Review Article
Review Article
Electromagnetic Interferences on Implanted Cardioverter Defibrillator from Apple and Huawei Smarthphones Magsafe Technology
Santomauro Maurizio1*, Riganti Carla2, Santomauro Mario Alberto3, Rapacciuolo Antonio4, Viggiano Aniello1, Iovino Gianluigi1 and Giovanni Esposito4
1Department of Cardiovascular Emergency, Internal Medicine and Geriatric, Medical School, Federico II University of Naples, Italy
2General Direction, Medical School, Federico II University of Naples, Italy
3Department of Clinical Medicine and Surgery, Medical School, Federico II University of Naples, Italy
4Department of Advanced Biomedical Sciences, Federico II University of Naples, Italy
Santomauro Maurizio, Department of Cardiovascular Emergency, Internal Medicine and Geriatric, Medical School, Federico II University of Naples, Italy.
Received Date:February 07, 2022; Published Date:February 22, 2022
Abstract
Background: Smartphones have become ubiquitous, nearly seamless extensions of ourselves and our daily lives. With >300.000 implanted cardioverter defibrillators (ICD) implanted each year worldwide and the continuing rise of smart device and wearable use, the question of interaction between smartphones and ICD is an important one.
Objective: The purpose of this study is to describe the possible magnet interference of new smartphones generation on ICD and has the potential to inhibit lifesaving therapy.
Method: We have tested in vivo the shock inhibiting therapies using two smartphones (iPhone12 and P30 Pro) on 51 patients. Tests were performed on a patient wearing a Medtronic and Boston Scientific ICD. Amongst the patients, 24 had an Medtronic ICD model and 27 the Boston Scientific ICD model implanted. The tests were conducted in hospital office. A correct position of the smarthphones was signaled by a beeping sound emitted by the device and by repeated device interrogation,
Result: With the two models of smartphones correctly positioned, the tested inhibited the shock therapies for both ICD models in 42 patients for the entire period for application (82.3%).
Conclusion: Patients with an ICD should be warned that some newer models of smartphones equipped with magnets (MagSafe), can disable their device inhibiting its lifesaving functions.
Keywords:Electromagnetic Interferences; Implantable Cardioverter Defibrillator; Smartphones with MagSafe technology; Deactivation Shock Therapy
Introduction
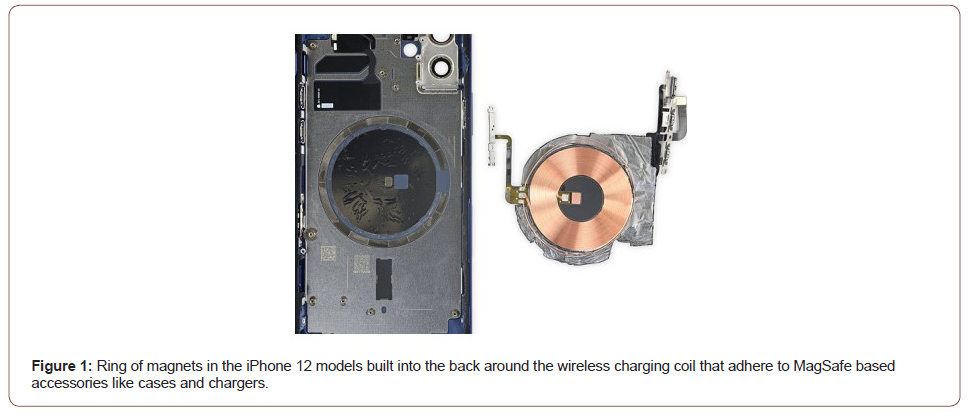
The implantable cardioverter defibrillator (ICD) was approved for the prevention of sudden cardiac death with indication for anti-bradycardia pacing or cardiac resynchronization therapy, recurrent monomorphic ventricular tachycardia responsive to antitachycardia pacing, or ventricular fibrillation responsive to shock. All ICD have a built-in magnetic reed switch that is designed to switch ‘ON’ or ‘OFF’ circuitry in response to clinical magnets [1- 6]. The magnet are accessories that may be used to temporarily inhibit the delivery of shock therapy from the device. A magnetic field effect of ≥ 10 Gauss aligned with the magnetic reed switch is required to activate the magnetic switch in order to alter the device function. The site of magnet placement is important since a poorly positioned magnet may not produce the desired effect. If the magnet is correctly placed over the device, beeping tones (R-wave synchronous) will be heard approximately one second after the magnet is applied. Shock therapy is not suspended until beeping tones are heard. Beeping will continue while the magnet is held in place and therapy continues to be inhibited unless the magnet has been removed. When the magnet is removed, arrhythmia detection resumes, and therapy delivery is no longer inhibited. Magnet application does not affect wireless communication between the device and the programmer. The iPhone12 series are smartphones developed by Apple Inc. Cupertino, California and the P30 Pro is smartphones developed by Huawei Technologies CO, LTD China. A magnetic connector known as MagSafe is introduced on these models, allowing accessories such as cases and charging cords to be attached to the rear of the device. Accessories can also be stacked together [7,8]. All of models have a ring of magnets built into the back around the wireless charging coil that adhere to MagSafe based accessories like cases and chargers. MagSafe uses a ring of magnets in the models to connect to accessories that also have magnets built inside. The MagSafe charger looks something like a larger Apple Watch Charging Puck with an aluminum body and a soft white material at the top of the charger (Figure 1). This technology can cause interference with ICD. More recently, attention has been directed by the American Heart Association (AHA) at serious EMI complications from new smartphones use on cardiac implanted electronic devices (CIED), raising important public health concerns. This has been echoed in the scientific literature by demands for stricter regulations for commercialization of these products. We present a case series of magnetic interference on ICD caused by Apples and Huawei MagSafe technology.
