 Research Article
Research Article
Extraction and Characterization of Baobab (Tebaldi) and Balanites (Laloub) Seeds oil in Sudan
Alia M A Ibrahim1* and Kamal E E Yassin2
1Department of Chemical Engineering, University of Khartoum, Khartoum, Sudan
Alia Mutasim Ali Ibrahim, Department of Chemical Engineering, College of Engineering, University of Khartoum, Khartoum, Sudan
Received Date: May 09, 2024; Published Date: June 06, 2024
Abstract
The objectives of this study were to obtain oils from Baobab and Balanites seeds, then converted the resulting oil into soaps. It is important to note that seeds are normally considered as waste, thrown away without being used despite its great benefit. The extraction was conducted using n-hexane as solvent. Oil and solvent were separated using rotary evaporator. The obtained physicochemical analysis results indicate excellent properties of extracted oils for use in nutrition, cosmetics and medicinal products, the extraction was carried out at room temperature. The physical state of oils extracted are non-volatile, liquids, reddish yellow color of Baobab oil and light-yellow color of Balanites oil with characteristic pleasant smells. Oils density was (0.760 and 0.781 g/m3), refractive index (1.451 and 1.460), and viscosity (62.79 and 56.34 mm2/sec) for Baobab and Balanites oil, respectively. The physicochemical properties were also determined, oil content were found to be (13.26 and 33.85 %), crude protein CP (13.475 and 8.23g/100g) saponification value SV (338.4925 g and 148.665 mgKOH/kg), acid value AV (3.927 and 4.488 mgKOH/g), free fatty acid FFA (1.9635 and 4.488 mgKOH/g), peroxide value PV (1.42 and 0.86 mg/kg), and iodine value IV (96.95 and 62.20g I2/100 g) for Baobab and Balanites oil respectively. The resulting oils have a good saponification number, especially in Baobab seed. Oils have been used to produce soaps, it suitable to insert in soap making. This reveals that the oil extracted from Baobab and Balanites can be used both domestically and industrially. It is therefore recommended that more and advanced studies should be conducted for these abundant sources of natural nutritious oil.
Keywords: Baobab Oil; Balanites Oil; Physicochemical; Saponification Value
Introduction
Fats and oils serve as rich sources of energy, yielding approximately nine kilo calories/gram. They serve as carriers of the fat-soluble vitamins (A, D, E and K), while certain components of fats such as unsaturated fatty acids are essential nutrients in the diet. Fats and oils play a distinctive role in the natural flavor of a wide variety of food commodities [1]. Baobab (Adansonia digitata L.) fruit in Sudan called “Tebaldi,” Baobab oil is used in many ways for food texturing and frying. Seeds oil is particularly essential sources of vitamins D and E. Which were founded in Baobab oil includes vitamins A, D, E and K. Baobab seeds also contain minerals and proteins. Baobab seed oil is one such ingredient, which has rapidly become popular on global markets [2]. Baobab is found Australia and in many African countries. Ninth Baobab species have been identified globally. In southern, western and eastern parts of Africa.
Which were founded in Baobab oil contains vitamins A, D, E and K. Baobab seeds also contain minerals and proteins. Baobab seed oil is one such ingredient, which has rapidly become popular on global markets [1].
Baobab is found Australia and in many African countries. Ninth Baobab species have been identified globally. In southern, western and eastern parts of Africa. Balanites aegyptiaca (L.) Del. fruit in Sudan called “Laluob, the seeds contain 30-60% oil, which is edible and used as cooking oil. Seed oil can also be used as biofuel as combustion energy of oil is comparable to that of diesel. Oil extracted is a high demand in villages, it uses for Cooking, medicinal, trade item and firewood/charcoal, oil is consumed for headache and to improve lactation. Traditionally, oil is used to medicine edema, abdominal pains, chest pain, skin infections, liver, skin diseases and snake bites [2]. The seed kernel is considered as an extremely useful edible product, it is rich in oil, protein, minerals and it has been reported to be used for over thousands of years. The extracted oil is used for many uses, and it is used in western Sudan remaining cake is used as animal feed [3]. It is widespread throughout tropical Africa. In Sudan, Baobab is most frequently found on sandy soils and by seasonal streams „khors‟ in short grass savannah. Balanites globally is found in Tropical land northern, eastern and western parts of Africa and Asia. It is. It is also found in Arabian Peninsula, India, Iran, Pakistan and Malawi. Baobab and Balanites are important trees found in states of Kordofan, Darfur and Blue Nile [4].
