 Research Article
Research Article
Probiotics as Biocontrol Agents for Safe Approach Against Mycotoxins Contamination
Patricia Daliu*
Department of Pharmacy, Faculty of Medical Science, Tirane, Albania
Patricia Daliu, Department of Pharmacy, Faculty of Medical Science, Tirane, Albania
Received Date: March 11, 2024; Published Date: May 06, 2024
Introduction
Nowadays the continuous growing interest of consumers in natural and safe products and the problems associated with the use of chemical pesticides and food preservatives make it necessary to search for suitable protective alternatives. The International standards and the European legislation well describe the so-called known mycotoxins such as aflatoxins (AF), ochratoxin A (OTA), and trichothecenes, present in a variety of crops. However, information about mycotoxins in fresh fruit and vegetables is limited. Thus, to mitigate mycotoxin contamination, control approaches are used. One of the safe methods proposed is Bio-preservation, defined as the use of microorganisms, as well as their metabolic products, to prevent fungal growth and improve the food shelf life [1]. Lactic acid bacteria (LAB) are microorganisms commonly used in numerous industrial fermentation processes [2] and also represent a promising strategy against mycotoxins, which are resistant to washing process and temperature, to prevent spoilage of fruits and vegetables [3,4]. The presence of mycotoxin contamination in food has adverse human health effects and nowadays has become of global interest. To explore the possibility to use lactic bacteria as a biocontrol agent against secondary metabolites from micro fungi contaminating crops, tomatoes have been chosen as a case study.
Tomatoes are rich in bioactive compounds and are highly susceptible to fungal contamination due to their soft epidermis. The industrial processing of tomatoes, such as heating, pasteurization, sterilization, and storage has no significant effect in reducing these mycotoxins in the final products. It has been observed that the exposure to these toxins may damage β cells triggering the progression to diabetes onset [5]. Moreover, some studies highlighted a bi-directional relationship existing between mycotoxins and gut microbiota. The gut microbiota plays a crucial role in diabetes. Recently, has been reported how the diabetogenic effects of Ochratoxin A (OTA), the most toxic product of Aspergillus ochraceous and Penicillium verrucosum, not only alter blood glucose and insulin levels in rats, but also leads to the increases in blood glucagon levels and causes pancreatic lesions [6].
For this purpose, considering the microbiological quality assessment, my research has been conducted to evaluate the efficacy of natural protective alternatives by using ten different strains of Lactobacillus spp. And Bacillus spp.as biocontrol agents against the mycotoxigenic fungi and identify-quantify the secondary metabolites produced from these strains, potentially responsible for the antifungal activity, such as organic acids, phenolic acids, and volatile organic compounds (VOCs). This step has been developed in order to check both quality and safety of a natural source like tomatoes, as a preliminary and important key step before the nutraceutical or functional food formulation.
Material and Method
Reagents
The standards of phenolic compounds: gallic acid, chlorogenic acid, caffeic acid, syringic acid, vanillin, p-coumaric, hydroxybenzoic acid, vanillic acid, hydroxycinnamic acid, sinapic acid, benzoic acid, DL-3-phenyllactic acid,1,2-dihydroxybenzene,3,4- dihydroxyhydrocinnamic acid and DL-p-hydroxyphenyl lactic acid were obtained from Sigma-Aldrich (Dublin, Ireland). Phenyllactic acid (PLA) was obtained from BaChem (Weil am Rhein, Germany). Ferulic acid was purchased from MP Biomedicals, and protocatechuic acid from HWI Pharma Services (Ruelzheim, Germany). All analytes had a purity of 95 %. Liquid chromatography grade solvents, including acetonitrile (I), methanol, ethyl acetate and formic acid (99%) were obtained from VWR Chemicals (Radnor, USA). Magnesium sulphate (MgSO4), C18, ammonium format and sodium chloride (NaCl) were obtained from Sigma-Aldrich. Potato dextrose broth (PDB), potato dextrose agar (PDA), de Man-Rogosa- Sharpe (MRS) broth, and MRS agar, Glucose, Malt extract broth, Yeast extract, Triptone soy broth, were obtained from Liofilchem (Teramo, Italy). Deionised water (<18 MW cm–1) was obtained from a Milli-Q purification system (Millipore Corp., Bedford, MA, USA).
