 Mini Review
Mini Review
Dengue Myocarditis Leading to Acute Cardiac Failure: Rare Manifestation of Expanded Dengue Syndrome
Richmond R Gomes*
Associate Professor, Medicine Ad-din Women’s Medical College Hospital, Bangladesh
Richmond R Gomes, Associate Professor, Medicine Addin Women’s Medical College Hospital, Dhaka, Bangladesh.
Received Date: February 02, 2022; Published Date: February 15, 2022
Dengue is a prevalent arthropod-borne viral disease in tropical and subtropical areas of the globe. Dengue clinical manifestations include asymptomatic infections; undifferentiated fever; dengue fever, which is characterized by fever, headache, retroorbital pain, myalgia, and arthralgia; and a severe form of the disease denominated dengue hemorrhagic fever/dengue shock syndrome, characterized by hemoconcentration, thrombocytopenia, and bleeding tendency. However, atypical manifestations, such as liver, central nervous system, and cardiac involvement, have been increasingly reported. Called expanded dengue syndrome. We report a 40 years old gentleman with atypical and rare presentation of dengue disease marked by a dramatic and fatal acute cardiac failure due to acute myocarditis. Condition improved after five days of conservative treatment. Cardiac complications in dengue are now increasingly observed with the most common case is myocarditis. The main mechanism of dengue myocarditis is still unknown though both direct viral infection and immune mediated damage have been suggested to be the cause of myocardial damage. The low incidence of dengue myocarditis is because it’s asymptomatic and diagnosis is easily missed. Almost all cases of dengue myocarditis are self-limiting and severe myocarditis leading to dilated cardiomyopathy is extremely rare. To avoid otherwise preventable morbidity and mortality, physicians should have a high index of suspicion for cardiac complications in patients with dengue illness and should manage this accordingly.
Keywords:Acute heart failure; Acute myocarditis; Cardiomyopathy; Expanded dengue syndrome; Dsengue fever
Introduction
Dengue, an arthropod-borne viral infection of humans, is endemic to tropical and subtropical regions of the world and represents an important public health problem. Dengue viruses are transmitted by the bite of the Aedesaegypti mosquito infected by the one of the four dengue virus serotypes: dengue 1, -2, -3, and -4. More recently, dengue disease has spread geographically to many previously unaffected areas and, as travelling around the world has become more accessible, physicians in temperate areas are more likely to see returning travelers with dengue infection [1,2]. World Health Organization (WHO) classification of symptomatic dengue infection, continuously evolved, first in 1997 it divided into dengue fever (DF), dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS). In 2009 it improved into dengue with or without warning signs and severe dengue [3]. However, in 2011, WHO Regional Office for Southeast Asia (SEARO) revised and further improving the classification, divided into DF, DHF without shock or with shock (DSS) characterized by increased vascular permeability, thrombocytopenia (platelets <100,000), bleeding tendency, and, in a small percentage of patients, circulatory shock [4-7] and expanded dengue syndrome [8].
Expanded dengue syndrome is a new entity added to the classification system to incorporate a wide spectrum of unusual manifestations of dengue infection affecting various organ systems that had been reported including gastrointestinal, hepatic, neurological, cardiac, pulmonary, and renal systems [8]. Of note, a variety of cardiac complications have been reported in dengue affected patients, which include atrioventricular conduction disorders [9], supraventricular arrhythmia [10], and myocarditis [11,12]. The most common complication is myocarditis. However, the study of myocarditis in dengue is still very lacking. The pathogenesis of myocarditis in dengue is still not clear. Clinical manifestation of myocarditis dengue is varied. Endomyocardial biopsy (EMB) is a deterministic diagnostic method but difficult to popularize. According to the ESC (European Society of Cardiologist) New Criteria, the combination of symptoms, electrocardiography, cardiac enzyme marker and cardiac imaging can use to diagnose the dengue hemorrhagic fever patient with myocarditis. The fatal complications of dengue myocarditis are arrhythmias, heart failure, cardiogenic shock until death [13].