Soaps are compounds that consist of a long chain of hydrocarbons attached with a carboxylic acid which is ironically bonded to the metal ion usually sodium or potassium, it is a combination of animal fat or plant oil with caustic soda. Soaps are therefore called the salts of fatty acids which are usually used as surfactants for washing, bathing and cleaning. Soaps are obtained by the treatment of vegetable oils or animal fats with a strong alkaline solution. The three molecules of fatty acids in the triglyceride gets attached to a single molecule of glycerol and results in a chemical reaction termed saponification. Soaps are used on a day-to-day basis in households and their physicochemical properties will be determined by their quality, efficiency and cleaning properties [3]. The objectives of this study were to obtain oils from Baobab and Balanites seeds using n-hexane as a solvent, study the physicochemical of the resulting oils and, converting the extracted oils to produce soaps.
Material and Methods
Samples Collection
Matured and fresh fruits of Baobab and Balanites were collected randomly from (El- Nuhud city) which located in Kordofan state, in the western side of Sudan. Kordofan state is one of the central states of Sudan, it occupies the center part although trends to be a little western between longitude 16,30 - 30,9ο‟ north 32,35 - 40,36ο‟ east.
Samples Preparation
Baobab shell was manually cracked using hammer to get fruits, seeds were soaked in water for about one hour, washed by hand to remove residual of pulp and fiber. Seeds were diffused on the drying trays mantled with an absorbent (paper towels). Left overnight on the laboratory bench to lose moisture gained during seeds washing. Seeds were put on the dryer at 70οC for 1 hour to reach constant moisture content. Dried seeds were ground to a fine powder using an electrical blender.
Balanites fruits were soaked in large bowl of clean water (overnight), to remove the glycoside of pulp coats seed. Then washed plenty of water to insured that any remaining layer of seed coat was removed, seeds dried in the oven at 60oC for 2hrs to reach constant moisture content, the wooden shell was cracked manually by hammer to removed, extracted seeds, which were converted into smaller particles by pestle to obtained seeds kernel. Both fruits seeds were packed in polyethylene bags and stored at room temperature for laboratory analysis Figures 2&3.


Cold Extraction
Extractions were carried out by soaking the powders of Baobab and Balanites seeds for 48hrs in an enclosed glass jar with n-hexane as solvent, with ratio of seed powder weight to solvent Volume 1:5. Leaching was done by solid extraction method, the final extracts were obtained filtered through a Whatman No. 1 filter paper, and solvent was separated from the extracted oils with the rotary evaporator at 40°C (Figure 4).

Oil Separation
Some chemical procedures require a quick and effective separation of substances through evaporation. The Rotary Evaporator is a tool which puts the separable substance under vacuum and heats evenly through a spinning motion, causing one component to evaporate and leaving the first component behind.
physicochemical analyses
Oil Content
Lipid was determined according to the method of [5] using Soxhlet apparatus follows: An empty clean and dry exhaustion flask was weighed. About 2 g of sample was weighed and placed in a clean extraction thimble and covered with cotton wool. The thimble was placed in an extractor. Extraction was carried out for eight hours with petroleum ether. The heat was regulated to obtain at least fifteen siphoning per hour. The residual ether was dried by evaporation. The flask was placed in an oven at 105°C till it dried completely and then cooled in a desiccator and weighed.
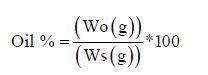
Crude Protein (CP)
Crude protein of the sample was determined by method according to [5] as:
1. Digestion: 0.2 g of sample was weighed and placed in small digestion flask (50 ml). About 0.4 g catalyst mixture (96% anhydrous sodium sulphate and 3.5% copper sulphate) was added, 3.5 ml of approximately 98% of H24 was added. The contents of the flask were then heated on an electrical heater for 2hrs till the colour changed to blue green. The tubes were then removed from the digester and allowed to cool.
2. Distillation: The digested sample was transferred to the distillation unit and 20 ml of NaOH (40%) were added. The ammonia was received in a 100ml conical flask containing10 ml of 2% boric acid plus 3-4 drops of methyl red indicator. The distillation was continued until the volume reached 50 ml.
3. Titration: The content of the flask was titrated against (0.02 M) HCL, then titration reading was recorded.
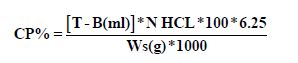
Where, T Titration Reading, B Blank Reading, N HCl Normality of HCl and Ws, sample weight.
