 Research Article
Research Article
Cardiac Helical Function. Fulcrum and Torsion
Trainini Jorge1*, Lowenstein Jorge2, Beraudo Mario3, Trainini Alejandro1,3, Mora Llabata Vicente4, Carreras- Costa Francesc5, Valle Cabezas Jesús6, Wernicke Mario7, Elencwajg Benjamín8, Lowenstein-Haber Diego2 and Bastarrica María Elena3
1Department of Cardiac Surgery, Hospital Presidente Perón, Argentina
2Department of Cardiology, Investigaciones Médicas, Argentina
3Department of Cardiac Surgery, Clínica Güemes, Luján, Argentina
4Department of Cardiology, Hospital “Dr Peset”, Spain
5Department of Cardiology, Hospital Sant Pau, Spain
6Naval Architect, Spain
7Department of Pathological Anatomy, Clínica Güemes, Luján, Argentina
8Department of Electrophysiology, Hospital Presidente Perón, Argentina
Jorge Trainini MD, PhD; Buenos Aires, Argentina.
Received Date:December 17, 2022; Published Date:January 05, 2023
Abstract
Background: The aim of this study was to investigate a) the starts and ends of the continuous myocardium; b) the slippage between the myocardial
segments, when performing both torsion and ventricular detorsion, implies that there should be an antifriction mechanism that avoids
dissipating the energy; c) the electrical activation of the endocardial and epicardial myocardium and secondarily understand ventricular twist and
the mechanism of active suction during the diastolic isovolumic phase.
Methods: Twenty-four hearts were used: a) Fitteen two-year-old bovine hearts weighing 800-1000 g; b) nine human hearts (two 16- and 23-
week gestation embryos, one from a 10-year-old child weighing 250 g and six from adults, with an average weight of 300 g); b) five patients with no
structural cardiac abnormalities and normal QRS complexes underwent three-dimensional endoepicardial electroanatomic mapping.
Results: We have found in all the bovine and human hearts studied a nucleus (fulcrum) underlying the right trigone, whose osseus, chondroid
or tendinous histological structure depends on the specimen analyzed. All the hearts studied presented myocardial attachment to the rigid structure
of the fulcrum. Hyaluronic acid was found in the cleavage planes between the myocardial bundles. Endo-epicardial mapping demonstrates an electrical
activation sequence in the area of the apex loop in agreement with the synchronic contraction of the descending and ascending band segments,
consistent with the mechanism of ventricular twist. The late activation of the ascending segment is consistent with its persistent contraction during
the initial period of the isovolumic diastolic phase (the basis of the suction mechanism).
Conclusions: The finding of the fulcrum gives support to the spiral myocardial band being the point of fixation that allows the helical torsion.
The hyaluronic acid would act as a lubricant and provide great resistance to mechanical pressures. This study explains the ventricular twist and the
active suction mechanism during the isovolumic diastolic and early ventricular filling phases.
Keywords:Cardiac anatomy; Ventricular band; Fulcrum; Friction; Hyaluronic Acid; Heart/physiology; Cardiac Electrophysiology
Abbreviations:TEM: Three-dimensional Electroanatomical Mapping; MS: Milliseconds; LVEF: Left Ventricular Eyection Fraction; LV: Left Ventricle
Introduction
The function of the heart corresponds to a mechanical dimension that should be addressed in terms of its structure, which is where we find the origin of the idea that led our research to explain its organicfunctional integrity. If we stop in classical descriptions of the heart we realize that anatomical attention was focused on its external and internal surfaces, granting scant importance to the intimate muscle conformation [1]. This was believed to be of a homogeneous solid nature with global uniform contraction, not considering that its mechanical capacity demanded a reinterpretation of its spatial anatomy and motions, leading us into other topics of its functioning that were completely disregarded by cardiology.
The anatomy of the heart was traditionally thought to be formed by spiraling muscle bundles, but these were never described in association with their physiology. Was Torrent Guasp who in 1970 [2] initiated the description and interpretation of the myocardial muscle band, starting point to understand its motions. This was demonstrated in multiple dissections showing that the ventricular myocardium is made up of a group of muscle fibers coiled unto themselves, resembling a rope, flattened laterally as a band, which by giving two spiral twists describes a helix that limits the two ventricles and defines their performance (Figures 1 & 2).
An explanation for this muscle homogenization with an intricate anatomical arrangement that hides the continuous myocardium, implies considering that its structural solidity is required in birds and mammals so that blood is ejected at high speed in a limited time span by an organ that must serve two circulations (systemic and pulmonary). The anatomical evolutionary state of the heart agrees with ventricular mechanics but lacked the understanding of an electrical propagation that could accurately explain the physiology. The studies on this topic aim to show the integrity of an essential cardiac structure-function [3-6]. The left ventricular endocardial and epicardial electrical activation performed in patients with three-dimensional electro anatomical mapping (TEM) allowed considering this fundamental topic to analyze it.
This pathway leading from structure to function induced
to research on topics poorly explained by their mechanical
organization [6-8], but which should be considered complementary
among them and essential for the physiology of the heart, to know:
1. Anatomical and histological investigation of the segmental
sequence of the continuous myocardium.
2. Support and insertion of myocardium.
3. How is ventricular torsion produced?
4. Is there a histology that explains the phenomenon of
friction between the layers of the myocardium? Is there an
organic lubricating source?
5. How is active protodiastolic ventricular suction produced?
6. Is it possible to consider in the heart a coupling phase
between systole and diastole where cardiac suction takes place?
Which is the energy mechanism in the active suction phase?
Material and Method
The methods used in this study to explain the hypothesis of the
anatomo-functional integrity of the heart were:
1) Cardiac dissection: Twenty-four hearts were used: a)
Fitteen two-year-old bovine hearts weighing 800-1000 g; b)
nine human hearts (two 16- and 23-week gestation embryos,
one from a 10-year-old child weighing 250 g and six from
adults, with an average weight of 300 g)
2) Histological and histochemical analysis of anatomical
samples.
3) Left ventricular endocardial and epicardial electrical
activation in humans by means of TEM (five adult patients). The
left ventricular endo and epicardial electrical activation sequence
was studied with a navigation system and Carto mapping,
enabling three-dimensional anatomical representation, with
activation and electrical propagation maps. Isochronic and
activation sequence maps were performed, correlating them
with surface ECG.
Since the left ventricular muscle structure is composed of
an endocardial and an epicardial portion, or descending and
ascending segments, respectively, in the anatomical classification
of Torrent Guasp (Figure 1), two access routes were used to
perform the mapping. The endocardial access was achieved by
conventional atrial transeptal puncture and the epicardial access
by percutaneous approach in the pericardial cavity with an ablation
catheter. Endocardial and epicardial mapping were immediately
and consecutively performed. In addition, the electrical activation
propagation times of the muscle band were measured in milliseconds
(ms). The study was performed at Hospital Presidente Perón
(Buenos Aires, Argentina) and included patients who had signed an
informed consent previously approved by the Institutional Ethics
Committee. All patients were in sinus rhythm, with normal QRS and
no verifiable cardiac disease by Doppler echocardiography and in
resting and stress gamma camera studies.
4) Echocardiography to corroborate previous studies and
the usefulness of these knowledge in clinical practice.
Result
Myocardic architecture:Torrent Guasp demonstrated through multiple dissections in hearts of different species, including the human, that the ventricular myocardium is made up of a helical band (Figures 1 & 2) [8]. This study approach correlates with a cardiac structure presenting the remarkable characteristic of being adriving-suction pump with the size equivalent to a human fist and an average weight of 270 grams, which ejects 4-6 liters/minute at a speed of up to 200 cm/s, consuming only 10 watts, and working without interruption for 80 years without maintenance, almost without noise, and no smoke. Its work is equivalent to the daily withdrawal of 1 ton of water 1 m deep with a mechanical efficiency (work/energy relationship) of 50%, not achieved by man-made machines which only attain 30% efficacy. This allows ejecting 70% of the left ventricular volume with only 12% shortening of its
Figure 1:Rope model of the myocardial band. It illustrates the different segments that form the band. In blue: Basal loop. In red: Apical loop. The site of cardiac fulcrum can be seen in the continuous myocardium (cord model). A: aorta; AP: arteria pulmonary.
