 Mini Review
Mini Review
Current View of Genetics in Coronary Artery Disease
Panagiotopoulos Evangelos1*, Bampouras Nikolaos1 and Gazouli Maria2
1Medical School, National and Kapodistrian University of Athens, Greece
2Department of Basic Medical Sciences, Laboratory of Biology, Medical School, National and Kapodistrian University of Athens, Greece
Panagiotopoulos Evangelos, Medical School, National and Kapodistrian University of Athens, Athens, Greece.
Received Date: June 01, 2023; Published Date: June 12, 2023
During the last two decades, the area of Cardiovascular Medicine has presented considerable advances regarding invasive and pharmacological therapies, trying to reduce cardiovascular complications and death rates. However, the diagnosis of early-stage cardiovascular disease is still challenging. Genome Wide Analysis Studies is our new tool aiming to identify candidate genes and their variants that are associated to hypercholesterolemia, atherosclerotic plaque progression and platelet dysfunction. This is a brief review describing the key points of these biomedical efforts.
Keywords:Single nucleotide polymorphisms; Genome wide association studies; MicroRNAs; Coronary artery disease
Introduction
Despite ongoing progress in pharmacological and clinical treatment, cardiovascular diseases (CVDs) are still the leading cause of morbidity and mortality worldwide. Based on WHO 2019 epidemiological reports, approximately 18 million people died from CVDs. This percentage actually represents one third of all global deaths, whereas the majority (85%) of those deaths was due to heart attack and stroke [1]. Symptoms, clinical signs, flow and vessel imaging techniques, molecular imaging (MI) and biomarkers associated to coronary artery disease (CAD) are at present our clinical tools for CAD diagnosis. However, the diagnosis of earlystage CAD using these methods is still less than optimal.
Familial standpoint
In the early 70s, researchers identified that CAD tended to aggregate in certain families along with familial hyperlipidemia and xanthomata with excess risk being greatest for younger relatives leading them to assume that index cases harbour a genetic factor which they share with their family members [2, 3]. The familial analysis method called linkage analysis was mainly used, which assumes that the disease is caused by a single, high-penetrance genetic mutation and is transmitted from generation to generation according to Mendelian laws. Diseases with this characteristic are also called monogenic disorders, but this form of inheritance has not been consistently observed in patients with CAD and explains only some of the genetic risk [4].
However, family-based analysis of the disease allowed us to observe a clear phenotype linked to CAD and to identify the causative genes, as in those patients with familial hypercholesterolemia (FH) who presented early-onset myocardial infarction (MI), high cholesterol levels and xanthomata [4]. The discovery of such families with a LDLR gene mutation led to the subsequent identification of genes associated with FH including APOB, PCSK9, LDLRAP1, ABCG5, and ABCG8. In addition, LRP6 gene mutations associated with LDL cholesterol, triglycerides, hypertension, diabetes mellitus, and osteoporosis as well as DYRK1B gene mutations associated with obesity, severe hypertension, and diabetes mellitus have been reported as familial onset MI genes that are different from FH and cause MI at a young age [4].
Genome Wide Analysis
Today, 50 years later, current research has been expanded through the introduction of arrays that enabled genomewide genotyping (Genome Wide Analysis Studies, GWAS) of an increasing number of genomic variants with subsequent imputation of millions of further variants; and secondly, through the formation of large international consortia jointly investigating the genetics of CAD [5]. Tanaka, Ozaki and their colleagues were the first in the world to conduct a GWAS using the information on approximately 90,000 single nucleotide polymorphisms (SNPs). The SNP identified in their study was on the gene located in the HLA region of chromosome 6q21, which encodes the lymphotoxin-alpha (LTA) which is a proinflammatory cytokine [6]. The chromosome 9p21 locus was independently identified by 3 research consortia at the same time and together with the LPA locus, as the loci with the strongest effect on CAD risk. However, chromosome 9p21 region does not contain protein-coding genes, making it difficult to elucidate the mechanism leading to the disease development [5].
Chromosome 6p24 is also a disease susceptibility locus strongly associated with CAD, carotid artery dissection, and hypertension and recent analyses have reported several disease pathogenic mechanisms, with two candidate genes, phosphatase action regulator 1 gene (PHACTR1) and endothelin-1 gene (EDN1) [4]. In addition, a meta-analysis of joint Japanese and European GWAS with a total of 650,000 subjects [7], which is the largest scale analysis in the world so far, identified 35 novel disease susceptibility loci. As such, genetic studies for CAD have shown a rapid increase in sample size and a concomitant rapid increase in the number of disease-susceptibility loci.