Method
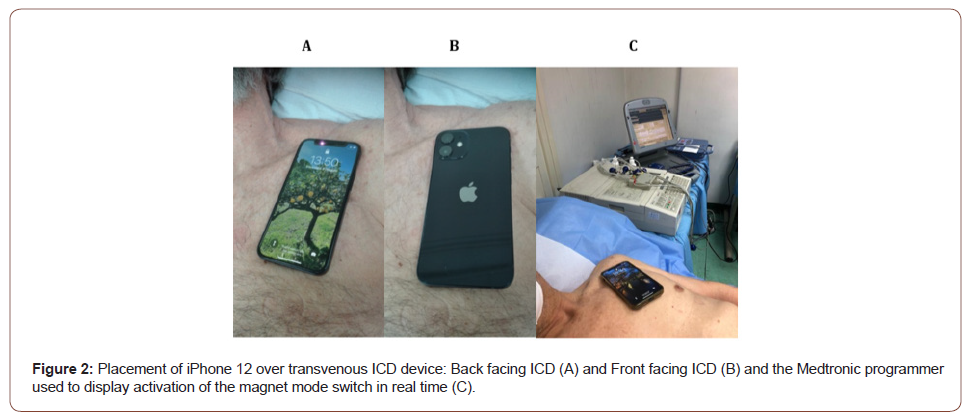
The study population includes 51 patients (39 males, mean age was 63,6 ± 13 years, body mass index of 24.7 ± 3.7), 24 patients with Medtronic traditional ICD (3 Maximo VR model, 4 Consulta CRT-D model, 17 Evera VR model) and 27 with Boston Scientific ICD: 18 transvenous ICD (3 Incepta F160 model, 5 Incepta F163 model, 10 Resonade X4 model) and 9 subcutaneous ICD (2 Emblem A219 model, 7 Emblem A 209 model) who presented to the hospital ambulatory for generator change or for interrogation. The study was a prospective single-center observational study evaluating potential magnetic interference (EMI) of ICD with the iPhone 12 and P30 Pro. Informed consent was obtained prior to the study. A baseline device interrogation was performed to note settings and ensure appropriate functions. Subsequently, an iPhone 12 Pro Max and P30 Pro was placed directly on the skin over the device of the patient, with implanted traditional or subcutaneous ICD devices were enrolled for testing and a programmer were used to check for activation of magnet mode (Figure 2). The tests were carried out with either the front or the back of the iPhone 12 and the P30 Pro facing the ICD. Magnetic interference with the ICD was defined as magnet response and suspension of ICD therapy delivery. In total, 204 tests were conducted in hospital office. There was not a specific requirement for temporary magnet deactivation therapies, nor direct clinical indication. Suspension of shock therapy in transvenous and subcutaneous ICD from Boston Scientific was detected by the audible tone from the ICD magnet alarm and from Medtronic also the programmer was used to display activation of the magnet mode switch in real time. All ICD were interrogated before, during and immediately after the tests. Patients with newly implanted ICD systems (< 6 weeks) were excluded. For this study, the Ethics Committee of Federico II University approved our clinical study.