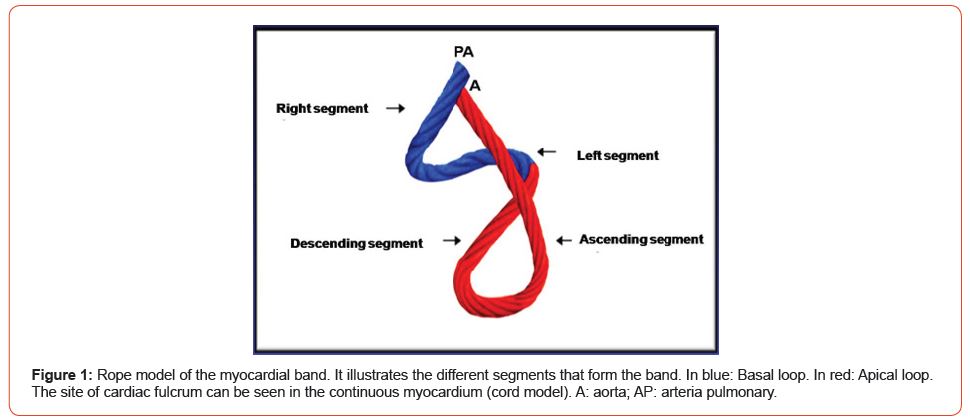
Figure 2:Helical myocardium. RV: Right ventricle; LV: left ventricle; PIS: Posterior interventricular system; AIS: Anterior interventricular system.
In view of the criticism or indifference met by the helical myocardial band, its dissection finds a structure with defined planes where the successive and related physiological heart motions of narrowing, shortening-twisting, lengthening-untwisting and expansion take place depending on the propagation of the electrical stimulus along its muscle pathways. It should be recalled that the myocardium constitutes a spiraling continuum in its fibers responding to the helical pattern in its muscle bundles. This arrangement indicates the need to generate mechanical work that dissipates little energy. Therefore, the fiber layers gradually shift their orientation, with more or less acute angles, to avoid abrupt changes in the spatial organization dissipate the necessary work for cardiac function [10].
This situation generates a tangle of fibers that allows the band to behave as a continuous transmission chain with the epicardial fibers taking an oblique direction, the intermediate fibers a transverse course and the endocardial fibers also an oblique direction, but contrary to that of the epicardial plane. The endocardial and epicardial plane access angle is approximately 60 degrees in relation to the transverse fibers. Fiber orientation defines function and thus the ejection fraction is 60% when the normal helical fibers contract and falls to nearly 30% if only the transverse fibers shorten, reaching 15-20% if only the subendocardial longitudinal fibers are deformed. This occurs when the left ventricle dilates in cardiac remodeling and the fibers miss their oblique orientation, losing muscular and mechanical efficiency [11].
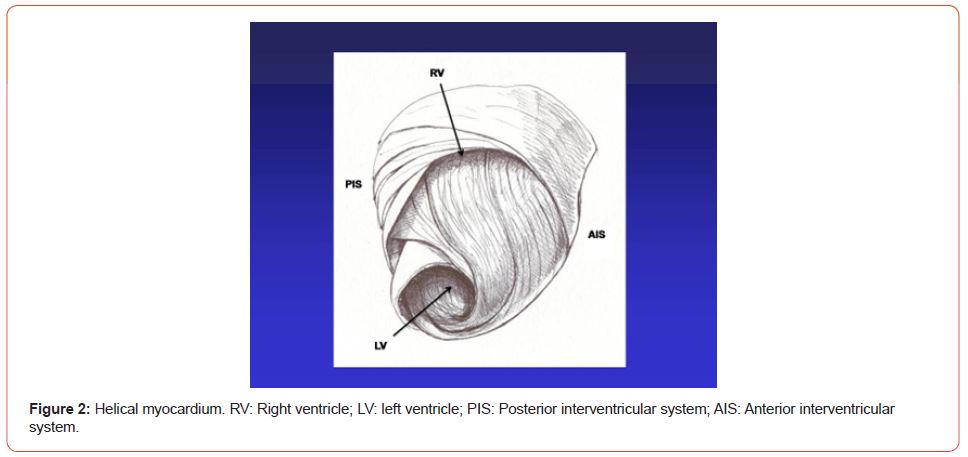
In the progression from the ventricular base to the apex, the number of horizontal fibers decreases in relation to the oblique fibers, showing that the heart is organized as a continuous muscle helix (Figure 3).
Figure 3:Spiral fibers. The ascending and descending segments can be seen in a cross section near the apex. They are correctly visualized as the fibers are spiraling along their path (bovine heart).
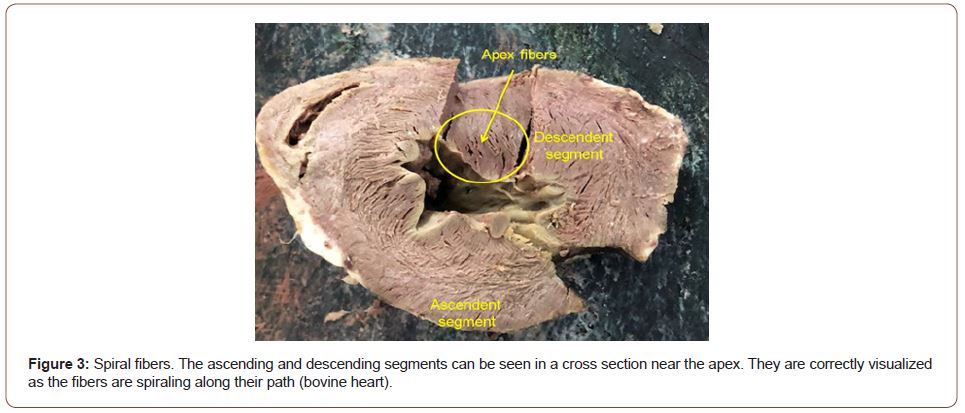
Myocardial muscle bundles and bands, which derive from phylogenetic development, essentially shape a master axis of precise dynamic requirement. The spatial muscle structure adopted by the myocardial muscle has a double function: a) to limit the ventricular chambers and b) to fulfill the suction and driving action in its role of cardiac pump.
Segmentation of the continuous myocardium
Figure 4:Myocardial unfolded in all its extension (bovine heart). PA: Pulmonary artery; RS: Right segment; LS: Left segment; DS: Descending segment; AS: Ascending segment; A: Aorta.

(Figure 4) shows the unfolded muscle band. Being able to unfold the myocardium with a similar thickness in all its extension proves that the band is real and not a “heuristic” or biased construction. In its course, the myocardial band adopts a helical configuration that defines the two ventricular chambers. The myocardial band is characterized by a descending and an ascending band. The former includes the right, left and descending segments, while the latter is formed by the remaining ascending segment (Figure 1).
The continuous myocardium describes two spiral turns with the insertion of its initial end along the line extending from the pulmonary artery to the orifice of the tricuspid valve, called the pulmo-tricuspid cord, in front of the aorta, while its final end attaches below the aortic root. Both ends are fixed by an osseous, chondroid or tendinous nucleus, depending on the different species (animal or human) used in the studies. This nucleus, which we have called cardiac fulcrum [1,11], is the only perceptible edge where the muscle band fibers originate and end (Figures 5 & 6). These insertions must be understood as the supporting point of the continuous myocardium to fulfill its hemodynamic function (Figure 7). In our investigations we have not found insertion of cardiomyocytes in the collagen matrix of the trigones (Figure 8).
Figure 5:Adult human heart. The ascending segment can be seen inserting into the cardiac fulcrum. References: LA: Left atrium; RA: Right atrium; PA: Pulmonary artery; RV: Right ventricle. A: Cardiac fulcrum resected.