Figure 1 presents known genes until 2021 and supposed mechanisms using a rough classification to a) initiation of plaque formation, b) plaque progression, c) platelet function, and d) unknown mechanism [5].
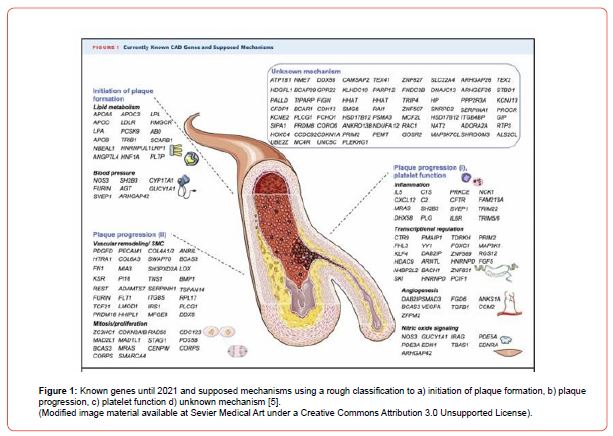
CAD-related polymorphisms present a small to moderate relative risk of about 18% because they are widely diffused in the general population with little biological impact (i.e., on average, CAD-related polymorphisms are found in half of the population) [8]. Possibly, gene expression variability from patient to patient is an explanation to the CAD phenotype dispersion. MicroRNAs (miR) are non-coding small RNAs that regulate gene expression by inhibiting translation or by leading their targets (mRNAs) to degradation. In a recent miR analysis study in carotid plaque specimens [9], miR21, miR122, miR146a and miR196a were up regulated in the carotid plaque region compared to adjacent healthy carotid regions, suggesting that these miRs are correlated with vascular inflammation.
Conclusion
In conclusion, the last several years’ research using GWA studies has provided deep insights into the genetic architecture of CAD, with implications for future prevention and treatment strategies. Polygenic risk scores are under development in order to identify patients at high risk for developing CAD early in their life. The accuracy of CAD polygenic risk scores will probably present considerable variation among different populations creating significant debates within the scientific cardiovascular societies in the near future.
Acknowledgment
None.
Conflict of Interest
No conflict of interest.
References
- (2021) https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds).
- Patterson D, Slack J (1972) Lipid abnormalities in male and female survivors of myocardial infarction and their first-degree relatives. Lancet 1(7747): 393-399.
- Epstein F (1976) Genetics of ischemic heart disease. Postgraduate Medical Journal 52(610): 477-480.
- Miyazawa K, Ito K (2021) Genetic Analysis for Coronary Artery Disease Toward Diverse Populations. Front. Genet 12: 766485.
- Kessler T, Schunkert H (2021) CAD Genetics Enlightened by GWASs. JACC: Basic to Translational Science 6(7): 610-623.
- Ozaki K, Ohnishi Y, Iida A, Sekine A, Yamada R, et al. (2002) Functional SNPs in the lymphotoxin-α gene that are associated with susceptibility to myocardial infarction. Nature Genetics December 32(4): 650-654.
- Koyama S, Ito K, Terao C, Akiyama M, Horikoshi M, et al. (2020) Population-specific and Trans-ancestry Genome-wide Analyses Identify Distinct and Shared Genetic Risk Loci for Coronary Artery Disease. Nat Genet 52: 1169-1177.
- Roberts R (2014) Genetics of Coronary Artery Disease: An Update. Methodist Debakey Cardiovasc J 10(1): 7-12.
- Sioziou A, Katifelis H, Legaki E, Patelis N, Athanasiadis D, et al. (2018) Expression of miR21, miR122, miR146a and miR196 in Symptomatic Carotid Disease. Int Cardiovasc Res J 12(1): 7-12.
-
Panagiotopoulos Evangelos*, Bampouras Nikolaos and Gazouli Maria. Current View of Genetics in Coronary Artery Disease. On J Cardio Res & Rep. 7(3): 2023. OJCRR.MS.ID.000664.
-
Coronary artery disease, Cardiovascular medicine, Pharmacological therapies, Cardiovascular disease, Single nucleotide polymorphisms, Genome wide association studies, MicroRNAs, Familial hypercholesterolemia, Myocardial infarction, High cholesterol levels, Xanthomata
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