Although reports of a more severe disease with progression to cardiogenic shock and death have been increasingly described [14- 16], the pathogenesis of myocardial lesions has not been elucidated. We present a rare case of a fulminant and fatal myocarditis leading to acute heart failure caused by dengue.
Case Report
A 40-year-old gentleman was admitted to Ad-din Women’s Medical College Hospital, Dhaka, Bangladesh with a history of progressive dyspnea in the last 3 days that had recently evolved to dyspnea at rest and orthopnea, atypical chest pain and palpitation. Eight days prior the admission, he complained of fever, headache, retro-orbital pain, generalized body ache and weakness for 6 days followed by afebrile for last 2 days. There are spontaneous bleeding complaints in the form of nosebleeds 1-day prior admission to hospital His past medical history was positive only for a diagnosis of primary hypothyroidism for last four years for which he was taking tablet thyroxin 50 mcg daily regularly. From the previous history of disease did not get the history of dengue fever, diabetes mellitus, bronchial asthma, hypertension, and heart disease. Patients do not smoke and do not consume alcohol.
On clinical examination, he was in severe condition, agitated, dyspneic with signs of poor peripheral perfusion, such as cold extremities and cyanosis. Heart rate was 105 bpm, blood pressures 60/30 mm of Hg, respiratory rate 28 breaths/min, axillary temperature 98.2ºF. SaO2 was 85% in room air. Bi pedal edema was present. Hemorrhagic suffusions or other skin lesions were absent except right subconjunctival hemorrhage and positive rumple leed test. On examination of the head and neck, there is no anemia or jaundice. No enlargement of lymph nodes nor increased jugular venous pressure. On precordial examination there was gallop with no murmur. On abdominal examination, there was no ascites with normal bowel sounds, liver and spleen were not palpable. On chest examination, Bi basal mid to late inspiratory coarse rales were present over both lung base with no rhonchi. Breath sound was vesicular.
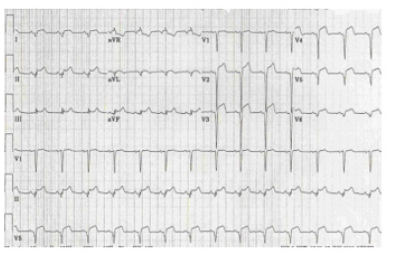
On laboratory investigations, hemoglobin 11.1 g/dL, leukocyte 5600/μL with normal differentials, platelet 45,000/μL, HCT 33.8%, SGPT 66U/L (normal less than 40U/L), Alb 4.8g/dL, serum creatinine 0.75mg/L, sodium 132mmol/L, potassium 3.6mmol/L, chloride 101mmol/L,HCO3 20 mmol/L. HbsAg (non-reactive), CKMB 77U/L (normal 7.0-25.0U/L) and troponin I 2.71ng/mL (normal <0.02 ng/mL). IgM Anti dengue antibody came positive. Chest x ray revealed bilateral opacities extending from both hilum with bat wing appearance suggestive of pulmonary edema (Figure 1). USG of whole abdomen revealed thickened GB wall with mild ascites. Urine routine examination was normal. NT-Pro BNP was 27332 pg/ml (normal less than 400 pg/ml). The 12-lead electrocardiogram detected diffuse ST-segment elevation (Figure 2). Emergency transthoracic echocardiography was performed at bedside and showed mild pericardial effusion, without signs of cardiac tamponade, dilated left ventricle with diffuse hypokinesia of left ventricular wall. There was severe left ventricular dysfunction with ejection fraction was 36%. Interventricular septum was normal. So, diagnosis of dengue hemorrhagic fever with dengue myocarditis with acute pulmonary edema was made as a part of expanded dengue syndrome.
Figure 1:showing chest x-ray suggestive of pulmonary edema.