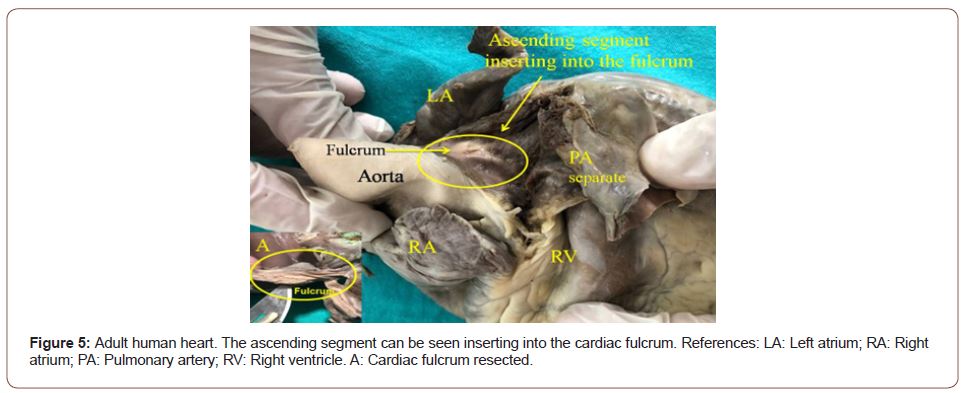
Figure 6:Fulcrum in the adult human heart (anatomical piece).
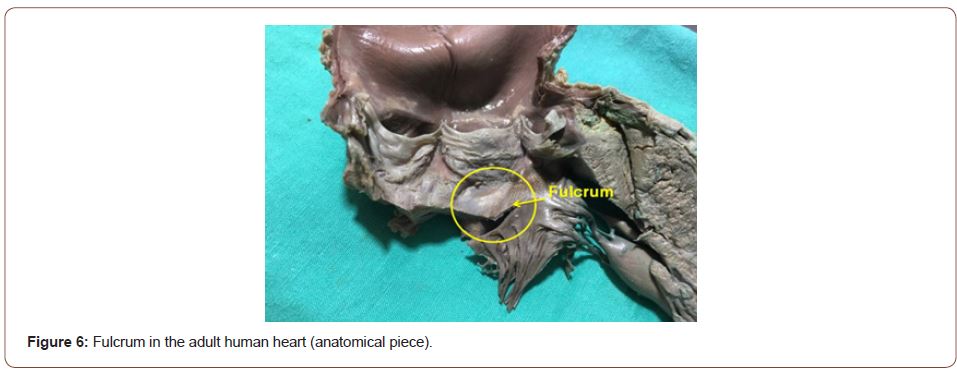
Figure 7:Cardiomyocytes penetrating in the the fulcrum (adult human heart). The circle details the insertion site. 1, cardiomyocytes; 2, fibrocolagenus matrix.
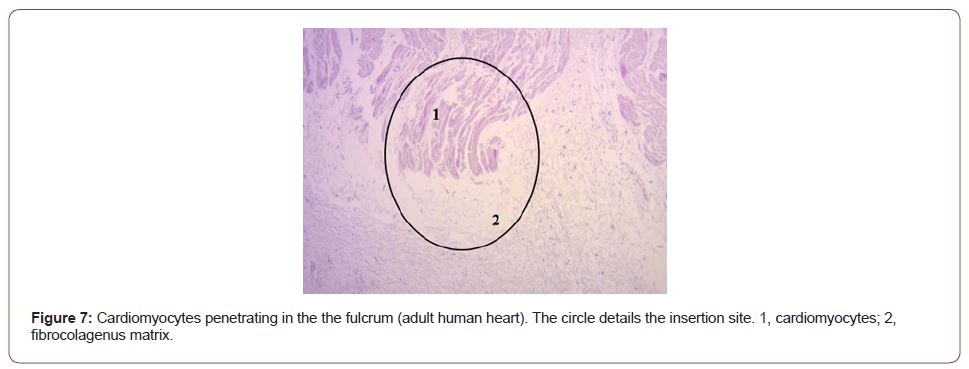
Figure 8:Left an right trígones (adult human heart). There is no inclusion of cardiomyocytes.
In the myocardium we can distinguish the basal and apical loops. The basal loop extends from the base of the pulmonary artery to the central band twist. On the other hand, the apical loop courses from this point of inflexion to the base of the aorta. In turn, each loop consists of two segments. The basal loop consists of the right and left segments and the apical loop of the descending and ascending segments (Figure 1). In the general loop configuration, the basal loop envelops the apical loop, so that the right ventricular chamber presents as an open slit in the muscle mass thickness forming both ventricles (Figure 2). These concepts indicate that the right ventricular free wall is formed by one loop (basal) and the left ventricular free wall by both loops (basal and apical). The fundamental point for cardiac mechanics is that the base and apical muscle fibers course in different directions. This disparity finds correlation with the fiber trajectories and the helical pattern of the myocardium limiting the ventricles.
Histological analysis of the continuous myocardium
The myocardium is a syncytial muscle with lateral bridges between its muscle fibers and a laminar histological arrangement of the muscle bundles. Due to its histological arrangement, the myocardium (syncytium) behaves like a auxetic biological material; in other words, it contracts simultaneously in two directions (longitudinal and circumferential) and thickens in a third direction (radial, towards the ventricular centroid). The histological analysis sequence of the unfolded continuous myocardium (Figure 9) demonstrates its linear orientation according to the segmental continuity of its spatial organization when the band is coiled, both in its internal and external surfaces. These orientations are identical in both surfaces (internal and external). It can be seen that the myocardial structure is not a lattice (crisscross of myocardial fibers) but a continuous muscle. The lattice concept used was developed due to the band folding resulting in overlapping segments, which are functionally independent and with friction between their surfaces [12]. This arrangement is essential to achieve myocardial torsion, a fundamental action of cardiac mechanics that would be impossible with the lattice structure.
Figure 9:Segment sequence from the myocardial band histological analysis (bovine heart). RS: right segment; LS: left segment; DS: descending segment; AS: ascending segment.
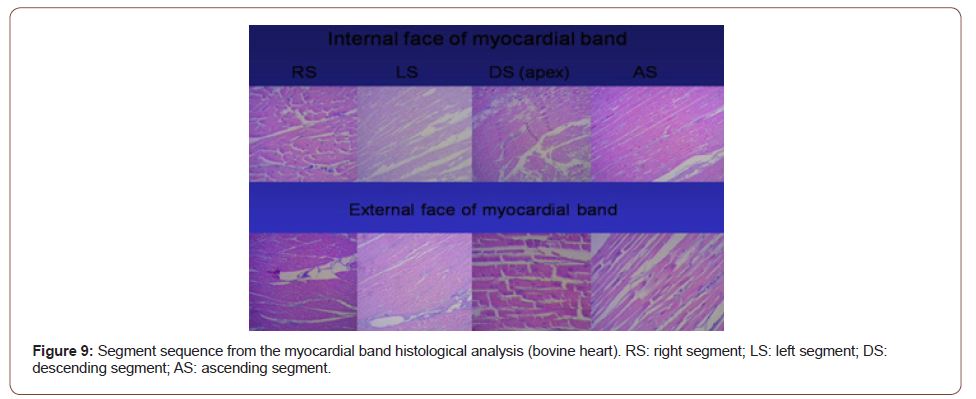
No segment of the myocardium histological sequence explored in our investigations presents a lattice organization. As the external surface of the distal descending segment (Figure 9, lower panel) twists to become the ascending segment, the cardiomyocytes generate in the planimetric histological sections a different architecture in their orientation from that of the internal surface, only site (cardiac apex) where this situation occurs. The rest of the orientation is always parallel. In the apex, the spiral course of the myocardial fibers, which shift from the periphery towards the center, determine a torsion where the subepicardial fibers become subendocardial, overlapping like the tiles of a roof, as evidenced in this image (Figure 3). As previously expressed, the opposite orientation and rotational movement of the left ventricular fibers, both at the basal and distal apical segment levels, explain the helical myocardium model.