Result
In total, clinically significant EMI between the iPhone 12 and P30 Pro and ICD occurred in 42 patients (82.3% of the study population). In all cases, magnetic interference with the iPhone 12 only occurred when the phone was placed in close proximity over the ICD pocket, with the back of the iPhone 12 facing the ICD. Among patients with EMI, 18 (35.2 % of the study population) were implanted with a transvenous Boston Scientific ICD, 24 (47.0 % of the study population) with Medtronic ICD (Table 1). In particular in all patients tested by iPhone 12 model, the temporarily triggering magnet reversion mode was able by placing the smartphone directly on the skin over an ICD from the back facing, on the contrary, in patients tested by P30 Pro model, from the front facing the ICD in transvenous Boston Scientific models (66.6 % of the Boston Scientific population). Notably, in all patients with Medtronic ICD, tested by two smartphone models, the temporarily activation of the magnet reversion mode occurred by placing the smartphone directly on the skin over an ICD both with the back and the front facing the ICD (100 % of the Medtronic population). No triggering magnet reversion mode delivery by any smartphone model application in all S-ICD patients tested (33.4 % of the Boston Scientific population). No anomalies were reported by electrocardiogram during the smartphone’s tests for any of the patients. By the programmer, there were no changes to battery voltage, ability to detect the QRS signal or stored diagnostic data. Shock thresholds cannot be assessed by the ICD system, so this was not evaluated. None of the patients reported any pulling or twisting of the can or pain from heating of the ICD electrode.
Table 1: Summary of patient clinical data.

Discussion
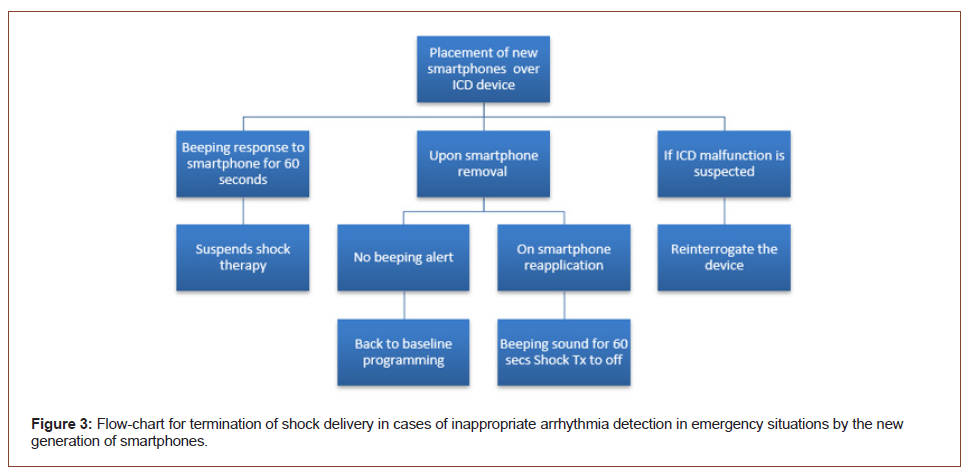
Our study demonstrates that magnet reversion mode may be triggered when the 2 popular smartphones (iPhone 12 Pro Max or P30 Pro with MagSafe Technology) are placed directly on the skin over an transvenous ICD and thus has the potential to inhibit shock therapies. Select ICD from all two major device companies were found to have magnetic susceptibility. Our case series has several clinical implications. In our study no EMI was observed in S-ICD. In fact, the EMI susceptibility does not depend on the ICD manufacturer but on the different models. Previous studies with a limited number of ICD models have confirmed that magnet reversion mode switches in ICD are susceptible to EMI standard mobile phone but have concluded that smartphones pose only minimal risk of EMI with ICD [9-13]. Instead, recent published case reports demonstrate the new iPhone 12 causing a magnet response in CIED [14-17]. The study by Lacour, et al. in 164 CIED patients (5.4% ICD), showed that the activation of the magnet-sensitive switch occurred in 18.3% of CIED patient when the iPhone 12 was placed in close proximity over the CIED pocket, and the back of the phone was facing the skin [15]. The article by Greenberg, et al. [16] highlighting the potential risks to patients from inhibition of therapies by the iPhone 12. In a patient with a cardiac resynchronization therapy ICD (Medtronic and Abbott), therapies were suspended when the iPhone 12 was brought within close proximity of the generator, which was a consistent observation throughout the testing procedure. The recent study by Nadeem, et al. [18] has an in vivo and an ex vivo component tested by iPhone 12 Pro Max. The in vivo component consists of consecutive patients who presented to the electrophysiology laboratory with previously implanted CIED (2/3 ICD, 1 Medtronic Amplia, 1 Abbott Medical 40 Fortify VR). For the ex vivo component, (5/11 ICD, 1 Medtronic, 2 Abbott, 2 Boston Scientific) were tested for magnetic interference caused by iPhone 12 Pro Max through unopened packages. iPhone 12 Pro Max resulted in clinically identifiable EMI in 3/3 (100%) patients in vivo and in 8/11 (72.7%) devices ex vivo. The study of Seidman, et al. [19] has measured the static magnetic field of the iPhone 12 models and Apple Watch to determine the separation distance between these electronic devices that may create EMI, and CIED where magnet mode can be triggered. All iPhone 12 and Apple Watch 6 models tested have static magnetic fields significantly greater than 10 gauss in close proximity (1 – 11 mm), which