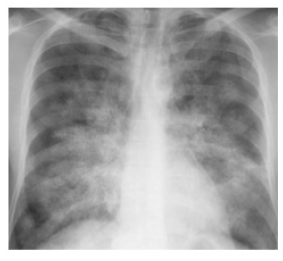
Figure 2:ECG with diffuse ST elevation respectively.
Resuscitation with intravenous frusemide, sodium bicarbonate, vasoactive drugs and digoxin were promptly initiated. Dopamine was used in progressive doses up to 20 μg/kg/min along with frusemide infusion 15 mg/hour along with high flow, high concentration oxygen. He continued receiving intravenous frusemide and dopamine for a further 2 days. With treatment his condition improved with decreasing shortness of breath. On 4th day dopamine infusion was stopped and frusemide infusion was substituted by bolus injection. On 7th day of admission, his condition further improved with no shortness of breath even on exertion. Repeat CKMB 17U/L (normal 7.0-25.0U/L), troponin I 0.7ng/mL (normal <0.02 ng/mL), NT-Pro BNP was 996 pg/ ml(normal less than 400 pg/ml). He was discharged on 9th day of admission with stable hemodynamic condition with no clinical signs of heart failure. He was advised to follow up on outpatient door after 2 weeks.
Discussion
Dengue is a worldwide public health problem and causes innumerous deaths. More than 40% of the world’s population lives in dengue endemic areas, and the World Health Organization estimates that about 2.5 billion people in 100 countries are at risk of infection and that as many as 100 million people are infected by dengue viruses every year. In the majority of infected people, dengue is an auto-limited disease that resolves in 5–7 days. However, approximately 500,000 people develop a severe form, leading to about 20,000 deaths annually. Consequently, approximately 0.5% of dengue patients develops a severe form and requires a specialized treatment [2,17].
Dengue virus infection is a disease that found in children and adults with the main symptoms of fever, muscle and joint pain that usually worsens after the first three days. This disease is an acute febrile illness accompanied by bleeding manifestations with potential shocking and can lead to death in children<15 years, but not likely to attack adults [18]. Signs of this disease are sudden high fever 2 to 7 days with no obvious cause, weakness, lethargy, anxiety, heartburn, accompanied by signs of bleeding in the skin (petechiae), bruising (ecchymosis) or rash (purpura). Sometimes there are other spontaneous bleeding manifestations such as nosebleeds, bleeding gums to dysentery. Severe symptoms can lead to decreased awareness or shock [19].
Laboratory results in dengue fever are found in thrombocytopenia (20% of the baseline on dengue hemorrhagic fever is a sign of plasma. Serological tests results in dengue are influenced by the type of dengue infection, whether it is the primary/ first, or secondary/reinfection. IgM antibodies are detectable by days 3–5 after the onset of illness, rise quickly in two weeks and decline to undetectable levels after 2–3 months, because this late appearance, the first five days of clinical illness are usually negative of IgM. In dengue secondary infection, the rise of IgM are not as high as primary infection, and sometimes absent / undetectable completely [20].
IgG antibodies in primary infection, evolves relatively slow, with low titers 8-10 days after fever onset, increase subsequently and remain for many years, whereas in secondary infection it evolves rapidly, with high titers soon after fever onset and persist to a lifelong period. Hence, a ratio of IgM/IgG is commonly used to differentiate between primary and secondary dengue infections. Ratio of IgM/ IgG titer less than 1.2 is considered a secondary dengue infection. But to be noted, titer ratio only could be validly use as a data if the IgG/IgM serological test is using pure quantitative means, not by qualitative or semi-quantitative [21].
NS1 antigen detection is widely used and cost-effective, NS1 could be detected from day 1-8 of fever onset, unaffected by a primary or secondary dengue infection. In conclusion, by combining the serological (IgG and IgM) and NS1 tests, clinicians could rapidly assess the dengue diagnosis with its types (primary or secondary infection) and applies the best treatment [22].