The interventricular septum consists of a ventral and a dorsal part. The first portion consists of the left descending segment, the intraseptal band (final segment of the continuous myocardium) and the anterior septal band. The first two belong to the left ventricle and the remaining one to the right ventricle. The posterior region of the septum is composed of the left descending segment (corresponding to the left ventricle) and the posterior septal band (corresponding to the right ventricle). The spatial arrangement and the rotational motion of the ventricular fibers, conform the architectural plan of the myocardium. Up to this moment, a classic interpretation has been made of blood circulation through the different heart chambers, which does not correlate with its muscle dynamics. And this is, ultimately, the engine for the circulation established by the continuous myocardium, which also defines with its muscle structure, the limits of the chambers through which blood flows. This spatial arrangement of the myocardium, mainly at the level of descending and ascending segments, is the one that grants the ventricular chambers the shortening-twisting and lengtheninguntwisting motions which are essential for cardiac function.
Muscle friction
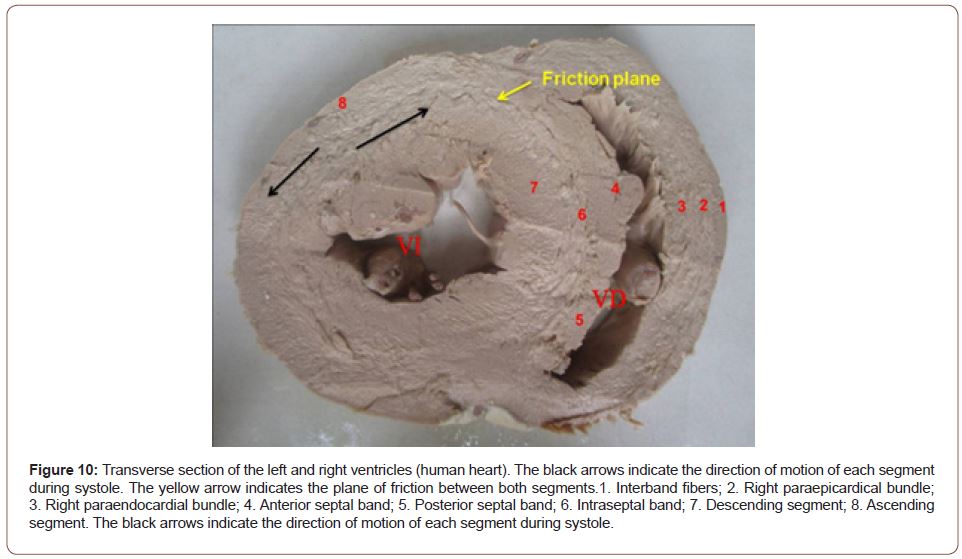
The opposing sliding motion of the left ventricular internal segments in relation to the external segments to achieve the mechanism of ventricular torsion, generates an inevitable friction between them (Figure 10). This would entail a high energy cost if the heart did not have a spongy system, with the participation of Thebesian and Langer venous conduits that act as a lubricating antifriction system: this is the hyaluronic acid, which flows across the myocardial thickness (Figure 11) [13-15].
Figure 10:Transverse section of the left and right ventricles (human heart). The black arrows indicate the direction of motion of each segment during systole. The yellow arrow indicates the plane of friction between both segments.1. Interband fibers; 2. Right paraepicardical bundle; 3. Right paraendocardial bundle; 4. Anterior septal band; 5. Posterior septal band; 6. Intraseptal band; 7. Descending segment; 8. Ascending segment. The black arrows indicate the direction of motion of each segment during systole.
Cardiac electric activation
As electro anatomical mapping corresponded to the left ventricle, the activation wave previously generated in the right ventricle was not obtained. Electro anatomical mapping took an average of 20 minutes. There were no complications related to the procedure itself or any of the approaches. (Figures 12-14) show the projection of the endocardial an epicardial electric activation. In all the Figures the right projection is observed in the left panel and the left anterior oblique projection is simultaneously observed in the right panel. The zones activated at each moment are detailed in red. On the lateral part, the activation of the descending and ascending muscle bands that make up the muscle structure of the continuous myocardium in the rope model is represented. In it, the area depolarized at that moment is represented in red and those that were previously activated and are in the refractory period are represented in blue. Below the rope model, the average electrical propagation time along the muscle band can be seen measured in ms at the analyzed site.
Figure 11:Dilated transverse veins in a condition of muscle relaxation, with hematoxylin-eosin stain technique (25x). The arrows indicate the presence of hyaluronic acid (HA) (human adult heart).
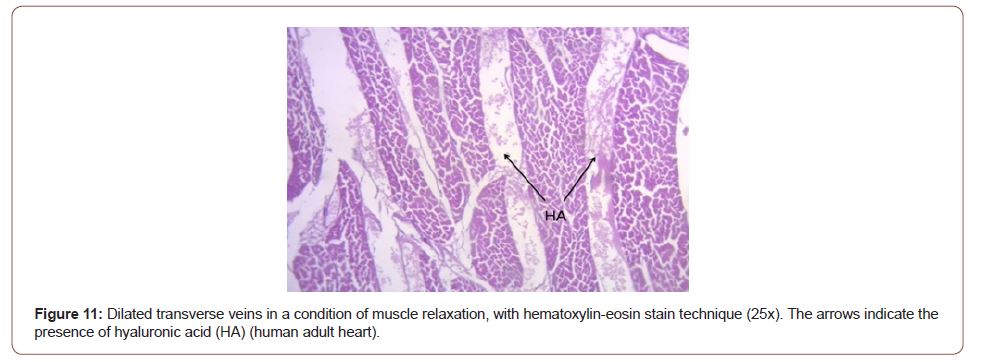
Figure 12:A: Onset of left ventricular activation. The left panel shows the depolarization of the interventricular septum, corresponding to the descending band. In the right panel, the ventricular epicardium (ascending band), has not been activated yet. B: Simultaneous band activation. Activation progresses in the left ventricular septum through the descending band (longitudinal activation) and simultaneously propagates to the epicardium (transverse activation) activating the ascending band. (Human investigation).