Crude Fiber (CF)
Crude fiber was determined according to [5]. A 2 g of defatted sample were treated successively with boiling solution of H2SO4 and KOH (0.26 N and 0.23 N, respectively). The residue was then separated by filtration, washed and transferred into a crucible then placed into an oven adjusted to 105°C for 18-24hrs. The crucible then with the sample was weighed and ached in a muffle furnace at 500°C and weighed.
Ash Content (AC)
Ash content of the sample was determined according to the method of [5] as follows: 2g of sample were placed in a clean dry pre-weighed crucible, and then the crucible with its content ignited in a muffle furnace at about 550οC for 3 hours or more until light grey ash was obtained. The crucible was removed from the furnace to desiccators to cool and then weighed. The crucible was reignited in the furnace and allowed to be cooled until a constant weight was obtained.
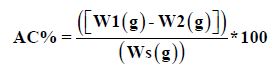
Where, W1weight of pot with ash, W2 weight of empty pot and Ws sample weight.
Iodine Value (IV)
300 ml conical flask with ground in stopper 0.1g sample was added. 20 ml of carbon tetrachloride were added, and the flask was sealed. 25 ml Hanus solution was also added, and the flask also sealed. The flask content shake for 1 minute. And kept sealed and left in a dark room (about 20οC) for 30 min with continuous shaking every 5 minutes. 10 M of 15% potassium iodide and 100 ml of water were added, and the flask sealed and shake for 30 seconds. The flask content titrated with 0.1 mol/L sodium thiosulphate to obtain iodine value. Likewise, perform a blank test to obtain blank level [5].
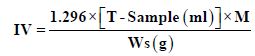
Where T, Titration of blank, M Molarity of stander and Ws, sample weight.
Peroxide Value (PV)
The method described by [5], 5 g of sample were delivered into conical flask with stopper. About 25 ml of solvent (15 ml acetic acid+10 ml chloroform) were added and gently shaken to dissolve the sample completely. The air inside flask gently replaces with nitrogen to remove remaining oxygen. One ml of saturated potassium iodide was added and immediately seals the flask and gently shakes it for one minute. The flask left at room temperature 15 to 20οC in dark room. 30 ml of pure water were added, and the flask sealed and stirred. Titration with 0.01 mol/L sodium thiosulphate was performed to measure peroxide value.
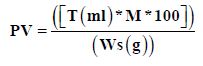
Where T, Titration of stander, M Molarity of stander and Ws, sample weight.
Saponification Value (SV)
Determining of saponification value method by [5], a 2 g of the oil was weighed in a 25 ml conical flask to which 5 ml of 0.5 ml alcohol and 20 ml of 0.5 M alcoholic KOH solution were added. Also 5 ml of 0.5 alcoholic KOH solution were added for the blank and both were refluxed for an hour, after cooling. The contents of the flasks were titrated against 0.5 M HCl using phenolphthalein as indicator. The difference in titer between that of the blank and the sample solution is equivalent to the amount of the fatty acid present.
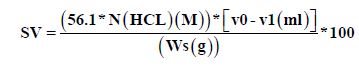
Where V0, V1, are the volume of hydrogen chloride required by blank and sample, respectively, N is the concentration of hydrogen chloride and Ws, sample weight.
Acid Value (AV)
About 5 of cooled oil sample accurately was weighed in a 250 ml conical flask and 50 ml added to 100 ml of freshly neutralized hot ethyl alcohol and about one ml of phenolphthalein indicator solution. The mixture was boiled for about five minutes and titrated while hot against standard sodium hydroxide shaking vigorously during the titration, determined by standard methods [6]. The oil mixed thoroughly before weighing. The weight of the oil taken for the estimation and the strength of the alkali used for titration shall be such that the volume of alkali required for the titration does not exceed 10 ml.
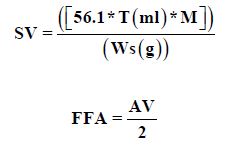
Where T Titration of stander, M Molarity of stander and Ws, sample weight, FFA Free Fatty acid.
Moisture content
5 g of each sample was accurately weighed using analytical balance (sensitivity 0.1 mg) into dried, tarred moisture dish and dried in an oven (Memmert, Germany) for 2 hours at 110˚C and repeated until a constant weight (difference be- tween two measurements not exceeding 0.5 mg/g of sample) was reached. The percentage moisture was then calculated using the method adopted by [6].