Fungi and bacteria isolation
Penicillium verrucosumVTT D-01847 was obtained from the VTT Culture Collection (Finland), Botrytis cinera (CECT 20973) was obtained from the Spanish Type Culture Collection (Spain), Aspergillus flavus ITEM 8111, Fusarium strains (Fusarium proliferatum ITEM 12072), Fusarium graminearumITEM 126, F. verticillioides ITEM 12044, F. sporotrichioidesITEM 12168, F. langsethiaeITEM 11031, F. poaeITEM 9151), Alternariaalternata(ITEM 8122) were obtained from the Agro- Food Microbial Culture Collection (Italy).
Microorganism culture preparation
Based on their specific growth medium, the studied bacteria as biocontrol agents were divided into two groups and given a code as shown in Table 1.
Table 1: Antifungal activity of isolated bacteria against Penicillium, Aspergillus, Fusarium and Alternaria species by overlay assay. Values are expressed as % of inhibition after 7 days ± standard deviation. ND- Not detected.
Medium ingredients
De Man, Rogosa and Sharpe (MRS broth): (meat peptone 10.0 gL−1; dextrose 20.0 gL−1; yeast extract 5.0 gL−1; beef extract 10.0 gL−1; disodium phosphate 2.0 gL−1; sodium acetate 5.0 gL−1; ammonium citrate 2.0 gL−1; magnesium sulfate 0.1 gL−1; manganese sulphate 0.05 gL−1; Tween 80 1.0 gL−1). Preparation notes: 26g have been solubilized in 1 L of distilled water.
Nutrient broth (NB): meat peptone 10.0 gL−1; beef extract 10.0 gL−1; sodium chloride 5.0 gL−1; Preparation notes: 28g have been solubilized in 1 L of distilled water.
Growth of strains for preparation of cell-free supernatant
The bacteria were cultivated in corresponding medium (MRS or Nutrient Broth) at 25-37 °C until the exponential phase growth (12 h). Then, the strains were inoculated at a concentration of 107 CFU mL-1 in 200 mL of each medium and incubated at 37 °C for 72 h. After fermentation, LAB were separated by centrifugation at 3200 g for 10 min. Cell-free supernatants (CFS) were stored at –80 °C for 24 h before ebulizeron (Free Zone 2.5 L, Labconco, Kansas City, MO, USA) and then stored at –19 °C.
Qualitative assay in vitro of antifungal activity in solid medium
Diffusion agar test
The effect of the bacteria on different fungal strains growth was evaluated using two qualitative methods. On one hand, the diffusion agar method was used to study the effect of fermented CFS against fungi. Potatoes dextrose agar (PDA) plates were inoculated with fungal spores using sterile cotton swabs. Then, the wells were made using sterile pipette tips, and each well was loaded with 100 mL of lyophilized CFS and suspended in Milli-Q water to a concentration of 250 mg mL-1. A well with lyophilized of medium was included as the negative control. Afterward, the plates were incubated at 25 °C for 48 h. Finally, the inhibition halo diameter was measured. Halos larger than 8 mm were considered positive for antifungal activity [7].
Dual Culture
The selection of biological control agents usually starts with an in vitro screening of a collection of strains against selected pathogens by a dual culture assay. Ten micro fungi strains were assigned a code as listed below and evaluated Figure 1.
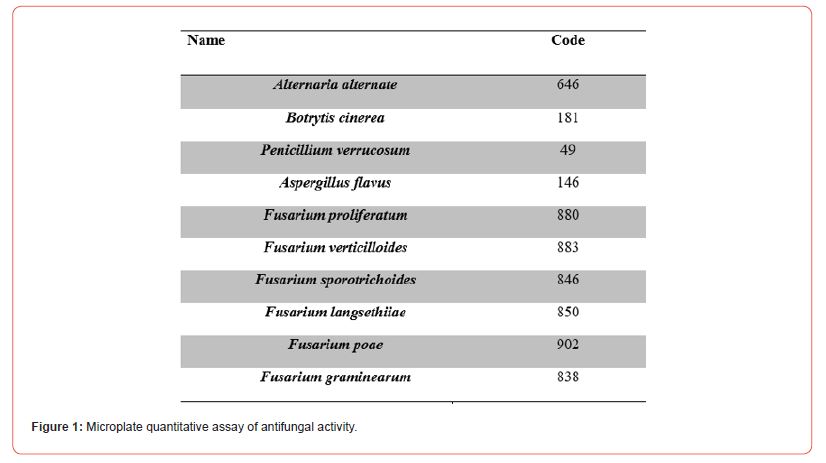
Microplate quantitative assay of antifungal activity
Minimum inhibitory concentration (MIC)/minimum fungicidal concentration (MFC)
The assays were performed as described by [8] with some modifications. A volume of 100 μL of fermented CFS at a final concentration ranging from 0.1 to 100 gL-1 was added to 96-well sterile microplates. Next, the microwells were inoculated with 100 μL taken from a 5×104mL-1spores suspension in PDB of the toxigenic fungi. The positive control consisted of inoculated PDB medium with non-fermented CFS (100 gL-1), and the negative control was a non-inoculated PDB medium without any treatment. Inoculated microplates were incubated at 25 °C for 72 h. The minimum inhibitory concentration is defined as the lowest concentration of the fermented CFS at which the fungi did not show any visible growth. Four replicates of each assay were done.