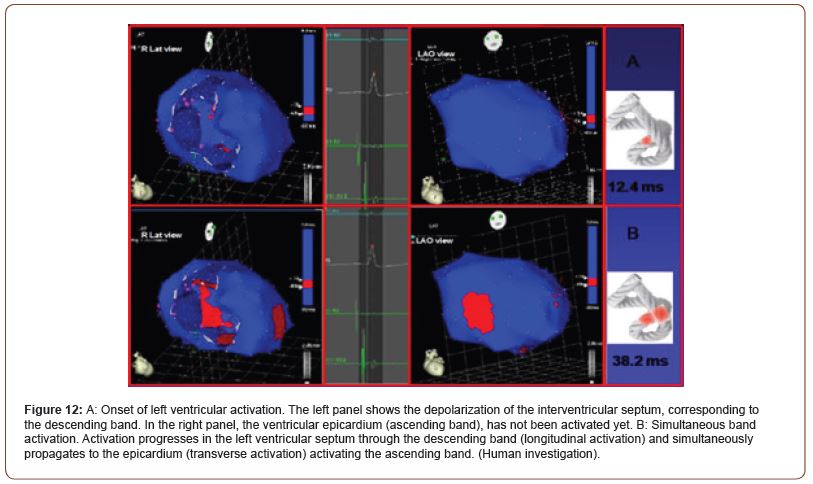
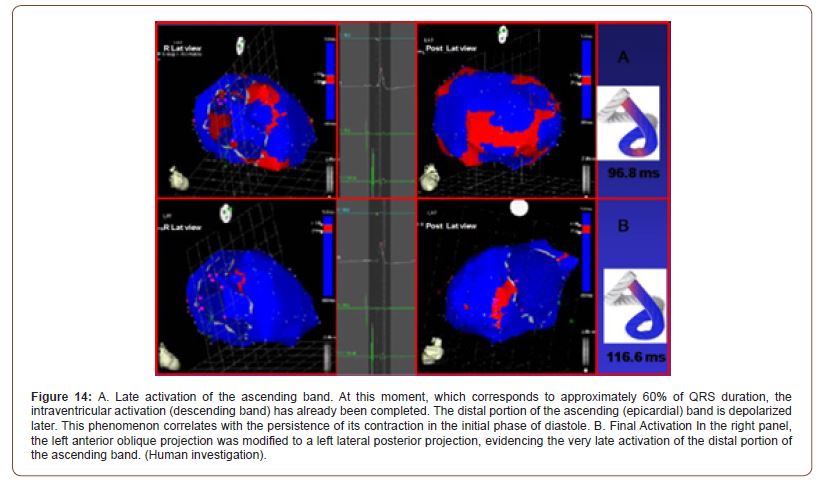
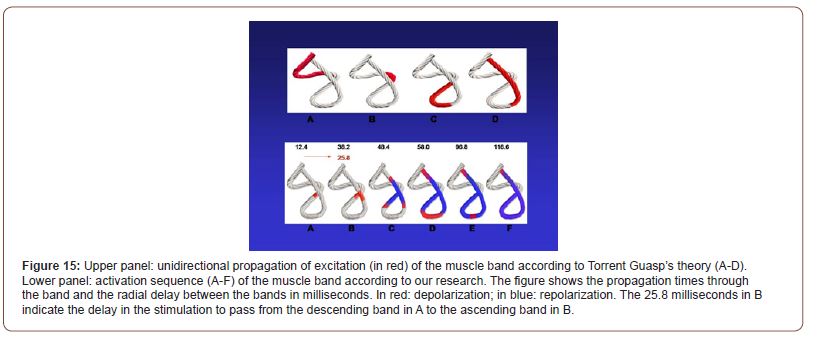
Activation of the left ventricle occurs 12.4 ms ± 1.816 ms after its onset in the interventricular septum (Figure 12A). At that moment it also spreads to an epicardial area - ascending bandsevidencing a radial activation at a point we call “band crossover” that occurs on average 25.8 ms ± 1.483 ms after septal stimulation (Figure 12B) and at 38.2 ms ± 2.135 ms from cardiac activation onset. Synchronously, following the anatomical arrangement of the descending band, the activation moves axially towards the ventricular apex reaching it at an average of 58 ms ± 2.0 ms (Figures 13A & 13B). At the band “crossover” the activation loses its unidirectional character and becomes slightly more complex. Three simultaneous wave fronts are generated: 1) the distal activation of the descending band towards the apical loop; 2) the depolarization of the ascending band from the crossover towards the apex and 3) the activation of this band from the crossover towards the end of the muscle band in the aorta. (Figures 13B, 14A & 14B) show the continuation and completion of this process. Intraventricular activation ends long before the termination of the QRS (Figure 14A). The rest of the QRS corresponds to the late activation of the distal portion of the ascending band, which justifies the persistence of its contraction during the diastolic isovolumic phase, constituting the basis of the ventricular suction mechanism (Figure 14B). A synthesis of the stimulation found in this study is shown with the rope model in (Figure 15, upper panel).
Figure 13:A: Bidirectional apex and the ascending band activation. The final activation of the septum is observed, progressing towards the apex, synchronously with the epicardial activation in the same direction. At the same time the epicardial activation is directed towards the base of the left ventricle. B: Progression of activation. Activation progresses in the same direction of the previous figure. (Human investigation).
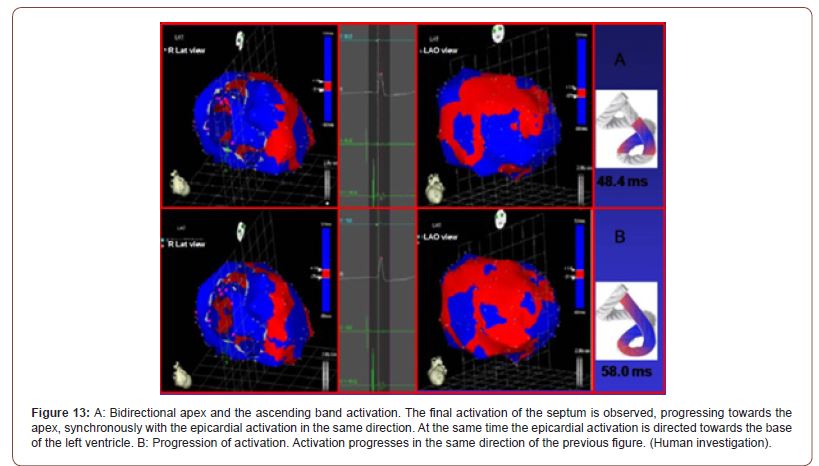
Figure 14:A. Late activation of the ascending band. At this moment, which corresponds to approximately 60% of QRS duration, the intraventricular activation (descending band) has already been completed. The distal portion of the ascending (epicardial) band is depolarized later. This phenomenon correlates with the persistence of its contraction in the initial phase of diastole. B. Final Activation In the right panel, the left anterior oblique projection was modified to a left lateral posterior projection, evidencing the very late activation of the distal portion of the ascending band. (Human investigation).
According to Torrent Guasp, longitudinal diffusion of stimuli along the continuous myocardium explained the performance of the heart (Figure 15, upper panel). However, this sequential “peristaltic” activation does not correlate with some currently well-known fundamental phenomena, as clockwise and counterclockwise twisting at the left ventricular apex and base, which are mainly responsible for its mechanical efficiency (Figure 15, lower panel). Our research modifies these concepts since stimulus propagation is simultaneously axial and radial. A relationship must be found between activation and the mechanical result. The explanation is given by the simultaneous axial and radial electrical conduction pathways when they reach the band crossover and is also supported by the spatial arrangement of the fibers, the subendocardial ones being located on the right side and the subepicardial ones on its left side.
Figure 15:Upper panel: unidirectional propagation of excitation (in red) of the muscle band according to Torrent Guasp’s theory (A-D). Lower panel: activation sequence (A-F) of the muscle band according to our research. The figure shows the propagation times through the band and the radial delay between the bands in milliseconds. In red: depolarization; in blue: repolarization. The 25.8 milliseconds in B indicate the delay in the stimulation to pass from the descending band in A to the ascending band in B.
Stimulus propagation and left ventricular twisting
As noted, left ventricular activation begins in the endocardial
descending band, which is almost simultaneously depolarized
axially and radially. At the crossover point of both bands, the
activation spreads from the endocardium to the epicardium (radial
propagation), that is, from the descending to the ascending band.
From this point onwards, the ascending band depolarizes in two
senses: towards the apex and towards the base, at the same time
that the descending band completes its activation towards the apex
(Figure 14). Thus, two essential phenomena occur:
1) As the apical loop depolarizes from the band crossover
in two simultaneous wave fronts (from the descending and
from the ascending bands) it generates their synchronized
contraction.
2) The activation of the ascending band propagates from the
band crossover in two opposing directions: towards the apex
and towards the base (Figure 14). The resulting mechanical
contraction will also have a divergent direction, giving origin to
the apical and basal clockwise and counterclockwise rotations,
respectively.
The contractile behavior like a auxetic biological material (contracts simultaneously in direction longitudinal and circumferential), together with the helical anatomical arrangement of the myocardial fibers around the ventricular cavity, results in thickening radial myocardial and left ventricular wringing, which is a combination of twisting and reduction of the longitudinal axis. This muscular action results in a squeezing of the left ventricle. Thickening occurs towards the ventricular centroid only and is not accompanied by external thickening towards the pericardium. Furthermore, there is a decrease in cardiac diameter, as would be seen in a M-mode section of the LV in the parasternal long axis plane; in such circumstances, the external cardiac diameter would be smaller in Telesis tole than in tele diastole, despite myocardial thickening. This is due to the helical arrangement of the myocardial fibers, which contribute to the thickening of the myocardium.
Active suction in the diastolic isovolumic phase
A fundamental issue we investigated, which did not have electrophysiological evidence, was to consider ventricular filling as an active phenomenon generated by a myocardial contraction that tends to lengthen the left ventricular apex-base distance after the ejective phase, thus producing a suction effect similar to a “suction cup”. This mechanism is explained by the persistence of the ascending segment contraction during the isovolumic diastolic phase [16-22].