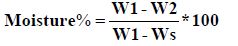
Where M1 initial weight of sample (g), M2 final weight of sample (g), Ms Weight of crucible (g).
Oxalate
1 g of sample was weighed into 100 ml conical flask. 75 ml 3MH2SO4 was added and stirred for 1hr with a magnetic stirrer. This was filtered by filter paper. 25 ml of the filtrate was then taken and titrated while hot against 0.05M KMnO4 solution until a faint pink colour persisted for at least 30 sec. Oxalate content was then calculated by taking 1 ml of 0.05M KMnO4 as equivalent to 2.2 mg oxalate [7].
Oxalate=2.2*Titration
Density (ρ)
The density of the oil was determined by the dry pycnometer filed with prepared sample in such a manner to prevent trap of air bubbles after removing the cap of the side arm. The stop per was inserted in pycnometer immersed immediately in water bath 30.0±0.2 and hold for 30 minutes. Any oil that came off the capillary opening for the pycnometer stopper was wiped out carefully. The bottle was removed from the bath, cleaned and dried thoroughly. The cap of the side arm removed and quickly the bottle weighed ensuring the temperature did not failed below 25°C [6].
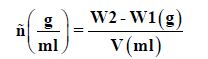
Viscosity
Cleaned viscometer well with acetone then dried. Put 10 ml of distil water in viscometer accurately of tube that wide party. In Elasticity tube sucked out water in other party (capillary tube party) utile water Surface concave above upper mark (T1) for viscometer to retched (T2). Secured it by putting forefinger on nozzle pipette, prepared stopwatch and started arithmetic time took water to flowing between upper and down mark (t1) time need for oil, repeated the previous experimental [8].
Where W2, bottle weight with oil, W1 weight of empty bottle and V volume of oil.
Refractive Index
The refractive index of oil was determined by method of [5]. The refract meter was first adjusted at 1.3330 at 25οC with pure distilled water as a blank reading. A drop of the oil was placed in the instrument and telescope was adjusted so that the cross hairs were distinct and in focus. The adjustment of the knob was rotated until the lower part of the field was dark and the upper part was light, and a clear definite boundary appeared. The coarse adjustment knob was moved first and then the fine adjustment knob until the boundary line coincided with the intersection of the cross hair in the telescope. The instrument was red when the temperature was stable. Refractive index value read directly from refract meter.
Preparation of Baobab and Balanites Oil Soap
Baobab Oil Soap
According to the method [9] were modified to Baobab and Balanites oils, for saponification procedure: 20 g of sodium hydroxide pellets was dissolved in 100 cm3 volumetric flask and volume made to the mark with distill water. The required quantity of alkali solution was mixed with Canary melon seed oil (ratio 1:1 v/v). Oils were warmed gently and poured into the beaker followed by the alkali solution to form an intimate mix and then stirred frequently using stirring rod until reaction reach equilibrium this took 5 minutes. The saponification mixture was then poured into mould and allowed to dry (cure) for 24hrs.
PH of Soap
2 g of soaps were added into 20 ml distilled water and shaken, and the soap suspensions were allowed to stay for at least 12 hours before the PH meter was inserted into a beaker, the readings were recorded.
Statistical analysis
The Statistical analysis of Baobab and Balanites seeds oils results were achieved using Microsoft Excel (2007) - version 12.0.4518.1014. The results officiated in three duplicating.
Results and Discussion

Table 1: Chemical Composition of Baobab and Balanites Seeds Oils.
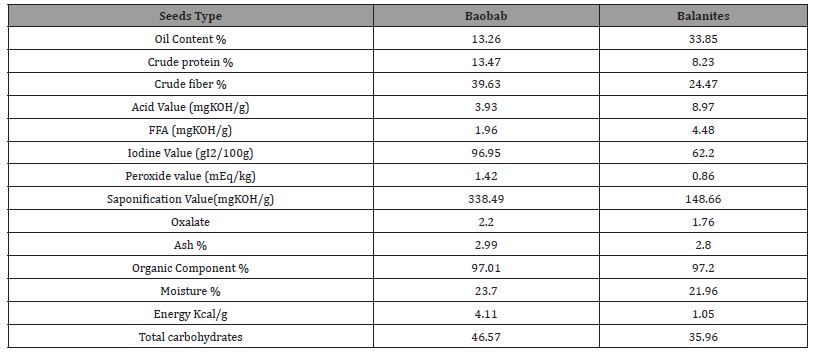
The obtained results showed that gained oil were reddish yellow (golden yellow) color for Baobab oil and pale yellow for Balanites oil, fixed and liquid at room temperature 25οC. Chemical Composition of Baobab and Balanites Seeds Oils in Table 1 & Figure 5. The properties of Baobab and Balanites seeds oil extracted showed that the seeds were a good source of oil, obtained oils were fixed, non-volatile, liquid at room temperature, Reddish yellow color, and with nuts smell of Baobab oil. Whereas Balanites oil was bright yellow color, with palatable flavor. Results showed that Baobab seed oil content was found to be13.26 %, that within the amounts which are 21.40 to 22.75% [17], 12.25 % [10] and 33.00 % [11].