In 2011, based on many reports of cases with dengue-related unusual manifestations and organ complications, WHO-SEARO further improved and revised 2009 WHO guidelines by adding a new entity, that is expanded dengue syndrome (unusual/atypical manifestation of dengue), these include neurological, hepatic, renal, cardiac and other isolated organ involvement, that could be explained as complications of severe, profound shock or associated with underlying host conditions/diseases or coinfections [8].
The incidence of cardiac complications in patients with dengue illness varies greatly from one series to another. From India, Agarwal, et al. reported that only one of 206 patients subjected to cardiovascular evaluation experienced cardiac symptoms [23]; Wali, et al. reported that 70% of 17 patients with DHF/DSS who underwent myocardial scintigraphy study suffered diffuse left ventricular hypokinesis with a mean ejection fraction of 40% [24]; and Kabra, et al. reported that 16.7% of 54 children with dengue illness had a decreased left ventricular ejection fraction of<50% 11A recent report from Sri Lanka showed that 62.5% of 120 adults with dengue fever (DF) had an abnormal electrocardiogram.14 These series suggest that cardiac complications in patients with dengue illness are not uncommon, and might have been under-diagnosed because most of the cases with cardiac complications are clinically mild and self-limited [25]. Weerakoon, et al. [26] performed autopsies on five patients who died due to dengue complications and showed histopathological evidence of myocarditis. In China from August to October 2014, from 1782 diagnosed dengue patients, there are about 201 cases patient were diagnosed with myocarditis and the prevalence of myocarditis in hospitalized dengue was 11.28%.
The clinical manifestations of cardiac complications in dengue illness vary considerably [9-12,14,24,25]. At one end of the clinical spectrum, patients are asymptomatic or have mild cardiac symptoms despite relative bradycardia, transient atrioventricular block, and/or ventricular arrhythmia [9,10,24,27,28]. At the other severe end, patients may experience acute pulmonary edema and/or cardiogenic shock due to severe myocardial cell damage with left ventricular failure [6,9,11,12,14,24,29]. Myocarditis can masquerade as acute myocardial infarction [29,30]. Clinical presentation in myocarditis is varied. The sign and symptoms are chest pain, dyspnea at rest or exercise, palpitation, syncope, cardiac shock, and sudden cardiac death.13Cardiac arrhythmias are other clinical manifestations of myocarditis. Various arrhythmias have been described during dengue virus infection such as atrial fibrillation, ventricular tachycardia, and even atrioventricular blocks. These arrhythmias are associated to syncope and even sudden death [10,27].
According to the diagnostic criteria from European Society of Cardiology 2013, dengue patients were subjected to electrocardiogram (ECG), echocardiography and cardiac enzyme test (CET) to make the diagnosis of myocarditis. Myocarditis was diagnosed if 1 or more clinical presentation and 1 or more auxiliary diagnosis method; 2 or more auxiliary diagnosis method should be met if the patient is asymptomatic. 12 leads ECG was considered abnormal with any of following, such as sinus arrest, AV-block, bundle branch block, atrial fibrillation, ST wave change (ST elevation, ST depression, T inversion), abnormal Q waves. Based on echocardiography usually found functional and structural abnormalities such as ventricular dilatation, increased wall thickness, diastolic function abnormality, pericardial effusion, left ventricular ejection fraction less than 55%, valvular regurgitation or vegetation. The cardiac enzyme was considered to be elevated and abnormal if CK-MB more than 25U/L and/or cTnI more than 0.02ng/m and/or NT-proBNP more than 450ng/L (age 75 years). The gold standard to diagnose myocarditis is EMB (Endomyocardial Biopsy), but it is not performed regularly [13]. As for the cardiac complication in this reported patient, the differential diagnosis included acute myocardial infarction and acute myocarditis; the former is characterized by a blockage of the coronary arteries, while the latter has patent coronary arteries. However, rapid clinical improvement after the development of hypotension and acute pulmonary edema unequivocally indicated that this was a case of myocarditis.
Figure 3:Pathogenesis myocarditis in dengue infection virus [34].