We have found that the endocardium is completely depolarized during the first part of the QRS. If according to our studies the depolarization of the ascending segment starts 25.8 ms on average after that of the descending segment and its contraction persists for the same period of time, the condition of ventricular contraction will last approximately 400 ms. On the other hand, as ventricular systole lasts about 300 ms, the remaining 100 ms correspond to the diastolic isovolumic phase (erroneously called isovolumic relaxation, because as we see there is ventricular contraction). Briefly, during the initial part of this phase the ascending segment remains contracted as a result of the depolarization that occurred during the QRS.
The final part of the QRS corresponds in our investigation to the activation of the ascending segment. In this way, during the diastolic isovolumic phase, the contraction necessary to generate suction (“suction cup effect”) occurs. With the onset of untwisting during the diastolic isovolumic phase the ascending segment progressively lengthens, generating negative intraventricular pressure with this segment still contracted (active process) as an energy residue of the twisting process.
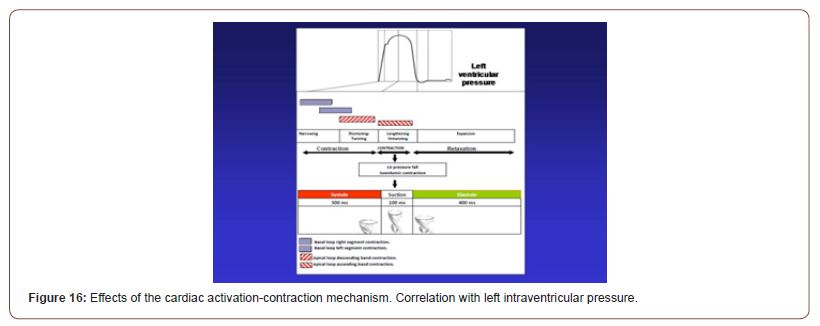
This suction phase between systole and diastole lasts around 100 ms and is active during muscle contraction with an intraventricular pressure drop below zero (Figure 16). The difference in the duration of mean systolic strain between the systolic and postsystolic phase in the echocardiographic work of Mora Llabata, et al. is 88 ± 7.1 ms, a value analogous to those found in our research in relation to the duration of the ascending segment activation in the suction phase [23,24]. We have measured the left intraventricular pressure curve in patients who underwent cardiac resynchronization therapy in order to show the improvement at the level of the suction mechanism in the diastolic isovolumic phase.
Figure 16:Effects of the cardiac activation-contraction mechanism. Correlation with left intraventricular pressure
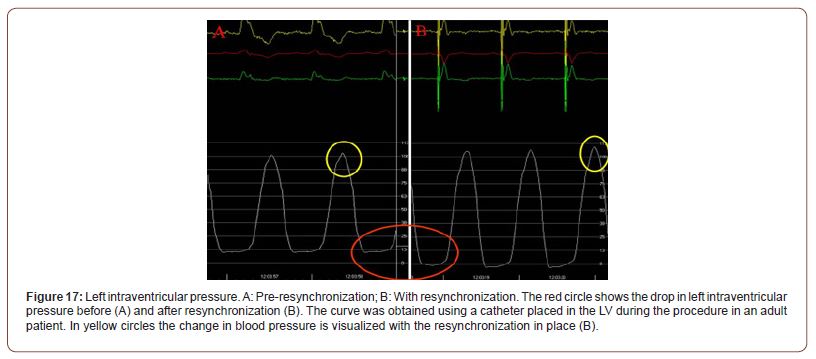
With the resynchronizer catheter positioned in the left ventricular endocardium (according to our research in the area of band “crossover”) normal electrical activation is restored, achieving normalization of the segments’ mechanical sequence and the restoration of the suction mechanism with the drop in left ventricular diastolic pressure [25]. In (Figure 17A), the curve corresponds to the left intraventricular pressure in a patient with left bundle branch block (LBBB) in which the resynchronizer has been turned off. In (Figure 17B), in the same patient, the function of the device has been restored. It can be seen on the intraventricular pressure scale, to the right of each panel (in mmHg), that the diastolic pressure has decreased from 13 to -3 mmHg with ventricular resynchronization. The increase in systolic pressure by 10 mmHg has also been observed.
Figure 17:Left intraventricular pressure. A: Pre-resynchronization; B: With resynchronization. The red circle shows the drop in left intraventricular pressure before (A) and after resynchronization (B). The curve was obtained using a catheter placed in the LV during the procedure in an adult patient. In yellow circles the change in blood pressure is visualized with the resynchronization in place (B).
Echocardiography
Echocardiography has the ability to provide noninvasive understanding about the complex mechanism of myocardial contraction. For over 15 years the speckle tracking technique has been used to assess strain or myocardial deformation. This tool of two-dimensional (2D) ultrasound provides a regional qualitative analysis of left ventricular function, confirming the relationship between the cardiac anatomy of the helical muscle band and diverse echocardiographic findings. According to Ballester, et al. [6] the different segments of the basal and apical loops of Torrent Guasp’s continuous myocardium correlate with 2D ultrasound findings.
The left ventricular eyection fraction (LVEF) results from the combined action of longitudinal and circumferential myocardic contraction. The distinctive helical structure of obliquely oriented myocardial fibers results in systolic rotation of the base and apex of the left ventricle (LV) in opposite directions around its longitudinal axis (the algebraic subtraction of this rotation causes twisting of the heart muscle) and the simultaneous shortening of the LV as the base is displaced towards the apex, all of which results in ventricular wringing. The combination of these events determines ventricular radial thickening and LVEF. Speckle tracking echocardiography allows us to study the muscular behavior of the myocardium by calculating Lagangrian strain. Strain is negative when de original distance between two points decreases, as occurs in myocardial muscle contraction (longitudinal and circunferential contraction) and is positive when the distance between them increases, as occurs in muscle thickening (radial thickening). Its accuracy has been validated with sonomicrometry and especially with nuclear magnetic resonance, respectively.
The anatomical arrangement described by Torrent Guasp and
the efficient myocardial function analyzed by our studies among
others, demonstrate the following consequences [23-24]:
1. The myocardial muscle mass is more important at the
mid-basal level where the basal and apical loop fibers join. This
explains that the maximum strain amplitude obtained by radial
strain occurs at this level (basal radial strain + 44 ± 18% vs.
apical strain + 23 ± 16.5%).
2. The descending fibers adopt a progressively more oblique
arrangement until they reach the apex. Effectively, as expected,
longitudinal strain is markedly greater at the ventricular apex
due to this oblique change in the spatial arrangement (apical
longitudinal strain -21.9% ± 2.4 vs. basal longitudinal strain
-19.6% ± 2.4).
3. Circumferential strain has greater amplitude at the apical
level facilitating apical rotation (-25.6% ± 6.6); therefore, at
the basal level the fibers are arranged more transversally, and
consequently, basal strain results less important (-16.8 ± 3.5).
4. To calculate the twist, the echocardiograph algorithm
performs an algebraic sum (it adds the positive value of apical
rotation to the negative one of basal rotation) In our experience,
the value in normal subjects is around +19 ± 9°, always with
predominant apical rotation
5. Post-systolic longitudinal strain, which indicates the
activity during relaxation, is basically produced in septal
segments and in the anterior basal region corresponding to the
anatomical location of the ascending segment [23].
The twist of an elastic and non-compressible material, such as the myocardium, involves a longitudinal shortening. In the case of the LV, the base and the apex rotate in opposite directions around the longitudinal axis of the LV at the same time as the base moves closer to the apex, which occurs due to the helical arrangement of the myocardial fibers. New parameters, possibly more adjusted to the current knowledge of physiology described, have been incorporated into our recent experience, including twist movements and the longitudinal shortening motions that are simultaneously developed during ventricular systole [24]. These are: a) the torsion index (twist/mitral annulus translational motion); and b) strain index (twist/left ventricular global longitudinal strain). Both parameters translate the active motions that are produced during systole “squeezing” the left ventricle, with a value of 13.1 ± 4°/cm for the torsion index and 0.96 ± 0.36°/% for the strain index in the healthy population studied.