Baobab seed contains a high amount of oil which indicates that it is an overgrown source of oil. The result of Balanites seed oil content result found to be 33.85%, proximal to 30.90% gained by [12] these values within the range of 45% - 46% reported [13]. These seeds contain high oil content indicating that processing of the oil for industrial or edible would be economical feasible. Balanites seeds are bearing more oil quantity than Baobab seeds. The crude protein earned of Baobab seed was 13.47%, which is lowest than values 20.13% and 36.60% scanted by [11] and [14] respectively. The loud amount of crude protein content in Baobab seed is a reflection of a high amount of amino acid embed the seed callus. Crude protein content of Balanites seed was 8.23%, this value lower than 30.9% which gained by [15]. The crude fiber gained of Baobab seeds was 39.63%, this value slightest than that get [11] and higher than values obtained by [16] which were 49.72% and 6.71%, respectively. Crude fiber of Balanites seed was 24.47%, this value higher than that 11.34% and 12.64% which gained by [15] and [12].
Acid value was determined to quantify the fatty acid found in the oil, AV of Baobab oil found is 3.93 mgKOH/g, it is more than values 0.43-0.84 found by [17], obtained result of acid value is high slightly than range of recommended for cooking oil which is 0.00- 3.00 mgKOH/g [18]. The acid value of Balanites seed oil was 8.97 mg KOH/g, it is higher than value 2.14 mg KOH/g obtained by [3]. Acid value was determined to quantify the fatty acid found in the oil as it measures the free fatty acids of oil. Low value of acid value means oil is stable [19]. Oils with high acid value, which will also mean high FFA will undergo rancidity due to the hydrolysis of the free fatty acids on storage. The acid value and FFA of Baobab and Balanites oils are high. The low FFA reduces the tendency of the oil to undergo hydrolytic activities.
The iodine value of Baobab oil is 96.95 gI2/100g, this value is equaled to 96.95 gI2/100g found by [20] and 96.95 to 115.97 gI2/100g [17]. Iodine value of Balanites oil is 62.20 gI2/100g, this value higher than 56.4 by [21] and lower than 76.8 [22] and 98.28 [23]. Iodine value was determined to quantify the degree of unsaturation in oil, and it was an identity characteristic of native oil. It indicates the degree of unsaturation in the fatty acids of triglyacyl glycerol. This value could be used to quantify the amount of double bonds present in the oil which reflects the susceptibility of oil to oxidation. When comparable to the standard of oil, it contains low degree of unsaturation and can therefore be classified Baobab and Balanites oil as non-drying edible oils because they fall in range of non-drying oils [19]. The peroxide value obtained of Baobab oil was 1.42mEq/kg this value was lower than that reported by [24] which is 6.6meq O2/kg and [17] 3.22meq O2/kg, it is within the range 0-10mEq/kg enforced for freshly vegetable oil, oils with PV below 10meq/kg are considered fresh oil, it is indicates to the rancidity process, low value of peroxide is an indication of low level of acidity of the oil and also suggests the high level of antioxidant.
The peroxide value determined of Balanites oil is 0.86mEq/g, this enables the oil to resist rancidity (autoxidation), obtained value lower than 6 and 8meq/kg found by [25] and [26] respectively. The result indicated that the oils had no rancidity, or they had good flavor quality and were considered stable. The peroxide value is defined as the amount of peroxide oxygen per 1 kilogram of fat or oil [1]. Detection of peroxide gives the initial evidence of rancidity in unsaturated fats and oils. Other methods are available, but peroxide value is the most widely used. The Saponification value was determined to quantify the amount of triglyceride. Saponification value measured of Baobab oil was 338.49mgKOH/g, sv gained had much higher saponification value reported in literature which ranged 133-200 mgKOH/g of oil according to [27], and 189.06 mgKOH/g [20]. The saponification value of the Balanites oil is 148.66 mg KOH/g, which within values 200.02 and 168.67 mg KOH/g found by [3]. This refers to oil also be used in soap making since its saponification value falls within the range of these oils the term “Unsaponifiable Matter” in oils or fats. When values are comparable to the values of certain vegetable oils like sesame, neem, groundnut, palm fruit and soya beans.
