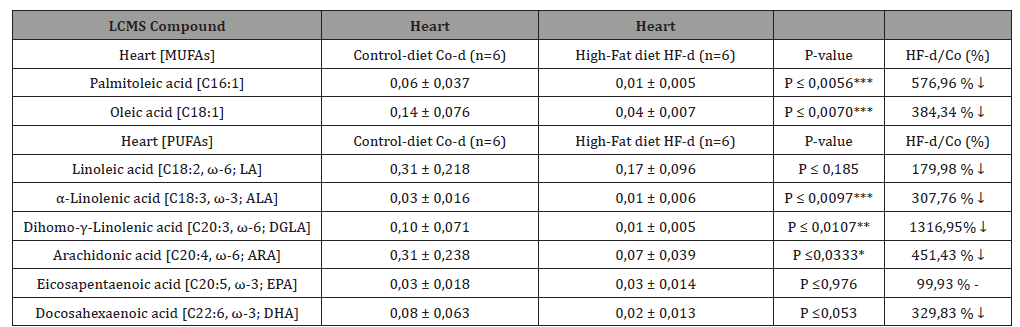
Table 4: Comparison between Control Chow- and a High-fat diet group for the elongase/desaturase derived enzymatic activities -based on product/ precursor ratios- in heart homogenate muscle tissue and for a C57bl6 High-Fat diet induced Insulin Resistant (IR) obese mouse model.
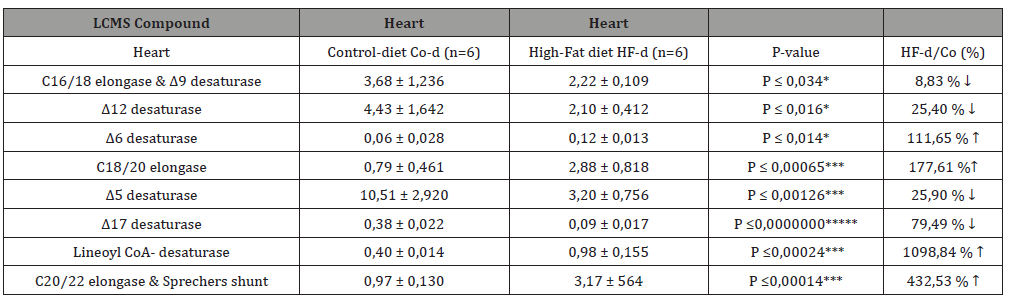
The path starts with the desaturation of α-linolenic acid (C18: 3, ω-3; ALA) to stearidonic Acid (C18: 4, ω-3; STA) by Δ6 desaturase, which is a rate limiting step. This is followed by extension to Eicosapentaenoic acid (20: 4n-3; ETA). Desaturation by Δ5 desaturase produces Eicosatetraenoic Acid (C20: 5, ω-3; EPA) and the following EPA is then extended by elongase-2 first to Docosahexaenoic acid (C22: 5, ω-3; DPA) and then Tetracosapentaenoic acid (C24: 5, ω-3). Tetracosapentaenoic acid then undergoes a second Δ6 desaturation to produce Tetracosahexaenoic acid (24: 6ω-3). These first steps take place in the endoplasmic reticulum; however, the final phase of DHA synthesis takes place in the peroxisome after translocation. In the peroxisome, 24: 6n-3 is shortened to DHA (22: 6n-3) by a single round of β-oxidation through the action of acyl-coenzyme A-oxidase, D- bifunctional enzyme and then peroxisomal thiolases [21] (Figure 2). α-Linolenic Acid (ALA), an 18-carbon omega-3 essential FA, is the precursor of Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). The term “essential” indicates that ALA cannot be synthesized by humans and must therefore be obtained entirely from exogenous sources. After consumption, the majority of ALA is catabolized via β- oxidation for energy generation, and a small portion undergoes conversion to produce two more powerful members of the omega-3 PUFA family: EPA and DHA [22].