After determining the MIC, the concentration corresponding to the MIC and higher concentrations were sub-cultured on PDA plates for the determination of the minimum fungicidal concentration. After incubation of the plates at 25 °C for 72 h, the MFC results were defined as the lowest extract concentration in which a visible growth of the subculture was prevented. The in vitro antifungal activity data were used to select LAB/Bacillus with a higher relative antifungal activity.
Identification of organic and phenolic acids in CFS
For the analysis of organic acids, ebulizer CFS was diluted in water and injected into the high-performance liquid chromatography (HPLC) system (Agilent 1100 Series HPLC System, Agilent Technologies, Palo Alto, CA, USA), equipped with a quaternary pump and a diode array detector, using a 20 μL sample injection loop [9]. The analytical separation was achieved with a Spherisorb S5 ODS2 (4.6 mm × 250 mm, 5 μm) reversephase column (Waters Corp., Milford, MA, USA) using an isocratic mobile phase of acidified water (pH 2.1) at a flow rate of 0.6 mL min-1 for 25 min. The chromatogram was monitored at 210 nm. Data were acquired by the HP-CORE ChemStation system (Agilent Technologies, Santa Clara, CA, USA).
For the identification of the phenolic acids, the CFS was purified using the QuEChERS method to remove possible interferents before the chromatographic analysis (Brosnan, Coffey, Arendt, &Furey, 2014). Ten mL of fermented CFS were extracted with 10 mL of ethyl acetate, 1 % formic acid, 4 g MgSO4 and 1 g NaCl, then vortexed for 1 min. The extract was then centrifuged. The supernatant was combined with 150 mg C18resin and 900 mg MgSO4 and vortexed for 1 min. The extract was centrifuged again, and the supernatant was evaporated under Nitrogen flow. Immediately before the chromatographic analysis, the purified extract was resuspended in 1 mL of a mixture H2O/I (90:10 v/v).
The HPLC system used for the chromatographic determination was an Agilent 1200 (Agilent Technologies, Santa Clara) equipped with a vacuum degasser, autosampler and binary pump. The column was a Gemini C18 (50 mm × 2 mm, 100 Å, 3-μm particle size; Phenomenex). The mobile phases consisted of water as solvent A, I as solvent B, both acidified (0.1% formic acid), with gradient elution, as follows: 0 min, 5% B; 30 min 95% B; 35 min, 5% B. The column was equilibrated for 3 min before every analysis. The flow rate was 0.3 mL min-1, and 20 μL of sample was injected. Mass spectrometry (MS) analysis was conducted using a Q-TOF-MS (6540 Agilent Ultra High Definition Accurate Mass), equipped with an Agilent Dual Jet Stream electrospray ebulizer (Dual AJS ESI) interface in negative ebulizer mode under the following conditions: drying gas flow (N2), 8.0 L min-1; ebulizer pressure, 30 psig; gas drying temperature, 350°C; capillary voltage, 3.5 kV; fragment or voltage, 175 V; scan range, m/z 20-380. Targeted MS/MS experiments were carried out using collision energy values of 10, 20 and 40 eV.
Analysis of VOCs of CFS
Lyophilized CFS (200 mg) was mixed with 2 mL of water and placed in a 10-mL glass vial. VOCs were identified by gas chromatography with a single quadrupole mass spectrometer detector (GC/MS) analysis. Prior to analysis, samples were incubated in a water bath at 55°C for 45 min, while being gently stirred with a rod. VOCs were extracted from the vial headspace by solid phase microextraction (SPME). The GC system was equipped with an HP-5MS (30m × 0.25 mm, 0.25 μm 5% diphenyl/95% dimethylpolysiloxane) capillary column (J&W Scientific, Folsom, CA, USA). The oven was programmed to start at 40 °C (held for 2 min) and to ramp up to 160 °C at 6 °C min-1, then increase to 260 °C at 10 °C min-1 (held for 4 min). Helium (99.999%) was used as the carrier gas, and the flow rate was 1 mL min -1. The flow was transferred from the column into an Agilent 5973 MS detector (Agilent Technologies, Palo Alto). The ion source temperature was set at 230°C, the ionizing electron energy was 70 eV, and the mass range was 40-450 Da in full scan acquisition mode. Compounds were identified using the NIST Atomic Spectra Database version 1.6 (Gaithersburg, MD, USA), considering spectra with 95% of similarity. Results were expressed as a percentage of the VOC by dividing the area of each peak by the total area of the chromatogram peaks [10]. The analysis was carried out in triplicate.