attenuates to below 10 gauss between 11 and 20 mm. The AHA has already cautioned that magnetic in iPhone series 12 fields can inhibit the ICD. Also the study of Censi, et al. [20] has investigated the risk of the magnetic interference of the iPhone 12 and its MagSafe accessories on a comprehensive set of PM and ICD. The devices were tested in vitro using demo models provided by the manufacturers. The Authors discovered that the magnets inside iPhone 12 trigger the magnetic mode in the 12 tested devices up to a distance of 1 cm. The AHA recommended that smartphones be used in the ear opposite the side of the body of an implanted device, and that the smartphones be kept at least 10 cm away from the device, therefore not in a shirt or coat pocket on the same side as the CIED [21]. Also the Food and Drug Administration (FDA) states that smartphones do pose a significant risk for patients with these devices [22,23]. Therefore, FDA emphasized the following recommandations: to avoid interference between smarphones and ICD, keep them at least 15 cm away from devices. Also, do not place smartphones close to implanted medical device. The FDA continues to monitor all relevant scientific information about this ongoing issue and will continue to take appropriate action, including informing the public and providing additional information, if the need arises based on its risk analysis. However, with the proliferation of small magnets in commercially available electronic devices, they can be integrated in ways that are difficult to recognize. Although manufacturers are not routinely required to specify the strength of the magnetic fields and safety information for interference with the CIED. Inclusion of a wider variety of smartphone brands (Apple, Samsung, Huawei, Garmin) for ICD EMI testing in future studies will help improve the applicability of findings. Therefore, Apple recommends keeping iPhone 12 and iPhone 13 models and all MagSafe accessories a safe distance away from CIED [8]. A safe distance is considered more than 15 cm apart or more than 30 cm apart if wirelessly charging. Paradoxically, the electromagnetic side effect of smartphones could be used by practical doctors in emergency situations outside the hospital in the event of an pseudo electrical storm and these is no magnet or programmer available (Figure 3). In this way, a critical point of news smartphones could be used as a resource for termination of shock delivery in cases of inappropriate arrhythmia detection.
Conclusion
This clinical study demonstrated the importance of public awareness regarding an interaction between ICD and a recently released smartphone models with magnetic charging capability. Patients should be counseled on this risk and advised to keep their wristbands at least 15 cm away from ICD, and not to wear them to sleep. Inclusion of a wider variety of smartphone brands for ICD EMI testing in future studies will help improve the applicability of findings.
Limitations
Our case series has several limitations. Our sample size is small, and we tested on selected device types and the results of our study may not be generalizable. A large-scale study of ICD should be performed to confirm our findings.
Acknowledgement
The authors thank the technicians Stefano De Maio and Michele Solimene for their excellent technical support.
Conflict of Interest
No conflict of interest.
References
- Claire E Raphael, Michael Koa Wing, Nolan Stain, Ian Wright, Darrel P Francis, et al. (2011) Implantable cardioverter-defibrillator recipient attitudes towards device deactivation: how much do patients want to know. Pacing Clin Electrophysiol 34: 1628-1633.
- Porres JM, Laviñeta E, Reviejo C, Brugada J (2008) Application of a clinical magnet over implantable cardioverter defibrillators: Is it safe and useful. Pacing Clin Electrophysiol 31: 1641-1645.
- Santomauro M, Petretta M, Riganti C (2020) Electrical Storm in Patients with Inappropriate Implantable Cardioverter-Defibrillator Therapy: Current Trends in Clinical Practice between Guidelines and Technology Progress. J Cardiol & Cardiovasc Ther 15: 123-133.
- Colleen M McFaul, Stefan Lombaard, Vivek Arora, William C Van Cleve, G Alec Rooke, et al. (2020) Unexpected Shocks from a subcutaneous implantable cardioverter-defibrillator despite attempted reprogramming and magnet use: a case report. A A Practice 14: e01178.