Conversion rates from ALA to EPA and DHA in humans are estimated at 8-20% and 0.5-9%, respectively [23]. Due to the fact that EPA and DHA can be synthesized in the body by ALA, these two FAs do not themselves meet the definition of essential FA. However, since this conversion is not efficient enough to meet health requirements, EPA and DHA are also considered essential FA (or conditionally essential FA). Although not convincing, the benefits of ALA appear to come primarily from EPA and DHA, and as a major consequence of ALA deficiency, it seems that EPA and DHA are not being sufficiently produced [24]. The clinical features of omega-3 PUFA insufficiency affects many physiological functions and are non-specific [25,26] and may also be due to the disruption in omega-6 PUFA homeostasis [27]. Increased intake of omega-3 PUFA, especially the long-chain omega-3 PUFA EPA and DHA, could lower the omega-6 / omega-3 tissue ratio to a level that was likely to exist during millions of years of human evolution [28]. This ratio has increased dramatically over the last millennia due to profound changes in dietary habits following the transition from the huntergatherer lifestyle to agricultural societies. This change could therefore be one of the crucial factors leading to the emergence of so-called civilization diseases, further skewed towards omega-6 PUFA by the agricultural revolution in the 19th century and the massive use of maize (with its high omega-6 PUFA content) in western societies during the 20th century.
In short, many high-quality preclinical (both observational and interventional) and clinical studies have been conducted to assess the potential cardiovascular benefit of omega-3 PUFA. A recent major meta-study re-analyzed and summarized these studies and found no clear benefit from omega-3 PUFA to protect against coronary artery disease in observational studies and in randomized controlled trials [29]. This meta-study also investigated the role of other fatty acids and concluded that the current evidence does not provide clear support for cardiovascular guidelines that encourage high consumption of omega-3 or omega-6 PUFA, or even low consumption of saturated fats.
Acknowledgement
Jaap & Marita van Meerveld for continuous help and support.
Conflict of Interest
No conflict of interest.
References
- Kaptoge S, Pennells L, De Bacquer D, Cooney MT, Kavousi M, et al. (2019) World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. The Lancet Global Health.
- Ruan Y, Guo Y, Zheng Y, Huang Z, Sun S, et al. (2018) Cardiovascular disease (CVD) and associated risk factors among older adults in six low-and middle-income countries: results from SAGE Wave 1. BMC public health 18(1): 778.
- Weylandt KH, Serini S, Chen YQ, Su HM, Lim K, et al. (2015) Omega-3 Polyunsaturated Fatty Acids: The Way Forward in Times of Mixed Evidence. BioMed research international pp. 143109.
- Fleming JA, Kris-Etherton PM (2014) The evidence for α-linolenic acid and cardiovascular disease benefits: Comparisons with eicosatetraenoic acid and docosahexaenoic acid. Adv Nutr 5(6): 863S-876S.
- Kris-Etherton PM, Harris WS, Appel LJ, for the American Heart-Association Nutrition Committee (2002) Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease [published correction appears in Circulation 2003;107:512]. Circulation 106: 2747-2757.
- van Ginneken V, de Vries E, Verheij E, van der Greef J (2016) Metabolomics in Hind Limb and Heart Muscle of a Mouse model after a High-Fat Diet. Anat Physiol pp. 2.
- Vincent van Ginneken, Elwin Verheij, Jan van der Greef (2019) Impact of the ‘wrong’ fats on the composition of the heart muscle in an obese High Fat diet (HF-diet) induced Insulin Resistant C57bl6 mouse model following a Lipidomics Systems Biology LCMS approach. EJPMR 6: 243-262.
- Mc Namara K, Alzubaidi H, Jackson JK (2019) Cardiovascular disease as a leading cause of death: how are pharmacists getting involved. Integrated pharmacy research & practice 8: 1-11.
- Abarca-Gómez L, Abdeen ZA, Hamid ZA, Abu-Rmeileh NM, Acosta-Cazares B, et al. (2017) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. The Lancet 390(10113): 2627-2642.
- Frieden TR, Berwick DM (2011) [Perspectives]: The “Million Hearts” Initiative – Preventing Heart Attacks and Strokes. N Eng J Med pp. e27(1)-e27(4).
- Lieberman LS (2003) Dietary, evolutionary, and modernizing influences on the prevalence of Type-2 diabetes. Annu Rev Nutr 23: 345-377.