Lactobacillus plant arum PP155 and Lactobacillus plantarum 850 as a tomato bio-preservative
To evaluate the antifungal activity using CFS fermented by L. Plantarum PP 155 and L. Plantarum 850, tomatoes were divided into three groups for 10 days, the first group served as a control, the tomatoes were not sprayed, 10 mL of CFS at a final concentration of 10.5 g lyophilized CFS Kg-1 of L. Plantarum PP 155 and L. Plantarum 850, were applied respectively in the second and third group. All experiments were repeated three times.
Statistical analysis
Data were statistically evaluated using the InfoStatsoftware version 2010. The differences between the groups were analyzed by one-way ANOVA. The significance level was set at p ≤ 0.01.
Introduction
Dual culture
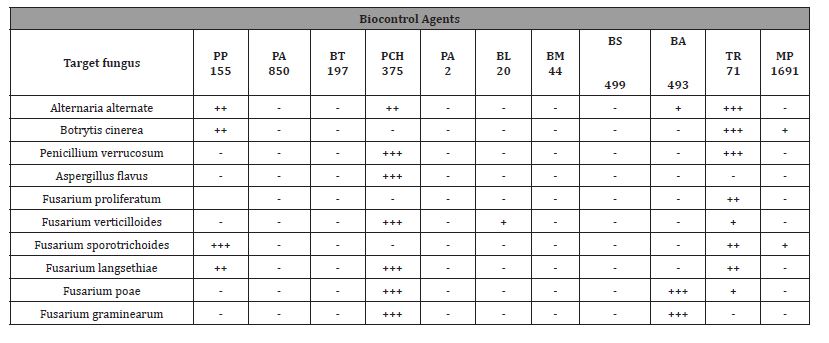
Several CFS of LAB and Bacillus showed antifungal activity against toxigenic fungi by the overlay method and in the solid medium diffusion agar test. In particular, the analysis of the activity and the percentage of mould inhibition range from 16.2% ±0.1 to 50.4% ± 0.1 as shown in Table 1. This result is most evident against the mycotoxigenic fungi belonging to Fusariumspp. (F. sporotrichoide, F. graminearum and F. verticillioides) and Botrytis cinerea, while a slight activity was observed for Aspergillus and Penicillium.
The Inhibition percentage has been calculated using the formula:
(Dmcrl-Dmstrain)/Dmcrl- 0.5 = % inhibition
Where Dm crl is the Diameter of control (cm), Dm strain is the- Diameter of strain (cm), 0.5 is the Diameter of fungi spot in the culture plate on the first day Figure 2.
Agar diffusion test
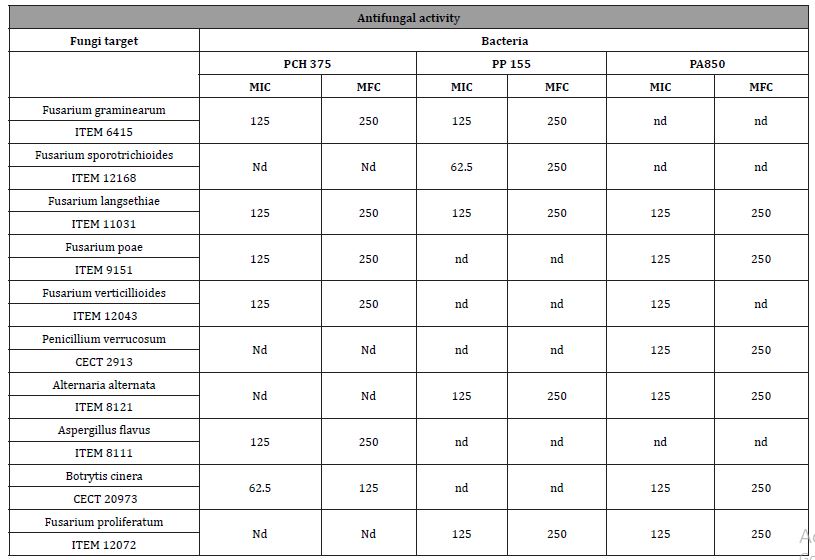
Among the ten strains evaluated, the highest antifungal activity was observed in cell-free supernatants (CFS) of the Lactobacillus plantarum 850 and Lactobacillus brevis PCH 375, which inhibited the growth of tested fungi genera in solid medium as shown in Table 2 and Figure 3, respectively. Only halos larger than 8 mm have been considered as positive result.
