 Opinion Article
Opinion Article
The Need for a Paradigm Shift in the Existing Strategies for Effective COVID-19 Control
Frank Adusei Mensah1,2*, Jussi Kauhanen1 and Carina Tikkanen Kaukanen3
1Institute of Public Health and Clinical Nutrition, University of Eastern Finland, Kuopio, Finland
2Public Health and Medicine Research Group, Center for Multidisciplinary Research Innovations, Finland, Ghana, and Nigeria
3Ruralia Institute, University of Helsinki, Mikkeli, Finland and Institute of Sustainability Sciences, University of Helsinki, Finland
Frank Adusei Mensah, Institute of Public Health and Clinical Nutrition, Yliopistonranta 1C, CA 3089 Canthia, 70210, Kuopio, Finland.
Received Date:November 25, 2022; Published Date:December 19, 2022
Abstract
The coronavirus pandemic is a global challenge affecting lives and livelihoods globally. With two years into the pandemic, hundreds of millions have been infected and mortality has reached millions despite the progress in COVID- 19 vaccination and vaccine availability. The continual emergence of new COVID-19 variants warrants a more comprehensive approach. The present opinion intends to get stakeholders and researchers aware of the possibility of natural products, ginseng and functional foods to be a game-changer in control of COVID-19 infection, if their continuous research against COVID-19 infection is supported. Emerging variants of COVID-19 have threatened the current COVID-19 vaccination campaigns demanding additional measures. There must be a paradigm in research, funding, and policies from prevention to treating the infected. Developing therapies for the infected is, therefore, a promising option, especially with multitargeted medicines. Functional foods and the combined use of natural and pharmaceutical products with multiple active compounds present a great alternative for consideration. Ginseng extracts and ginsengbased products have proved effective in reducing severity and hospitalization times in multiple clinical trials for acute respiratory viral infections. Natural functional foods, honey and berries and their numerous bioactive components possess huge potential against coronavirus disease. Funding and research of natural products for effective COVID-19 control should be promoted.
Keywords:COVID-19; Coronavirus; Therapies; Natural products; Acute respiratory illness; Ginseng; Functional foods
Abbreviations:ACE2: Surface angiotensin-converting enzyme-2; CFR: Case fatality rate; CoV: Coronavirus; COVID-19: Corona virus disease 2019 caused by SARS-CoV-2 virus; H1N1: Influenza virus; MERS: Middle east respiratory syndrome; MERS-CoV: MERS caused by coronavirus; MGO: Methylglyoxal; SARS: Severe acute respiratory syndrome; SARS-CoV: SARS caused by coronavirus; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; URI: Upper respiratory illness
Introduction
Being one of the most important advances in modern medicine, vaccines undoubtedly have saved the lives of millions from vaccine-preventable deaths. Credit to the success of vaccination, some of the world’s devastating pandemics including smallpox has been controlled and eradicated [1-3]. Vaccination is undeniably one of the world’s efficacious public health arsenals of modern medi cine. However, it is important to note that having safe and effica cious vaccines alone is not enough, but rather, vaccination together with therapies for the infected in addition to other public health measures do! COVID-19 (SARS-COV-2) vaccines coupled with therapeutic, functional foods and other health measures will give more dividends and progress in controlling the COVID-19 pandemic. For instance, the COVID-19 vaccinations rate regarding single and multiple shots vaccines has exceeded 70% of the population in many developed countries [4]. However, many of these countries continue to document thousands of COVID-19 infections daily. The resurgence of the pandemic and the continuous emergence of new variants ranging from the devastating Delta variant (B.1.617.2) to a more infectious Omicron variant (B.1.1.529) undeniably require a wide spectrum and a multifactorial approach to curb the COVID-19 disease. There also remain unanswered questions about the degree of protection for single and multiple shots, and the duration of waning of the vaccine triggered immunity [5]. There has been a hot debate on whether at all the 3rd and/ or 4th doses are necessary and whether it is indeed enough for the emerging variants.