- Römers H, Van Dijk V, Balt J (2017) Erroneous magnet positioning leads to failure of inhibition of inappropriate shock during fast conducting atrial fibrillation episodes. Pacing Clin Electrophysiol 40: 741-743.
- Apfelbaum JL, Schulman PM, Mahajan A (2020) Practice advisory for the perioperative management of patients with cardiac implantable electronic devices: Pacemakers and implantable cardioverter- defibrillators: An updated report by the American Society of Anesthesiologists task force on perioperative management of patients with cardiac implantable electronic devices. Anesthesiology 114: 247-261.
- Accessory design guidelines for Apple devices (2021) Apple Inc website.
- Apple Support (2021) About the magnets inside iPhone 12, iPhone 12 mini, iPhone 12 Pro, iPhone 12 Pro Max, and MagSafe accessories.
- Lee S, Fu K, Kohno T, Ransford B, Maisel WH (2009) Clinically significant magnetic interference of implanted cardiac devices by portable headphones. Heart Rhythm 6: 1432-1436.
- Maria Tiikkaja, Aapo Aro, Tommi Alanko, Harri Lindholm, Maila Hietanen (2012) Inappropriate implantable cardioverter-de fi brillator magnet-mode switch induced by a laptop computer. Pacing Clin Electrophysiol 35: e177-e178.
- Valerie Zaphiratos, Francois Donati, Pierre Drolet, Andrea Bianchi, Bruno Benzaquen, et al. (2013) Magnetic interference of cardiac pacemakers from a surgical magnetic drape. Anesth Analg 116: 555-559.
- Haran Burri, Louis Paulin Mondouagne Engkolo, Nicolas Dayal, Abdul Etemadi, Anne-Marie Makhlouf, et al. (2016) Low risk of electromagnetic interference between smartphones and contemporary implantable cardioverter-defibrillators. Europace 18: 726-731.
- Carsten Lennerz, Herribert Pavaci, Christian Grebmer, Verena Semmler, Felix Bourier, et al. (2017) Electromagnetic interference in cardiac implantable electronic devices: is the use of smartphones safe. J Am Coll Cardiol 69: 108-110.
- Zara Patterson, Sam Straw, Michael Drozd, Maria F Paton, Charlotte Cole, et al. (2021) To the editor — New phones, old problem? Interference with cardiovascular implantable electronic devices by phones containing magnets. Heart Rhythm 18: 1041.
- Philipp Lacour, Abdul Shokor Parwani, Franziska Schuessler, Felix Hohendanner, Frank R Heinzel, et al. (2020) Are contemporary smartwatches and mobile phones safe for patients with cardiovascular implantable electronic devices. JACC Clin Electrophysiol 6: 1158-1166.
- Greenberg JC, Altawil MR, Singh G (2021) Letter to the editor — Lifesaving therapy inhibition by phones containing magnets. Heart Rhythm 18: 1040-1041.
- Evan B Asher, Nikhil Panda, Cao Thach Tran, Michael Wu (2021) Smart wearable device accessories may interfere with implantable cardiac devices. Heart Rhythm Case Reports 7: 167-169.
- Nadeem F, Nunez Garcia A, Thach Tran C, et al. (2021) Magnetic interference on cardiac implantable electronic devices from Apple iPhone MagSafe technology. J Am Heart Assoc 10: e020818.
- Seidman SJ, Guag J, Beard B, Zane Arp (2021) Static magnetic field measurements of smartphones and watches and applicability to triggering magnet modes in implantable pacemakers and implantable cardioverter-defibrillators. Heart Rhythm 18: 1741-1744.
- Censi F, Mattei E, Onder G (2022) iPhone 12 MagSafe technology and cardiac implantable devices: assessment of the actual risk.
- American Heart Association Report (2021) Magnets in iPhone® series 12 can interfere with some implanted cardiac devices.
- Center or Devices, Radiological Health (2020) Potential cell phone interference with pacemakers and medical devices.
- FDA (2021) Magnets in Cell Phones and Smart Watches May Affect Pacemakers and other Implanted Medical Devices.
-
Santomauro Maurizio, Riganti Carla, Santomauro Mario Alberto, Rapacciuolo Antonio. Electromagnetic Interferences on Implanted Cardioverter Defibrillator from Apple and Huawei Smarthphones Magsafe Technology. On J Cardio Res & Rep. 6(3): 2022. OJCRR. MS.ID.000639.
-
Heart, Cardiology, Cardiac contraction, Vascular pulsation, Pericardium, Vascular system, Hidden intrathoracic diseases, Aortic valve
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.