The pathogenesis myocarditis in dengue patient is still unclear. The mechanism of myocardial damage in dengue could be the release of inflammatory mediators and the direct action of the virus on cardiomyocytes, as seen in acute myocarditis caused by other viruses [31]. Using immunofluorescence confocal microscopy in heart tissue, Salgado, et al. [32] reported that myotubes were infected by dengue virus in one child with fatal DHF, although the myocardium sections appeared morphologically normal, with minimal cellular, infiltrates (Figure 3).
The gold standard of myocarditis in dengue patient is Endomyocardial Biopsy, the fulminant course of clinical dengue myocarditis was associated with intense interstitial edema, several multifocal areas of necrosis, and diffuse inflammatory infiltration. Interestingly, the myocytolitic necrotic areas were replete with virus particles; therefore, providing detailed histological evidence of a possible dengue direct action in cardiomyocytes. Further clinical and experimental studies are necessary to better understand the molecular mechanism of dengue virusinduced lesions on the myocardium [33]. With respect to volume replacement for DHF patients with a 20% increase in hematocrit, the World Health Organization recommends intravenous infusion with 5% glucose in physiological saline at 6 ml/h/kg for the initial 1–2 h, followed by 3–5 ml/h/kg, which may be discontinued at 24 to 48 h depending on the normalization of hematocrit, pulse rate, and blood pressure. Overhydration may lead to fluid overload, resulting in respiratory distress in patients with dengue. In the present case, despite improvement in the serial hematocrit after fluid therapy, hypotension developed on the third day of treatment suggesting that this resulted from cardiac dysfunction rather than insufficient intravenous fluid replacement, thus indicating that the patient’s pulmonary edema was cardiogenic due to impairment of left ventricular function [34,35].
Myocardial dysfunction has been reported to be more severe inpatients with DSS when compared to those with DF or nonshock DHF [36]. The pathophysiology of myocardial cell injury in dengue illness is not yet fully understood. Myocardial involvement in dengue may result either from direct DEN invasion of the cardiac muscles or a cytokine-mediated immunological response, or both [37,38]. The upsurge in serum tumor necrosis factor-a, interleukins 6, 13 and 18, and cytotoxic factors in patients with dengue illness led to increased vascular permeability and shock [39,40]; whether these cytokines play a role in the development of myocardial cell injury is unknown. Of note, only DEN-2 and DEN-3 were reported to be the culprit viruses in dengue patients with cardiac complications where the DEN serotype was mentioned [9,14,24,25]. Further studies are needed to clarify the role that DEN serotype plays, if any, in cardiac complications in dengue-affected patients.
Our review shows that cardiac complications are not uncommon in dengue illness. Although it was self-limiting in our patient under supportive treatment, acute myocarditis in dengue may be clinically severe to such an extent that it has a fatal outcome [6,29]. Early recognition of myocardial involvement in dengue illness, prompt restoration of hemodynamic instability while avoiding fluid overload, and sparing unnecessary invasive management are important in treating dengue-affected patients with severe myocarditis.
Conclusion
Dengue virus can produce atypical manifestations as acute myocarditis leading to cardiogenic shock and death by a possible direct virus action on cardiomyocytes. Physicians taking care of dengue patients should be aware of this possible complication. Early recognition of myocardial involvement in dengue illness, prompt restoration of hemodynamic instability while avoiding fluid overload, and sparing unnecessary invasive management are important in treating dengue-affected patients with severe myocarditis.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Lupi O (2011) Mosquito-borne hemorrhagic fevers. DermatolClin 29: 33-38.
- Simmons CP, Farrar JJ, Nguyen V, et al. (2012) Dengue. N Engl J Med 366: 1423-1432.
- World Health Organization (2011) Comprehensive guidelines for prevention and control ofdengue and dengue haemorraghic fever (India: World Health Organization) p. 23-32.
- Da Fonseca BA, Fonseca SN (2002) Dengue virus infections. CurrOpinPediatr 14: 67-71.