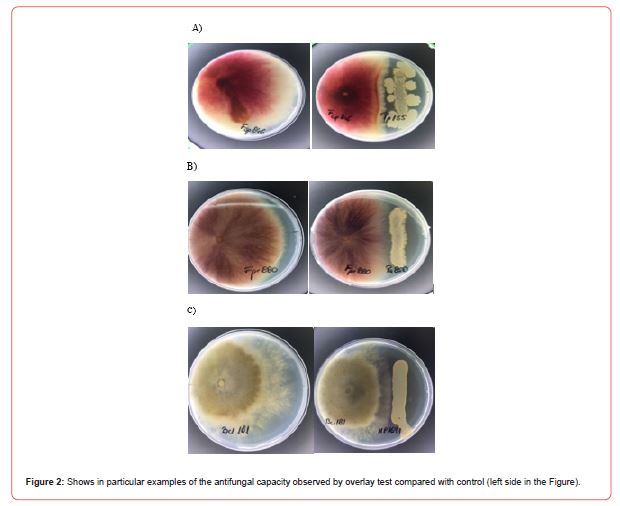
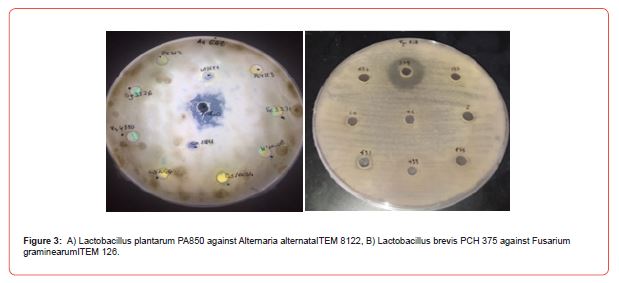
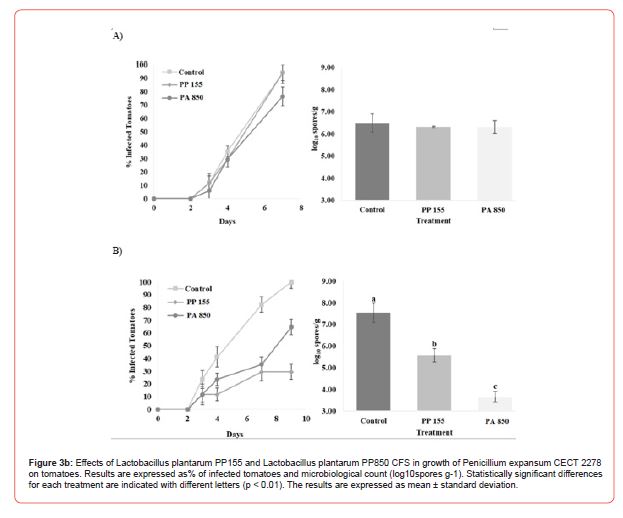
Table 2: Antifungal activity of CFS against Penicillium, Aspergillus, Fusarium Botrytis and Alternaria species by diffusion agar method. –non detected activity; + less activity; ++ middle activity; +++ strong activity.