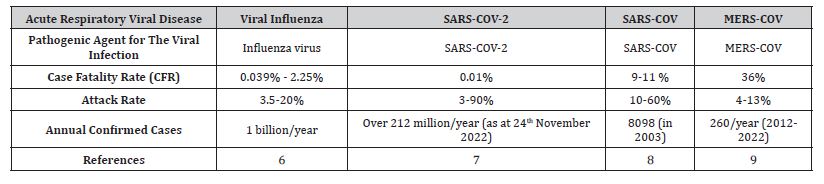
The COVID-19 virus resembles genetically and epidemiologically the previous acute respiratory viruses (Table 1). This gives hopes for translational medicine and translation of knowledge from the previous outbreak to the current COVID- 19 pandemic.
Table 1: Pathogens of acute respiratory infections.
Injections and combined pharmaceutic-complementary medicine for SARS-COV
Combination of pharmaceutical drugs, ginseng decoctions and extracts (5-15 g) and ginseng mixtures have been administered to SARS-COV patients during the 2003 epidemic clinical phase with impressive findings [10]. In SARS- COV patients with prostration syndrome: intravenous Shenfu or Shenmai injection (100 ml - 200 ml) per day was administered in addition to a decoction of American ginseng, red ginseng, and Panax ginseng (3-15 g) in a combination approach to manage the disease. In a clinical trials by WHO, the administration of integrated multiherbal Traditional Chinese Medicinal product for anti-fever, anti-toxins and dampness removing ‘‘Guoyao’’ (main ingredients were Sypsum Fibrosum 30 g, Semen Armeniacae Amarum 10 g, Radix Scutellariae 10 g, Rhizoma Atractylodis 10 g, Rhizoma Pinelliae 10 g and Radix Arnebiae seu Lithospermi 15 g and other Guoyao formulations) and pharmaceutical medicine (methylprednisolone 80–160 mg/day and ribavirin 0.75 g–1.5 g/day for a course of 2 weeks) < 28 days after the onset of SARS symptoms reduced hospitalization time compared to un-administered group [10]. SARS patients treated with integrated Guoya herbal mixture, methylprednisolone and ribavirin showed significant temperature reduction (p < 0.01) 2 - 3 days after the start of treatment compared to the control group treated with pharmaceutical medicine (methylprednisolone and ribavirin) only [10]. In the same trial, better cellular immunity and pulmonary inflammatory response and decreasing tendency in the occurrence of secondary infection were reported for the integrated treatment group as compared to the group treated with pharmaceutical medicine only [10]. Based on the above report, it is therefore imperative that, curative effects of the integrated SARS treatment are superior to pharmaceutical medicine treatment alone [10].
In a clinical trial in the USA by McElhaney, the standardized North American ginseng proved safe and effective with an overall 89% reduction in relative risk of preventing viral acute respiratory illness [11]. In a separate clinical trial, McElhaney and colleagues reported that, a proprietary extract of the roots of North American ginseng (Panax quinquefolium) significantly reduced incidence of viral influenza during the peak season (November - December) by 30% and recovery time by 7 days compared to the placebo group [12]. Additionally, a third clinical trial by McElhaney et al. [13], reported a significant reduction in the Jackson-confirmed upper respiratory illness (URIs) symptoms in the treated groups. The supplementation of 800 mg North American ginseng proved safe and reduced the viral influenza severity and duration of the URIs among the ginseng group compared to the placebo group [13]. Predy and colleagues studied another standardized North American ginseng containing 80% poly-furanosyl-pyranosyl-saccharides on viral upper respiratory tract infections in a clinical tria [14]. They observed 25% reduction in the incidence of viral URIs in the ginseng group compared to the placebo group. Significantly low viral upper respiratory tract re-infection rate (13% less), 25% reduction in disease severity, and 2 days faster recovery were reported in the ginseng group compared to the placebo group [14]. For pediatric use, the Panax quinquefolius (American ginseng root extract) was tested in a separate clinical trial and was reported to be safe and effective among children [15]. These observations suggest that early provision of combined treatment with pharmaceutical- ginseng/complementary products could be more beneficial to SARS-CoV-2 patients (Figure 1).