The saponification value of oil serves as an important parameter in determining the suitability of the oil for soap making. High amount of saponification value of Baobab seed oil puts it in the list of oils appropriate for soap manufacturing. Ash content analysis measured the amount of minerals present in the seeds; ash value of Baobab seed found was 2.99%. This is lower when compared with values obtained by [11] which are 7.50% and 7.61% respectively, but closer to 3.45% [20]. The ash content of Balanites seed was 2.80%, ash content close to 3.19 % [12] and 3.55% by [15].
The moisture content is important to determine whether seeds ready for use, moisture content of the seed was determined to be 23.70% which much louder than values 5.02% and 4.80% obtained by [11] and [14] consecutive. Moisture content of the Balanites seed was 21.96%, moisture content close to 17.65% found by [25], but very high than 3.27% found by [15], when oil has high moisture content this indicates that seeds oil can have long shelf live, the low moisture content indicated the activities of the microorganisms would be reduced and thereby increases the shelf life of seed [19]. The total carbohydrate amount is 46.57 % which is within the scope acquired by [14] and [28] which are 11.2% and 56.75%. While total carbohydrate of Balanites seed is 35.96%, this earned amount higher than obtained by [15] 8.9% table 1.
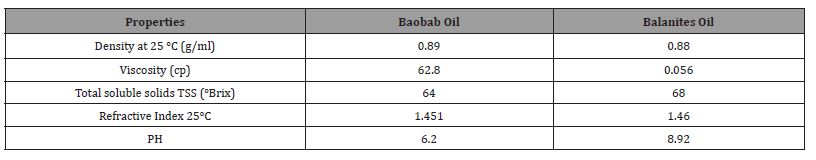
The physical properties were studied according to four different aspects as physical situation of density, viscosity, refractive index and PH. Table 2 shows the physical analysis of the Baobab and Balanites seeds oil. Baobab oil is viscous oil, slight acidity with density was 0.89 g/cm3, viscosity 62.80 mm2/s, refractive index 1.4510 and PH 6.2. While the previous studies had reported that Baobab oil density values ranged from 0.1950 to 1.0240 g/cm3 [29], this is agreed to the obtained value. Gained baobab oil viscosity was higher than 32.53 mm2/s that earned by [20]. RI lower than 1.4666 found by [1].
Balanites oil is viscous oil, slight basicity with density 0.88 g/ cm3, viscosity 56.34 mm2/s, refractive index 1.4600 and PH 8.92. Obtained density is closer to 0.9109 that reported by [12], viscosity higher than 19.63 and 34 cp reported by [12], obtained RI lower than 1.483 reported by [12]. The refractive index gained was within the normal range of vegetables oils. RI of Baobab seeds oil was close to that reported by [20] 1.4666 it was acceptable within values studies. Refractive Index is the ratio of light in a vacuum to its velocity in a specified medium. The Refractive Index can be used as an objective method for evaluation of rancidity in edible oils and fats.
Table 2: Physical Composition of Baobab and Balanites Seeds Oils.
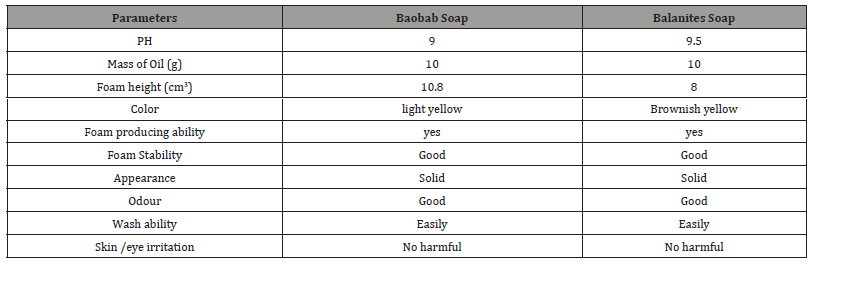
The physicochemical properties of the soap are summarized in Table 3. Oils were selected for excellent properties, high fat content (∼13.26%) for Baobab seed and (∼33.85%) for Balanites seed. The saponification value of Baobab seed oil is 338.4925 mgKOH/g and for Balanites seed is 148.66 mgKOH/g, the high saponification values indicated that oils are suitable for soap making.