The “combined strain parameter” has also been postulated to assess left ventricular function. It includes the “strain product (twist × longitudinal strain)”, which is probably better to evaluate contractility, and the previously described “strain index (twist/ global longitudinal strain), which would establish the type of predominant disease. Thus, a normal “strain product” (-387 ± 137° x %) would translate into a preserved left ventricular global function, either by normality of both parameters or by the compensation provided between them (“pseudoformal” product). A reduced “strain product” could be due to the decrease of longitudinal strain, twist, or both, and would be characterized by the “strain index” (high, low or normal, respectively). Then, this “combined strain parameter” could help to better differentiate the disorder and in addition monitor the myocardial impairment derived from treatments or secondary to different cardiac diseases.
There are previous reports in the literature which have used the strain product (twist × longitudinal strain) as the best predictor of future cardiotoxicity by anthracyclines, due to a more global assessment of subendocardial and subepicardial fibers. The incorporation of strain parameters have allowed the recent classification of heart failure according to left ventricular mechanical function disorders. After observing specific anomalous models of ventricular mechanics in different subgroups of heart failure patients, an alternative approximation has been proposed to characterize it. Thus, cardiac failure can be classified into two big subgroups: 1) dominant longitudinal dysfunction; 2) transmural dysfunction (longitudinal and circumferential). This classification is based on the double helix oblique orientation of left ventricular myocardial fibers. Probably, the integration of pump and myocardial function parameters need to be jointly valued for a more accurate assessment of ventricular systolic function.
Future studies should assess whether, during longitudinal follow-up of patients, values of myocardial torsion have prognostic value in terms of predicting ventricular failure. On the other hand, in processes that affect the entire myocardium from the early stages onwards, and not just the subendocardium, myocardial torsion could provide extra information if both components are evaluated together. In each different pathology, rotation, twisting and untwisting, are affected in different ways depending on myocardial torsion and the distribution of the most involved fibers, with clearly manifested characteristics in ventricular hypertrophy, diabetes, amyloidosis and heart valve and pericardial diseases. With a special technique known as Diffusion Tensor Magnetic Resonance Imaging with Multiresolution Tractography in a objective analysis, Poveda, et al. [3] in 2013 reveals a continuous helical myocardial fiber arrangement of both right and left ventricles, supporting the anatomical model of the helical ventricular myocardial band described by Torrent-Guasp and confirmed in our disecctions.
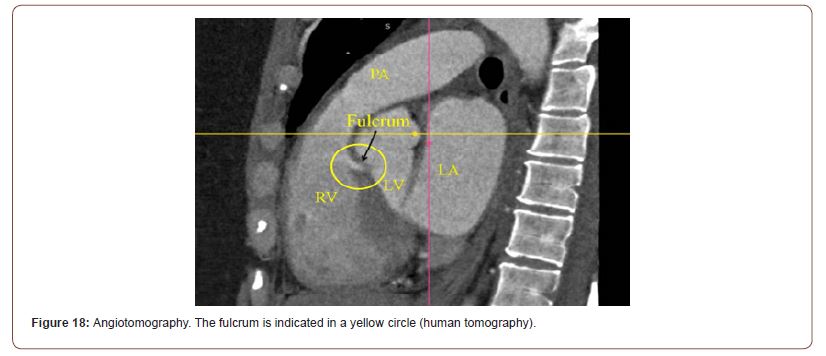
In the multislice coronary angiotomography studies, we have found that the analysis of the region described as cardiac fulcrum in the dissections performed, have an intensity in Hounsfield units above 110 UH, while the adjacent muscle has units below 80 UH. In the patient of (Figure 18), the area described as fulcrum has an average of 132 ± 4.5 HU and the adjacent areas of myocardial muscle between 47.96 ± 12.5 and 77.59 ± 21.64.
Figure 18:Angiotomography. The fulcrum is indicated in a yellow circle (human tomography).
Discussion
Regarding the argued difficulty to dissect the myocardium, which is more apparent than real, we should consider that once the myocardial band originated as a loop in the arterial semicircle of amphibians and reptiles to adapt to the physiological demands of aerial life, the muscle bundles became firmly attached to their contact surfaces, hampering the necessary cleavage planes necessary for their anatomical dissection. The evolutionary goal was to develop a sufficiently solid hemodynamic structure with the strength to generate the suction and pumping of the blood volume that supplied the whole organism. Thus, every attempt to dissect an anatomical segment from the rest of the myocardium, avoiding the real cardiac arrangement, always turns into an obstacle due to the structural plan of the axes where the orientation of the myocardial band courses. Its spatial helical arrangement is in agreement with the mechanical function evidenced by the different segments that compose it. No segment of the continuous myocardium sequential histology, explored in our investigations, presents a mesh arrangement.
The myocardial band cannot be anatomically suspended and free in the thoracic cavity because it would be impossible to eject blood at a speed of 200 cm/s. Therefore, there must be a point of attachment, which was identified as the cardiac fulcrum (supporting point of leverage). In this supporting site, the muscle fibers are inevitably forced to “intertwine” with the connective, chondroid or osseous fulcrum, and our anatomical and histological investigations have shown that this insertion attaches both the origin and end of the myocardial band [18, 26].
This structural composition keeps correspondence with the activation of the myocardial band. The stimulus runs by its muscle pathways, but in order to fulfill the function proposed by its helical arrangement, it is essential for it to simultaneously activate the left ventricular descending and ascending segments. The transmission of the stimulus between them generates the necessary ventricular torsion (a situation similar to “wringing a towel”) that enables the ejection of the blood content in a limited time span with the necessary force to adequately supply the whole body. The circulatory duct of annelids works with a peristaltic mechanism in its contractile progression. The impulse along its length preserves a pattern of axial transmission, but after the cardiac duct twists in birds and mammals, radial transmission of the impulse is added, allowing the helical motion indispensable to produce the successive twisting-shortening motions during systole and untwistinglengthening in the subsequent suction phase.
The ventricular narrowing phase at the beginning of systole is shaped by the contraction of the basal loop right and left segments. The overlapping shortening phase due to the descent of the base while twisting occurs, is produced longitudinally, as the ring contracts before the apex. The fact that the apex remains fixed is due to the movement of the base, descending in systole and ascending in diastole. This is better explained because the ascending band, rigid at the beginning of diastole, acts as a tight tutor keeping the apex immobile. The pressure generated to eject the highest amount of blood at the onset of ejection during an interval lasting 20% of the systolic phase is feasible due to the twisting motion. This action is achieved because the electrical stimulation propagates towards the descending band (axial propagation) and simultaneously to the ascending band (radial propagation). Although the electrical conduction progresses along the myocardial band, radial propagation towards the ascending band plays an essential role in ventricular twisting by allowing opposing forces on its longitudinal axis to generate the necessary intraventricular pressure to achieve abrupt blood ejection. Thus, a twisting mechanism similar to “wringing a towel” would be produced.
The following suction phase of the heart is not feasible due to the small difference with peripheral pressure. Neither can it be passive. The untwisting of the heart in the first 100 ms of diastole (isovolumic diastolic phase) generates the negative intraventricular force to draw blood into the left ventricle, even in the absence of the right ventricle, as shown in experimental animals [22,27]. This suction phase (“suction cup” mechanism) is active with energy expenditure, and implies that the heart cycle consists of three stages: systole, suction and diastole.
Diastolic activation explains the insertion of a suction active coupling phase between systole and diastole, with muscle contraction, energy consumption, aexpenditure and of intraventricular pressure. This effect draws blood into the ventricular chamber through a pressure difference with respect to the external pressure and is responsible that in only 20% of the total filling time it should achieve 70% of the total filling volume. The new echocardiographic proposed parameters integrate values of longitudinal shortening and twisting and provided reference strain values obtained in a population of healthy subjects allowing a complete physiological assessment of cardiac systolic function and could be used for the early detection and characterization of its alteration. There is a consensus that future clinical practice should adopt a combined approach in which changes in LV rotational mechanics and longitudinal shortening are considered and interpreted together. Likewise, the integration of complementary parameters of pumping and myocardial function should be considered for a more accurate evaluation of LV systolic function.
Limitations
All this research is necessary to replicate with a greater number of hearts and patients.
Conclusion
1. We consider there is enough evidence that the fiber
orientation and the opopposed base apex rotacional movement
of the heart justiy the helical myocardium model
2. The finding of the fulcrum gives support to the spiral
myocardial band being the point of fixation that allows the
helical torsion.