Antiadhesion therapy using natural products and their phytochemicals is a modern approach against infectious diseases [17- 20]. Withania somnifera, Indian ginseng, is a well-known antiviral, immunomodulatory, anti- inflammatory and antioxidant plant. Molecular docking studies in silico suggested two potential antiadhesive leads, with anoside X and quercetin glucoside, from W. sominifera with favorable interactions at the binding site of the virus SARS-CoV-2 [21]. Quercetin glucoside was also effective in clinical trial [22].
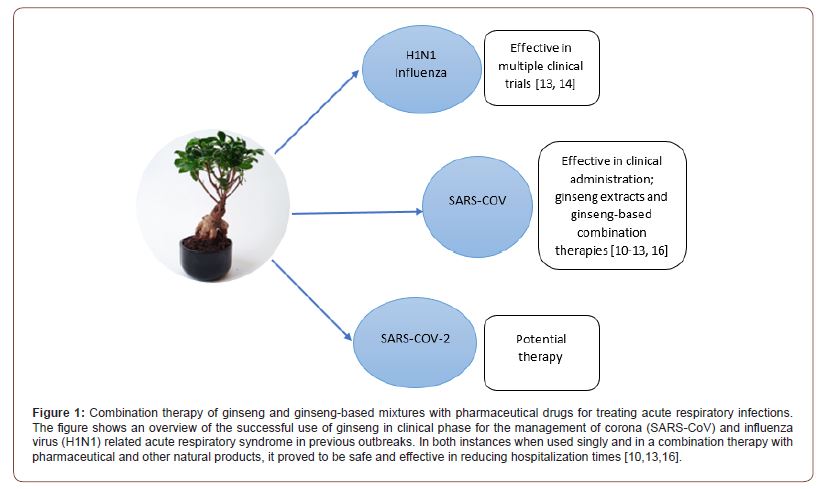
In our previous study, we observed the potential of the natural functional food (ginseng) and its extracts to promote the recovery of viral based acute respiratory infections in humans [3]. Ginseng was found to be effective in the reduction of risk of infection by 38%. In addition, the ginseng treatment group had 3-days shorter duration of acute respiratory illness than placebo group in all the reviewed randomized placebo-controlled trials [3]. As the world continues to race to find a cure, it is important to reconsider furthering research into the use of natural and functional foods like ginseng for the treatment of acute respiratory illnesses including COVID-19. Further studies are needed to determine the right dosage to improve efficacy and prevent adverse events.
Natural functional foods against COVID-19
Honey is unique natural functional food with remarkable antimicrobial properties against respiratory bacterial pathogens [17,23], and viral respiratory infections [24,25]. Honey methylglyoxal (MGO) activity has been described against both bacterial and viral infections [25,26]. In-silico screening has revealed hesperidin flavonoid activity in inhibition of SARS-COV-2 viral protease enzymes blocking the interaction with surface angiotensin-converting enzyme-2 (ACE2) [27]. Quercetin, isoquercetin and rutin flavonoids associated with reduction of viral load have been reported [27]. Immunostimulatory effects of honey have been found [28]. Honey saccharides [28,29], apigenin and kaempferol flavonoids [30] and proteins [31,32], induced cell-mediated immune responses. Honey bee products, including honey, propolis, royal jelly, bee pollen and bee venom, or their bioactive chemical constituents have therapeutic potential in the improvement of the immune response shown both in vitro, in vivo and in clinical studies [33]. Based on the antiviral, immune-boosting potency and phytochemical components of honey, the evidence for its role as a potentially effective natural product against COVID-19 exists.
Berries and their numerous polyphenols are known to have antimicrobial activity inhibiting the growth of several human bacterial pathogens [34-37]. Berries also can have antiadhesive properties [38,39], or both antimicrobial and antiadhesive activity [40]. Berry polyphenols include resveratrol, which reduces replication of coronavirus in vitro [41]. Quercetin flavonoid, also found in berries [42], was effective agent against SARS-CoV-2 in vivo [22]. Berries can impact gut microbiota and related metabolites [43-45]. Immunomodulatory wild blueberry anthocyanin fraction has been reported [46].