- Jacqueline L Deen, Eva Harris, Bridget Wills, Angel Balmaseda, Samantha Nadia Hammond, et al. (2006) The WHO dengue classification and case definitions: time for a reassessment. Lancet 368: 170-173.
- GN Malavige, S Fernando, DJ Fernando, SL Seneviratne (2004) Dengue viral infections. Postgrad Med J 80: 588-601.
- Teixeira MG, Barreto ML (2009) Diagnosis and management of dengue. BMJ 339: b4338.
- World Health Organization, Regional Office for South-East Asia (WHO-SEARO) (2011) Comprehensive guidelines for prevention and control of dengue and dengue hemorrhagicfever (India: World Health Organization) 20: 18-20.
- Khongphatthallayothin A, Chotivitayatarakorn P, Somchit S, Mitprasart A, Sakolsattayadorn S, et al. (2000) Morbitz type I second degree AV block duringrecovery from dengue hemorrhagic fever. Southeast Asian J Trop Med PublicHealth 31: 642-645.
- HortaVeloso H, Ferreira Junior JA, Braga de Paiva JM, FariaHonorio J, JunqueiraBellei NC, et al. (2003) Acute atrial fibrillation during dengue hemorrhagicfever. Braz J Infect Dis 7: 418-422.
- Kabra SK, Juneja R, Madhulika, Jain Y, Singhal T, et al. (1998) Myocardialdysfunction in children with dengue haemorrhagic fever. Natl Med J India 11: 59-61.
- Promphan W, Sopontammarak S, Pruekprasert P, Kajornwattanakul W, Kongpattanayothin A (2004) Dengue myocarditis. Southeast Asian J Trop Med Public Health 35: 611-613.
- Yingying Li, Zhongwei Hu, Yuli Huang, Jianping Li, Wenxin Hong, et al. (2016) Characterization of the myocarditis during the worst outbreak of dengue infection in China. Med J 95(27): 4051-4056.
- SAM Kularatne, Manoji MK Pathirage, UAB Medagama, Sunethra Gunasena, Maya B Gunasekara (2006) Myocarditis in three patients with dengue virus type DEN 3 infection. Ceylon Med J 51: 75-76.
- Nagaratnam N, Siripala K, de Silva N (1973) Arbovirus (dengue type) as a cause of acute myocarditis and pericarditis. Br Heart J 35: 204-206.
- Obeyesekere I, Hermon Y (1973) Arbovirus heart disease: myocarditis and cardiomyopathy following dengue and chikungunya fever – a follow-up study. Am Heart J 85: 186-194.
- Pinheiro FP, Corber SJ (1997) Global situation of dengue and dengue haemorrhagic fever, and its emergence in the Americas. World Health Stat Q 50: 161-169.
- Suhendro, Nainggolan L, Chen K, Pohan HT (2014) Dengue hemorrhagic fever Medical faculty of University of Indonesia study book 6 th edition (Jakarta: Interna Publishing) p. 539.
- Mediastianto E (2015) Extraordinary event in East Java and South Sumatra province in 2015. Jakarta: Health Department of Republic Indonesia.
- Chawla P, Amrita Y, Viney C (2014) Clinical implications and treatment of dengue. Asian Pac J TropMed. 7(3): 169-178.
- Guzman MG, Eva H (2015) Dengue infection. Lancet J Trop Med 385(9966): 453-465.
- Rathakrishnan A, Sekaran SD (2015) New development in the diagnosis of dengue infections. Expert Opin Med Diagn 7(1) 124-133.
- Agarwal R, Kapoor S, Nagar R, Misra A, Tandon R, et al. (1999) A clinical study of the patients with dengue hemorrhagic fever during the epidemic of 1996 at Lucknow, India. Southeast Asian J Trop Med Public Health 30: 735-740.
- Wali JP, Biswas A, Chandra S, Malhotra A, Aggarwal P, et al. (1998) Cardiac involvement in dengue haemorrhagic fever. Int J Cardiol 64: 31-36.