The PH of the soaps were determined to be 9 for Baobab oil soap and 9.5 for Balanites oil soap which place soaps as a moderately basic soap, this value is slightly higher than 9.38 for cotton seed oil soap, it can be overcome by the addition of excess fat or oil or any other super fatting agent to reduce the harshness of the soap. High alkalinity in soap could expose the skin to various quandaries because the soap produced fats and oils on the skin during reacting with water producing a soluble organic salt which will be washed away by the bath water [30]. All this indicates that the prepared soap is not corrosive to the skin. As the salt of a weak acid (fatty acid) and strong base (NaOH), soap is alkaline (pH~10) in aqueous solution [20]. The quality assessment of the soap is formulated from the seeds of Baobab and Balanites. The soap tends to have good leathering, stable foam, moderate foam size, washing easy and a creaming feeling when used to wash hands.
Table 3: Quality evaluation of Baobab and Balanites oil soaps..
Conclusion
The outcomes of this study proposed that Baobab and Balanites seed oils can have worthwhile impact for nutrition and beauty implementations to corroboration health. Feature of high yield oil was investigated from Baobab and Balanites seeds, the purposiveness of extracted oils and physicochemical properties to elevating the values of growled sources, which experiments undeceives the benefit stored in these thrown seeds. The overall results of this study show that Baobab seed contains valuable amounts of oil, energy, protein, fiber, FFA and saponification value. Obtained results in this study were acceptable and similar to previous studies. High amount of saponification value of Baobab seed oil puts it in the list of oils appropriate for soap manufacturing and high oil content of Balanites oil gives importance as natural important exporter for oil. Soaps formulated from Baobab and Balanites oils have good texture, property and potency. Therefore, Baobab and Balanites oils can be inducts, applicable and additional to soap making and other cosmetics industries.
Recommendation
It recommended that more advanced studies should be conducted for these abundant sources of natural nutritious oil.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this paper.
Acknowledgment
None.
References
- Ibrahim AMA, Yssin KEE (2024) The Variation Effect of Solvents on the Physicochemical Properties Baobab (Ophelussitularius) Seeds Oil. Open Access Journal of Agricultural Research 9(1): 000347.
- Ibrahim AMA, Yssin KEE (2018) Extraction of Oils from the seeds of Baobab (Ophelussitularius) and Balanites (Aegilopsgeniculata). University of Khartoum, Sudan.
- Zang CU, Jock AA, Garba HI (2018) Application of Desert Date (Balanites aegyptiaca) Seed Oil as Potential Raw Material in the Formulation of Soap and Lotion. American Journal of Analytical Chemistry 9: 423-437.
- Wiehle M, Goenster S, Gebauer J, Mohamed SA, Buerkert A, et al. (2014) Effects of transformation processes on plant species diversity in home gardens of the Nuba Mountains, Sudan. Agroforestry System 88: 539-562.
- AOAC (1990) Official Methods of Analysis, 15th Association of Official Analytical Chemists, Arlington, VA, USA.
- Milwidsky BM, Gabriel DM (1994) Detergent Analysis: A Handbook of Cost-Effective Quality Control. Micele Press, 160-161.
- Usher G (1984) A Dictionary of Plant Used by Man. CBS Publishers and Distributors, New Delhi. pp. 74-80.
- Onwuka GI (2005) Food Analysis and Instrumentation; Theory and Practice, 219- 230.
- Warra AA, Wawata IG, Umar RA, Gunu SY (2012) Soxhlet extraction, Physicochemical Analysis and Cold process saponification of Nigerian Jatropha curcas L. Seed oil. Canadian Journal of Pure and Applied Sciences 6 (1): 1803-1807.
- Osman MA (2004) Chemical and nutrient analysis of baobab (Adansonia digitata) fruit and seed protein solubility. Plant Foods for Human and Nutrition 59 (1): 29-33.
- Ajayi IA, Oderinde RA, Kalogbola DO, Ukponi JU (2006) Oil of Under-utilized Legumes from Nigerian Journal Food Chemistry 99(1): 115-120.
- Freon SME (2015) Physicochemical Properties of Balanites aegyptiaca (Laloub) Seed oil. Sudan University of Science and Technology, Sudan).
