 Mini review
Mini review
Mitochondrial Uncoupling Proteins: A Potential Target for Disease-Modulation in Cardiovascular Disorders
Zakaria A Almsherqi, Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
Received Date: May 25, 2019; Published Date: May 28, 2019
Abstract
Mitochondria provide cellular energy through oxidative phosphorylation, but as an integral part of this process, superoxides and other reactive oxygen species (ROS) are also produced. When the balance between the production of free radicals and antioxidant capacity of the cardiac cells altered, oxidative stress is induced. Oxidative stress has been linked to the development of ischemic heart disease, atherosclerosis, congestive heart failure, ischemic-reperfusion injury, and vascular endothelial dysfunction. Although several clinical trials over the past decades employed different strategies of antioxidants supplementation, the results were generally negative in the setting of chronic preventative therapy. Less attention has been paid to the modulation of ROS production, despite the fact that prevention, rather than cure, would appear to be the logic approach to attenuate the oxidative damage. There is increasing evidence that endogenous myocardial uncoupling proteins (UCPs) can play a vital role in regulating ROS generation to protect against various pathogenic stresses. Their expression, which can be induced, may well be a potential therapeutic target for various drugs to alleviate the harmful effects of pathogenic processes and hence modify the progression of cardiovascular diseases (CVDs).
fuel excitation-contraction coupling. More than 90% of cardiac cells energy is produced in the mitochondria from oxidative phosphorylation activity [1]. Oxidative phosphorylation is the process by which energy from fuel oxidation is converted to the high-energy phosphate bonds of adenosine triphosphate (ATP). The chemiosmotic hypothesis suggests that the ATP synthesis is provided by the electrochemical gradient across the inner mitochondrial membrane. This electrochemical gradient is maintained by constituents of the electron transport chain (ETC), which acts to pump protons from the mitochondrial matrix to the intermembrane space of the mitochondria as they accept and donate electrons in a prescribed manner Figure. This process of electron transport and oxidative metabolism in cardiac cells are accompanied by the reduction of oxygen to superoxide and other ROS which considered as a by-product of mitochondrial respiration.
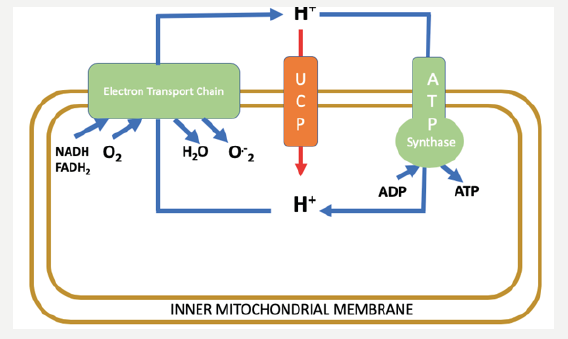
Figure 1: The chemiosmotic proton cycle across the inner mitochondrial membrane. The coupling cycle, consisting of substrate oxidation (NADH, FADH2) and the enzymes of ATP production (ATP synthase), results in coupled oxidative phosphorylation and superoxide (O-2) generation as a by-product. The uncoupling cycle, consisting of substrate oxidation and proton conductance pathway through the uncoupling proteins (UCPs), results in uncoupled respiration and low levels of superoxide generated.
-
Zakaria A Almsherqi. Mitochondrial Uncoupling Proteins: A Potential Target for Disease-Modulation in Cardiovascular Disorders. On J Cardio Res & Rep. 2(1): 2019. OJCRR.MS.ID.000527.