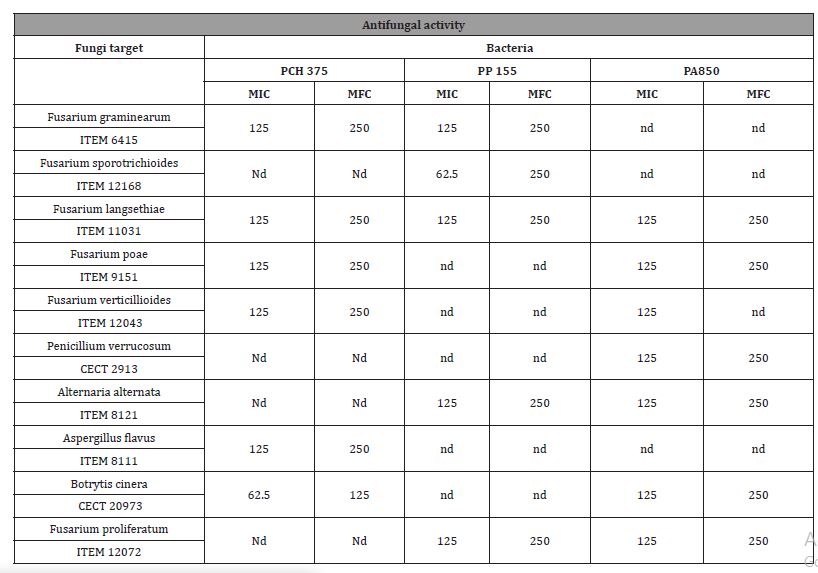
MIC (Minimal Inhibitor Concentration)/ MFC (Minimal Fungicide Concentration)
In order to quantify the potential antifungal activity of CFS, the MIC and MFC values were determined, and the results are shown in Table 3. The aim was to find stains effective in low levels of MIC and MFC. The MIC and MFC values of CFS on Fusarium spp., Penicillium spp, Alternariaspp, Aspergillussspp, were in the range 62.5-250 gL- 1. The Fusarium genus was the most sensitive to the compounds present in the fermented CFS, presenting the lowest average MIC and MFC values.
Table 3: Minimum Inhibitory Concentration (MIC) and Minimum Fungicidal Concentration (MFC) for lyophilized CFS (mg mL-1) fermented by bacteria against: a) Penicillium, b) Aspergillus, c) Fusarium and Alternaria species. The result is expressed as (g L-1).
Identification of antifungal compounds in CFS
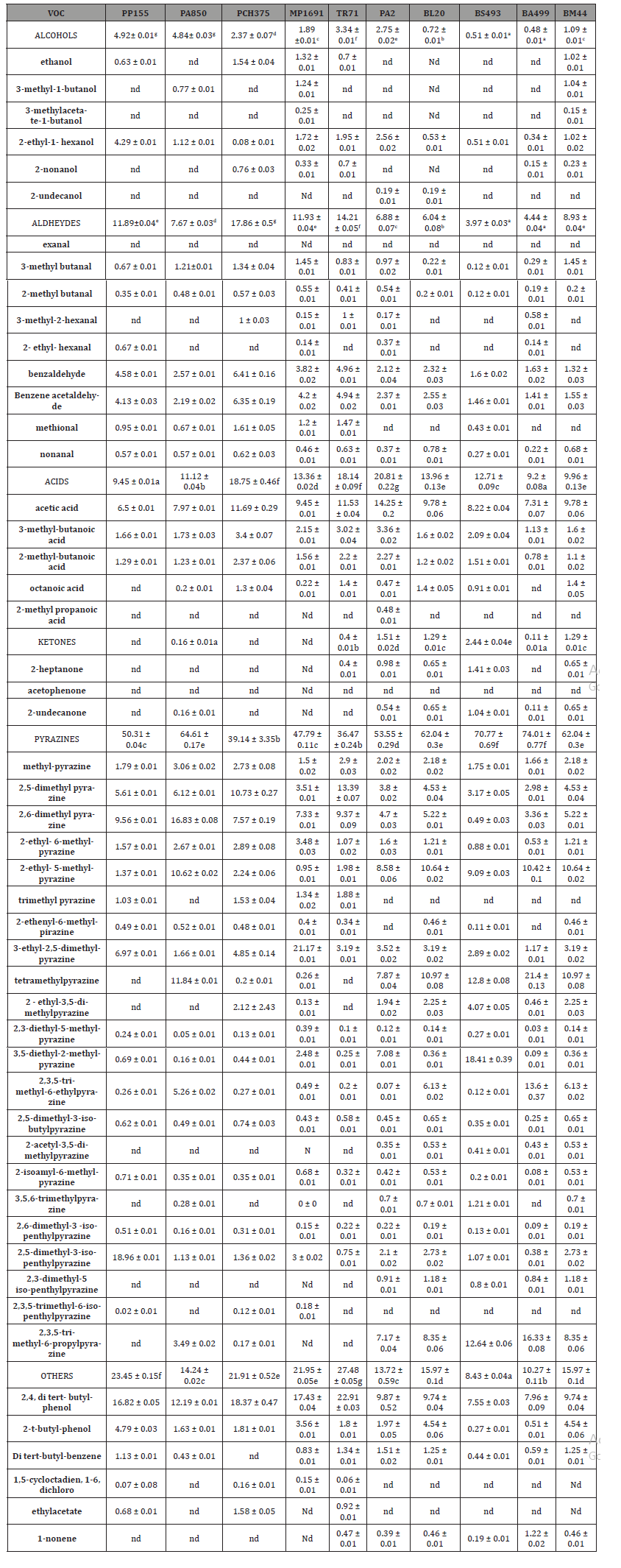
According to the literature, the antifungal activity of the bacteria as biocontrol is not only due to a type of compound but also depends on the synergistic action between all metabolic substances present in the fermented media [11]. A total of four organic acids were determined, namely, lactic acid, acetic acid, succinic acid, and malic acid. According to our results as shown below in Table 4a), all isolated stains produced lactic acid and acetic acid with concentration in the range24-282± 0.64g Kg-1and 21–77 ± 0.32 g Kg-1, respectively. The LAB with the highest lactic acid production were L. Plantarum PP155 (282.31 ± 0.84 g Kg-1), L. Plantarum PA850 (227.74 ± 0.29 g Kg-1), L. Plantarum MP1691 (143.83 ± 0.45 g Kg-1). These values could explain the fact that high antifungal activity has been detected from these strains. The production of succinic acid (14-45g Kg-1) and malic acid (6-13 g Kg- 1) was determined only in five and six CFS, respectively. In addition, these two acids had a lower average concentration than lactic acid and acetic acid. The phenolic acids detected in fermented CFS are listed in Table 4b. Among the eighteen targeted compounds, six phenolic acids were detected and quantified in CFS (benzoic acid, 1-2-dihydroxybenzene, DL-3-phenyllactic acid (DLA), p-coumaric acid,3-4-dihydroxyhydrocinnamic, vanillic acid). These compounds have been reported as antifungal agents produced by LAB [12]. Like organic acid production, the phenolic acids data correlated with the