- Ravindral S, Kanagasinham A, Neomali A, Amerasena, Uditha B, et al. (2007) Asymptomatic myocardial involvement in acute dengue virus infection in a cohort of adult Sri Lankans admitted to a tertiary referral centre. Br J Cardiol 14: 171-173.
- Kosala Gad Weerakoon, Senanayake Am Kularatne, Deepthika H Edussuriya, Sarachchandra Ka Kodikara, Laxman Pg Gunatilake, et al. (2011) Histopathological diagnosis of myocarditis in a dengue outbreak in Sri Lanka, 2009. BMC Res Notes 4: 268.
- . Chuah SK (1987) Transient ventricular arrhythmia as a cardiac manifestation in dengue haemorrhagic fever—a case report. Singapore Med J 28: 569-572.
- Lateef A, Fisher DA, Tambyah PA (2007) Dengue and relative bradycardia. Emerg Infect Dis 13: 650-651.
- Lee CH, Teo C, Low AF (2009) Fulminant dengue myocarditis masquerading as acute myocardial infarction. Int J Cardiol 136: e69-e671.
- Mascarenhas DA, Spodick DH (1993) Acute myocarditis masquerading as acute myocardial infarction. N Engl J Med 328: 1714-1715.
- Cooper LT Jr(2009) Myocarditis. N Engl J Med 360: 1526-1538.
- Doris Martha Salgado, José Miguel Eltit, Keith Mansfield, César Panqueba, Dolly Castro, et al. (2010) Heart and skeletal muscle are targets of dengue virus infection. Pediatr Infect Dis J 29: 238-242.
- Lee IK, Lee WH, Liu JW, Yang KD (2010) Acute myocarditis in dengue hemorrhagic fever: a case report and review of cardiac complications in dengue-affected patients. Int J Infect Dis 14: 919-922.
- Yacoub S, Wertheim H, Simmons CP, Screaton G, Wills B (2014) Cardiovascular manifestation of the emerging dengue pandemic. Nature Rev Cardiol 11: 335-345.
- Ware LB, Matthay MA (2005) Acute pulmonary edema. N Engl J Med 353: 2788-2796.
- Khongphatthanayothin A, Lertsapcharoen P, Supachokchaiwattana P, La-Orkhun V, Khumtonvong A, (2007) Myocardialdepression in dengue hemorrhagic fever: prevalence and clinical description. Pediatr Crit Care Med 8: 524-529.
- Hober D, Poli L, Roblin B, Gestas P, Chungue E, et al. (1993) Serumlevels of tumornecrosis factor-alpha (TNF-alpha), interleukin-6 (IL 6), andinterleukin-1 beta (IL-1 beta) in dengue-infected patients. Am J Trop Med Hyg 48: 324-331.
- Hober D, Delannoy AS, Benyoucef S, De Groote D, Wattre P (1996) High levels of sTNFRp75 and TNF alpha in dengue-infected patients. MicrobiolImmunol 40: 569-573.
- Chen RF, Yang KD, Wang L, Liu JW, Chiu CC, et al. (2007) Different clinical andlaboratory manifestations between dengue haemorrhagic fever and denguefever with bleeding tendency. Trans R Soc Trop Med Hyg 101: 1106-1113.
- Chen RF, Liu JW, Yeh WT, Wang L, Chang JC, et al. (2005) Altered T helper 1reaction but not increase of virus load in patients with dengue hemorrhagicfever. FEMS Immunol Med Microbiol 44: 43-50.
-
Richmond R Gomes. Dengue Myocarditis Leading to Acute Cardiac Failure: Rare Manifestation of Expanded Dengue Syndrome. On J Cardio Res & Rep. 6(3): 2022. OJCRR.MS.ID.000637.
-
Acute heart failure, Acute myocarditis, Cardiomyopathy, Expanded dengue syndrome, Dsengue fever, Pulmonary, Renal systems, Arrhythmias, Heart failure, Cardiogenic shock, Dengue fever, Diabetes mellitus, Bronchial asthma, Hypertension, Heart disease
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