- Author CC (1995) Lipid-Based Fats Substitutes. Critical Review Science Nutrients Journal 35: 405-430.
- Murray SS (2001) Nutritional Composition of some wild Plant Foods and Honey used by Hadza Forages of Tanzania. Journal of Food Composition and Analysis 14: 3-13.
- Ali AE, Mohammed B, Ali HAM, Malik M, Ali MAM, et al., (2021) Biochemical Properties and Nutritional Value of Balanites Aegyptiaca (Laloub). Seed Oil. Insights into Chemistry and Biochemistry 2(1): 2694-1708
- Nkafamiya II, Osemeahon SA, Dahiru D, Umaru HA (2007) Studies on the chemical composition and physicochemical properties of the seeds of baobab (Adansonia digitata). African Journal Biotechnology 6: 756-759.
- Ibrahim AMA, Yssin KEE (2021) Effects of Storage on Fatty Acid composition and Chemical Properties of Sudanese Baobab (Adansonia digitata L.) Seed oil in Kordofan and Blue Nile states. International Journal of Agriculture and Biological Sciences pp: 68-77.
- Oderinde RA, Ajayi IA, Adewuyi A (2009) Characterization of seed and seed oil of Hara crepitans and kinetics of degradation of the oil during heating. Electronic Journal Environment Agriculture Food Chemistry 8: 201-208.
- AOAC (2008) Official Methods of Analysis, Association of Official Analytical Chemists, Washington D.C. (15th ed).
- Ibrahim AMA, Yssin KEE (2021) Formulation of Cosmetics Containing Sudanese Baobab (Andasonia Digitata L.) Seed Oil in Kordofan State. Greener Journal of Agricultural Sciences 11(3): 195-203.
- Babagana G, Shittu S Bamidele, Idris M Bugaje (2011) Characterization and Composition of Balanites aegyptiaca seed oil and its potential as biodiesel feed stock in Nigerian Journal of Tapplied Phytotechnology in environmental sanitation 1 (1): 29-35.
- Manji AJ, Sarah EE, Modibbo UU (2013) Studies on the potential of Balanites aegyptiaca seed oil as raw material production of liquid cleansing agents. Department of Industrial Chemistry, Modibbo Adama University of Technology, P. M. B. 2076, Yola, Adamawa State, Nigeria.
- OkIai CA, Kwetegyek JA, Okiror P, Kimondo JM, Teklehaimanot Z, et al. (2013) Physicochemical characteristics and fatty acid profile of desert date kernel oil 21: 723-734.
- Erwa YI, Ali MA, Khalid AE, Omer BA, Ishag OO (2019) Proximate Composition, Mineral Elements Content and Physicochemical Characteristics of A. Digitata L Seed Oil. International Journal of Parma and Bio Sciences 10(4):119-126.
- Alemayehu H G (2015) Physico-Chemical Characterization and Extraction of Oil from Balanites Aegyptiaca Plant (Seed). World Journal of Pharmaceutical Research 4(11): 1723-1732.
- Babeker MA (2013) Physicochemical Properties of Laloub Seed Oil. University of Khartoum, Sudan.
- NKafamiya II, Osemeahon SA, Dahiru D, Umaru HA (2007) Studies on the Chemical Composition and Physicochemical Properties of the Baobab (Adansonia digitata). African Journal of Biotechnology 6(6): 756-759.
- Poll J, Petzk KJ, Ezeagu IE, Merges CC (1998) Nutritional Quality of Unconventional Tropical Seed in Rats. Journal Nutritional 128(11): 2014-2022.
- Singh HK, Yusup S, Wai CK (2016) Physicochemical Properties of Crude Rubber Seed Oil for Biogasoline Production. Procedia Engineering 148: 426-431.
- Oyeleke GO, Salam MA, Adetoro RO (2012) Some Aspects of Nutrient Analysis of Seed, Pulp and Oil of Baobab (Adansonia digitata L.). IOSR Journal of Environmental Science, Toxicology and Food Technology 1(4): 32-35.
-
Alia M A Ibrahim* and Kamal E E Yassin. Extraction and Characterization of Baobab (Tebaldi) and Balanites (Laloub) Seeds oil in Sudan. Onl J of Conf Procee. 1(2): 2024. OJCP.MS.ID.000506.
-
Baobab Oil, Balanites Oil, Physicochemical, Saponification Value.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