observed antifungal activity of CFS. A synergism between lactic acid and acetic acid has been documented [13], as well as between these and DLA in the potential antifungal effect [14]. A total of fifty-one VOCs has been identified in the lyophilised CFS fermented by LAB as According to the literature, the antifungal activity of the bacteria as biocontrol is not only due to a type of compound but also depends on the synergistic action between all metabolic substances present in the fermented media [11]. A total of four organic acids were determined, namely, lactic acid, acetic acid, succinic acid, and malic acid. According to our results as shown below in Table 4a), all isolated stains produced lactic acid and acetic acid with concentration in the range24-282± 0.64g Kg-1and 21–77 ± 0.32 g Kg-1, respectively. The LAB with the highest lactic acid production were L. Plantarum PP155 (282.31 ± 0.84 g Kg-1), L. Plantarum PA850 (227.74 ± 0.29 g Kg-1), L. Plantarum MP1691 (143.83 ± 0.45 g Kg-1). These values could explain the fact that high antifungal activity has been detected from these strains. The production of succinic acid (14-45g Kg-1) and malic acid (6-13 g Kg- 1) was determined only in five and six CFS, respectively. In addition, these two acids had a lower average concentration than lactic acid and acetic acid. The phenolic acids detected in fermented CFS are listed in Table 4b. Among the eighteen targeted compounds, six phenolic acids were detected and quantified in CFS (benzoic acid, 1-2-dihydroxybenzene, DL-3-phenyllactic acid (DLA), p-coumaric acid,3-4-dihydroxyhydrocinnamic, vanillic acid). These compounds have been reported as antifungal agents produced by LAB [12]. Like organic acid production, the phenolic acids data correlated with the observed antifungal activity of CFS. A synergism between lactic acid and acetic acid has been documented [13], as well as between these and DLA in the potential antifungal effect [14]. A total of fifty-one VOCs has been identified in the lyophilised CFS fermented by LAB as
Table 4: Identification and quantification of organic acids (g kg-1) produced by LAB in CFS. The results are expressed as mean ± standard deviation. Statistically significant differences for each fermentation are indicated with different letters (p < 0.01).
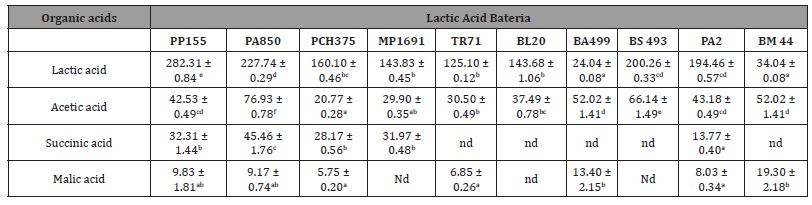
Table 5: Identification and quantification of phenolic acid (mg mL -1) produced by LAB in CFS.