Discussion
When using natural products as anti-infectives against SARSCOV- 2 and other serious viral infections it is important to reveal their anti-infective compounds and mechanisms. Coronavirus infection is triggered by receptor recognition, membrane fusion, and successive viral entry mediated by the SARS-CoV-2 spike (S) glycoprotein. The receptor-binding domain of S protein initiates host attachment to the host’s ACE2 receptor proteins. S protein contains major antigenic determinants being the target of most COVID-19 vaccines [47,48].
We aim to recall for the need to redirect attention to therapeutic interventions including natural products and functional foods for the control of the COVID-19 pandemic. We present in brief, the outcome of our recent review of meta-analysis study carried on ginseng- based clinical trials on acute respiratory viral infections [3]. This observation was also recommended by Ratan and colleagues [49]. The intent is to inform stakeholders and researchers about the possibility of ginseng being a game-changer in Covid-19 management and it needs continuous research and attention [49].
Consuming regularly available natural products might lead to reduced and less serious COVID-19 infections based on their potential antimicrobial and antiadhesive effects against SARS-COV-2, or through boosting immune responses [20]. Characterization of the bioactive components will be essential to find effective leads and drugs in the prevention and treatment of the devastating viral diseases, like SARS-COV-2.
There must be a paradigm shift in the direction of research, funding and policies from prevention to treating the infected. Therapeutic development and management strategies to treat the COVID-19 infected population should be the core objective of both pharma and biologic companies while further attention is given to the funding and of natural products and functional foods’ research for effective control. This shift would be of great importance most especially as the world hope for a move from the pandemic phase to the endemic phase of the COVID-19 disease. There must be a paradigm in research, funding, and policies from prevention to treating the infected. Developing therapies for the infected is, therefore, a promising option, especially with multitargeted medicines.
Conclusion
Natural products, functional foods, and probiotics have shown remarkable findings in both animal and clinical studies against acute respiratory infectious diseases [50]. Recent meta-analysis revealed favorable impact of honey against viral respiratory infections [24]. Although less studied against viral infections, different berry components have shown activity against bacterial respiratory pathogens in several in vitro studies [38,40]. Probiotics, functional foods and healthy living should be encouraged to boost the natural immune defense mechanism.
Ginseng and ginseng-based products have high safety records at high dosage (3-15 g) based on evidence from multiple studies and centuries of continual usage. Ginsonoids from ginseng have been used singly and in combination with other pharmaceutical products for treating acute viral respiratory infections without any serious documented adverse health effects in humans and were proved to be effective in reducing disease severity and hospitalization times. Further research into the possibility of using these combinations for COVID-19 treatment could present great potentials.
Developing therapies for the infected is, therefore, a promising option, especially with multitargeted medicines, we therefore call for a paradigm shift in research, funding, and policies from prevention to treating the COVID-19 infected patients.
Acknowledgment
None.
Conflict of Interest
Authors declares no conflict interest.
- Onyegbule FA, Ilouno IO, Eze PM, Abba CC, Chigozie VU (2014) Evaluation of the Analgesic, Anti-Inflammatory and Antimicrobial Activities of Leaf Extracts of Breynia Nivosa. Chemical Science Review and Letters. 3(12): 1126-1134.
- Conway D (1973) The magic of herbs. Jonathan cape, London.
- Roja G, Rao PS (2000) Anticancer compounds from tissue cultures of medicinal plants. J Herbs Spices Med Plant 7: 71-102.
- Umar AH, Mabrouk M, Danjuma NM, Yaro A (2013) Studies on the Analgesic and Anti-inflammatory Properties of Hydro-alcohol Extract of Caralluma dalzielii NE Br (Asclepiadaceae) in Rats and Mice. British Journal of Pharmacology and Toxicology, 4(5): 169-175.
- Khalifa NS, El Hallouty SM, Barakat HS, Salim DM (2013) In Vitro Cytotoxic and Antioxidant Activities of Some Plant Extracts on Different Human Cancer Cell Lines. Egypt J Exp Biol (Bot) 9(1): 137-144.