- Kitano H (2002) Systems Biology: A brief Overview. Ridgway ND, McLeod RS (2016) (eds.), Book (6th eds.), “Biochemistry of Lipids, Lipoproteins and Membranes”. Science 295: 1662-1664.
- Siri-Tarino PW, Chiu S, Bergeron N, Krauss RM (2015) Saturated Fats Versus Polyunsaturated Fats Versus Carbohydrates for Cardiovascular Disease Prevention and Treatment. Annual review of nutrition 35: 517-543.
- Rehm CD, Drewnowski A (2019) Replacing Dairy Fat with Polyunsaturated and Monounsaturated Fatty Acids: A Food-Level Modelling Study of Dietary Nutrient Density and Diet Quality Using the 2013-16 National Health and Nutrition Examination Survey. Frontiers in nutrition 6: 113.
- Chen M, Li Y, Sun Q, Pan A, Manson JE, et al. (2016) Dairy fat and risk of cardiovascular disease in 3 cohorts of US adults−3. Am J Clin Nutr 104: 1209-1217.
- Blondeau N, Lipsky RH, Bourourou M, Duncan MW, Gorelick PB, et al. (2015) Alpha-linolenic acid: an omega-3 fatty acid with neuroprotective properties-ready for use in the stroke clinic. BioMed research international pp. 519830.
- Kromhout D, Bosschieter EB, de Lezenne Coulander C (1985) The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N Engl J Med 312: 1205-1219.
- Albert CM, Hennekens CH, O’Donnell CJ, U A Ajani, V J Carey, et al. (1998) Fish consumption and risk of sudden cardiac death. JAMA 279: 23-28.
- Lee JH, O’Keefe JH, Lavie CJ, Marchioli R, Harris WS (2008) Omega-3 fatty acids for cardio protection. Mayo Clin Proc 83: 324-332.
- Dyall SC (2015) Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Frontiers in aging neuroscience pp. 7(52).
- Arterburn LM, Hall EB, Oken H (2006) Distribution, interconversion, and dose response of n-3 fatty acids in humans. American Journal of Clinical Nutrition 83(6): 1467S-1476S.
- Stark AH, Crawford MA, Reifen R (2008) Update on alpha-linolenic acid. Nutrition Reviews 66(6): 326-332.
- Burdge GC (2006) Metabolism of α-linolenic acid in humans. Prostaglandins Leukotrienes and Essential Fatty Acids 75(3): 161-168.
- Holman RT (1998) The slow discovery of the importance of ω3 essential fatty acids in human health. The Journal of Nutrition 128(2 supplement): 427S-433S.
- Scholtz SA, Colombo J, Carlson SE (2013) Clinical overview of effects of dietary long-chain polyunsaturated fatty acids during the perinatal period. Nestlè Nutrition Institute Workshop Series 77: 145-154.
- Smit EN, Muskiet FAJ, Boersma ER (2004) The possible role of essential fatty acids in the pathophysiology of malnutrition: a review. Prostaglandins Leukotrienes and Essential Fatty Acids 71(4): 241-250.
- Simopoulos AP (2008) The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases. Experimental Biology and Medicine 233(6): 674-688.
- Chowdhury R, Warnakula S, Kunutsor S, Francesca Crowe, Heather A Ward, et al. (2014) Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta- analysis. Annals of Internal Medicine 160(6): 398-406.
-
Dr. Dr.Ir. Vincent van Ginneken, Elwin Verheij, Evert de Vries. How to Combat the Pandemic of Cardiovascular Disease? Vegetable Α-Linolenic Acid or Omega-3 “Fish Oil” EPA & DHA. On J Cardio Res & Rep. 4(3): 2020. OJCRR.MS.ID.000586.
-
Cardiovascular diseases, Heart, Metabolic syndrome, LC-MS, Lipidomics, Essential fatty acid (EFA), α-linolenic acid, Elongase/desaturase activity, Eicosatetraenoic acid (EPA), Docosapentaenoic Acid (DHA), Fish oil, Stroke, Epidemiological studies
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