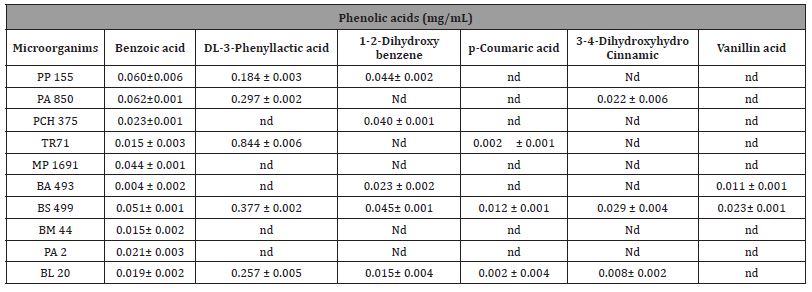
Table 6: Identification and quantification of Volatile Organic Compounds (VOC) (%) produced by LAB in CFS.
Lactobacillus plantarum PP155 and Lactobacillus plantarum PA850 as a tomato bio preservative

Since activity has been observed only for the Lactobacillus plantarum PP155 and Lactobacillus plantarum PA850 these two strains have been considered as case study using tomatoes as food matrix. Figure 3 shows the bio-preservation effect of CFS fermented by L. plantarum PP155 and L. plantarum PA850 on tomatoes against Penicillium expansum, during storage. Microbiological analysis of the population of the fungal confirmed the absence of inhibition of fungal growth in tomatoes treated with CFS. In the control experiment, the percentage of infected tomatoes on day 10 of incubation was 100 %, whereas, when CFS fermented by L. plantarum PP155 and L. plantarum PA850 were used, the values were 29 % and 65 %, respectively. The observed shelf-life data of tomatoes inoculated with P. expansum and treated with CFS were confirmed by microbiological analysis. The control experiment at 10 d of incubation, presented a fungal population of 7.56 log10 spores g-1, whereas in the tomatoes treated with CFS fermented by L. plantarum PP155, significant (p< 0.01) fungal growth of 5.58 log10 spores g-1 was observed. In the treatment with CFS fermented by L. plantarum PP850, infected tomatoes incubated for 10 d, presented a significant (p< 0.01) fungal count of 3.67 log10 spores g-1 Figure 4.
Discussion
The possible mycotoxins contamination in raw materials and/ or in transformed foods is a serious risk. The vegetal origin of phytocomplex and possible mycotoxins occurrence may affect the processes of extraction and concentration of the active principles, representing a critical point to be monitored to provide, safe and mycotoxins free nutraceutical and/or functional food. Despite the efficiency of synthetic chemical compounds in eliminating mycotoxin-producing fungi and mycotoxin reduction, the residues of many chemicals pose health risks to humans and animals. Due to the toxicity of these exogenous xenobiotics used to reduce mycotoxin production and fungal growth, numerous studies have been conducted to identify effective natural alternatives. This study provides insights into suitable alternatives to reduce possible toxicological risk associated with mycotoxin-contaminated phytocomplex components of a nutraceutical or functional food. Antifungal in vitro experiments demonstrated that CFS fermented by different strains of Lactobacillus have significant antifungal activity against a broad spectrum of toxigenic fungi. These effects may be due to the bacteriocins and to the secondary metabolic compound produced by these strains. Furthermore, the application of CFS as a novel bio-preservative in tomatoes, chosen as a case study whose results can be extended to other vegetal food matrices, evidenced a reduction in the spoilage associated with Penicillium expansum growth. Thus, the promising application presented in this paper to decrease micro fungi contamination and increase the post-harvest shelf-life of tomatoes, is efficient to protect from the mycotoxin’s contamination. This contributes to meeting the demand of consumers to reduce the agricultural use of synthetic compounds and increase the choice of natural alternatives.
-
Patricia Daliu*. Probiotics as Biocontrol Agents for Safe Approach Against Mycotoxins Contamination. Onl J of Conf Procee. 1(1): 2024. OJCP.MS.ID.000505.
-
mycotoxins, microbiota, Aspergillus ochraceus, Penicillium verrucosum, hydroxybenzoic acid, antifungal, phenolic acids, Gaithersburg, Bacillus, Aspergillussspp
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