3. The hyaluronic acid would act as a lubricant and provide
great resistance to mechanical pressures in a functional
association with the venous ducts of Thebesius and Langer,
that would intervene as tributaries of the necessary hydration
to said element and thus could counteract the friction of the
surfaces by exercising an anti-friction mechanism.
4. This study explains the ventricular twist and the active
suction mechanism during the isovolumic diastolic and early
ventricular filling phases, in contrast with the traditional
concept of passive relaxation during the diastolic isovolumic
phase and the vis a tergo as mechanism for ventricular filling.
5. The deep anatomical, histológical, electrofisiological and
functional invetigations that our group have carried out at the
level of the differents segments of the continuous myocardium
allows a better interpretation of cardiac physiology and its
clinical application is unquestionable in light of new cardiac
images teccniques.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Trainini JC, Lowenstein J, Beraudo M, Mora Llabata V, Carreras-Costa F, et al. (2022) Fulcrum and Torsion of the Helical Myocardium. Ed Biblos, Buenos Aires; Argentina pp. 27-105.
- TorrentGuasp F (1998) Estructura y función del corazó Rev Esp Cardiol 51: 91-102.
- Poveda F, Gil D, Martí E, Andaluz A, Ballester M, et al. (2013) Estudio tractográfico de la anatomía helicoidal del miocardio ventricular mediante resonancia magnética por tensor de difusió Rev Esp Cardiol 66: 782-790.
- Carreras F, Ballester M, Pujadas S, Leta R, Pons-Lladó G (2006) Morphological and functional evidence of the helical heart from non-invasive cardiac imaging. Eur J Cardiothoracic Surg 29(Suppl 1): S50-5.
- Trainini JC, Elencwajg B, López-Cabanillas N, Herreros J, Lago N (2015) Electrophysiological Bases of Torsión and Suction in the Continuous Cardiac Band Model. Anat Physiol 5: S4-001.
- Ballester M, Ferreira A, Carreras F (2008) The myocardial band. Heart Fail Clin 4(3): 261-272.
- Trainini JC, Elencwajg B, López Cabanillas N, Herreros J, Lago N, et al. (2015) Stimuli propagation, muscle torsión and cardiac suction effect through electrophysiological research. In “Basis of the New Cardiac Mechanics. The Suction Pump”, Trainini JC and col. Lumen, Buenos Aires pp. 39-61.
- Torrent Guasp F, Buckberg G, Carmine C, Cox J, Coghlan H, et al. (2001) The structure and function of the helical heart and its buttress wrapping. I. The normal macroscopic structure of the heart. Seminars in Thorac and Cardiovasc Surg 13: 301-319.
- Trainini JC, Elencwajg B, López-Cabanillas N, Herreros J, Lowenstein J, et al. (2017) Ventricular torsion and cardiac suction effect: The electrophysiological analysis of the cardiac band muscle. Interventional Cardiol 9(1): 45-51.
- Biesiadecki B, Davis J, Ziolo M, Janssen P (2014) Tri-modal regulation of cardiac muscle relaxation; intracellular calcium decline, thin filament deactivation, and cross-bridge cycling kinetics. Biophys. Rev 6: 273-289.
- Trainini JC, Beraudo M, Mora Llabata V, Wernicke M, Lowenstein J, et al. (2020) Nuevos descubrimientos que apoyan el complejo mecanismo de la torsión miocá RETIC 3: 14-18.
- Anderson R, Ho S, Redman K, Sanchez-Quintana D, Punkenheimer P (2005) The anatomical arrangement of the myocardial cells making up the ventricular mass. Eur J Cardiothoracic Surg 28: 517-525.
- Bignami A, Asher R (1992) Some observations on the localization of hyaluronic acid in adult, newborn and embryonal rat brain. Int J Dev.Neurosci 10(I): 45-57.
- Hong-yan Ding, Ya-nan Xie, Qiang Dong, Koji Kimata, Yoshihiro Nishida, et al. (2019) Roles of hyaluronan in cardiovascular and nervous system disorders. J Zhejiang Univ-Sci B (Biomed&Biotechnol) 20(5): 428-436.
- Lorén C, Dahl C, Do L, Almaas V, Geiran O, et al. (2019) Low molecular mass myocardial hyaluronan in human hypertrophic cardiomyopathy. Cells 8(2): 97.
- Cosín-Aguilar J, Hernándiz Martínez A, TuzónSegarra MT, Agüero Ramón-Llin J, TorrentGuasp F (2009) Estudio experimental de la llamada fase de relajación isovolumétrica del ventrículo izquierdo. Rev Esp Cardiol 62: 392-399.
- Buckberg GD, Coghlan HC, Torrent Guasp F (2001) The structure and function of the helical heart and its buttress wrapping. V. Anatomic and physiologic considerations in the healthy and failing heart. SeminThorac Cardiovasc Surg 132: 358-385.
- Trainini JC, Lowenstein J, Beraudo M, Wernicke M, Trainini A, et al. (2021) Myocardial torsion and cardiac fulcrum (Torsion myocardique et pivot cardiaque). Morphologie 105 :15-23.
- Arvidsson M, Töger J, Carlsson M, Steding-Ehrenborg K, Pedrizzetti G, et al. (2017) Left and right ventricular hemodynamic forces in healthy volunteers and elite athletes assessed with 4D flow magnetic resonance imaging. Am J Physiol Heart Circ Physiol 312: H314-H328.
- Pedrizzetti G, Arvidsson PM, Töger J, Borgquist R, Domenichini F, et al. (2017) On estimating intraventricular hemodymamic forces from endocardial dynamics: A comparative study with 4D flow MRI. Journal of Biomechanics 60: 203-210.
- Maksuti E, Carlsson M, Arheden H, Kovács S, Broomé M, et al. (2017) Hydraulic forces contribute to left ventricular diastolic filling. Scientif reports 7: 43505.
- Trainini JC, Trainini A, Valle Cabezas J, Cabo J (2019) Left Ventricular Suction in Right Ventricular Dysfunction. EC Cardiology 6(6): 572-577.
- Mora V, Roldán I Romero E, Saurí A, Romero D, Perez-Gozabo J, et al. (2018) Myocardial contraction during the diastolic isovolumetric period: analysis of longitudinal strain by means of speckle tracking echocardiography. J Cardiovasc Dev Dis 5: 4.
- Mora V, Roldán I, Romero E, Romero D, Bertolín J, et al. (2018) Comprehensive assessment of left ventricular myocardial function by two-dimensional speckle-tracking echocardiography, Cardiovascular Ultrasound 16: 16.
- Elencwajg B, López-Cabanillas N, Cardinali EL, Barisani JL, Trainini J, et al. (2012) The Jurdham procedure endocardial left ventricular lead insertion via a femoral transseptal sheath for cardiac resynchronization therapy pectoral device implantation. Heart Rhythm 9: 1798-1804.
- Moittié S, Baiker K, Strong V, Cousins E, White K, et al. (2020) Discovery of cordis in the cardiac skeleton of chimpanzees (Pan troglodytes). Sci Rep 10: 9417.
- Trainini J, Valle Cabezas J, Carreras-Costa F, Beraudo M, Wernicke M, et al. (2022) Cardiac Energy. Clin Exp Invest 3(1): 7-7.
-
Trainini Jorge, Lowenstein Jorge, Beraudo Mario, Trainini Alejandro, Mora Llabata Vicente, Carreras-Costa Francesc, Valle Cabezas Jesús, Wernicke Mario, Elencwajg Benjamín, Lowenstein-Haber Diego and Bastarrica María Elena. Cardiac Helical Function. Fulcrum and Torsion. On J Cardio Res & Rep. 7(1): 2023. OJCRR.MS.ID.000653.
-
Cardiac anatomy, Ventricular band, Fulcrum, Friction, Hyaluronic Acid, Heart, Physiology, Cardiac Electrophysiology, Cardiology, Pulmonary artery, Myocardium, Cardiac dissection, Epicardial plane, Cardiac fulcrum
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