- Cameron SI, Smith RF, Kierstead KE (2005) Linking medicinal/nutraceutical products research with commercialization. Pharmacetical Biology 43(5): 425-433.
- Vukovic N, Milosevic T, Sukdolak S, Solujic S (2007) Antimicrobial Activities of Essential Oil and Methanol Extract of Teucrium montanum. Evid Based Complement. Altern Med 4: 17-20.
- Evans WC (2002) Trease and Evans Pharmacognosy. 15th Edition. London: Elsevier Science Ltd.
- Chetty KM, Sivaji K, Rao KT (2008) Flowering plants of Chittoor district, Andhra Pradesh, India. 1st eds. Student Offset Printers, Tirupati, India.
- Haque SM, Ghosh B (2013) In vitro completion of sexual life cycle: production of R1 plants of Ipomoea quamoclit L. Propagation of Ornamental Plants 13: 19-24.
- Lowell C, Lucansky TW (1990) Vegetative anatomy and morphology of Ipomoea quamoclit (Convolvulaceae). Bulletin of Torrey Botanical Club 117: 232-246.
- Rajendran K, Srinivasan KK, Shirwaikar A (2007) Pharmacognostical identification of stem and root of Ipomoea quamoclit (Linn). Natural Product Sciences 13: 273-278.
- Sajem AL, Rout J, Nath M (2008) Traditional tribal knowledge and status of some rare and endemic medicinal plants of North Cachar Hills district of Assam, Northeast India. Ethnobotanical Leaflets 12: 261-275.
- Pullaiah T, Chennaiah E (1997) Flora of Andhra Pradesh. 1st ed. Scientific Publishers, Jodhpur, India.
- Sorathia KD (2014) Ethnomedicinal explorations for certain Convolvulaceae members of Anjar Taluka. Life Sciences Leaflets 49: 28-33.
- Khare CP (2007) Indian medicinal plants. 1st eds. Springer, New Delhi, India.
- Hasan SR, Hossain MM, Akter R, Jamila M, Mazumder MEH et al. (2009) DPPH free radical scavenging activity of some Bangladeshi medicinal plants. Journal of Medicinal Plants Research 3: 875-879.
- Trease GE, Evans WC, Bralliar TC (2002) In Pharmacognosy, 11th edition. Macmillian Publishers: New York, NY, USA, 3 110.
- Udegbunam RI, Nwaehujor CO, Udegbunam SO (2014) Evaluation of the anti-arthritic effect of Sterculia tragacantha (lindl.) Leaf extract in rats. American Journal of Pharmacology and Toxicology 9 (2): 107-113.
- Ardhie AM, (2011) Radikal Bebas dan Peran Antioksidan dalam Mencegah Penuaan. Medicinus 24(1): 4-9.
- Sanchez A, Calpena AC, Clares (2015) Evaluating the Oxidative Stress in Inflammation: Role of Melatonin. International Journal of Molecular Sciences 16(8): 16981-17004.
- Meliala L, Pinzon R, (2007) Breakthrough in Management of Acute Pain. Dexa Media Jurnal Kedokteran dan Farmasi 4(20): 151-155.
- Kristanti CD, Simanjuntak FPJ, Pramita NK, Dewi A, Tianri SV, et al. (2017) Anti-Inflammatory and Analgesic Activities of Avocado Seed (Persea americana Mill). Journal Farmasi Sains dan Komunitas, 14(2): 104-111.
- Renuka K, Ravishankar K (2014) Preliminary phytochemical investigation and in vitro study of antioxidant antimicrobial and anticancer activities of ethanolic extract of Ipomoea quamoclit. World Journal of Pharmaceutical Research 3: 612-626.
- Moin S, Shibu BS, Wesley PS, Devi BC (2012) In vitro screening of antibacterial protein activity from medicinal and economically important plants seed. Drug Invention Today 4: 533-536.
- Reddy RJ, Kumar SA, Gupta RMV (2015) Anti-diabetic activity of Ipomoea quamoclit in streptozotocin induced diabetic rats. Journal of Pharmacognosy and Phytochemistry 4: 68-71.
- Prodhan ZH, Biswas M, Rahman M, Islam N, Golam F (2012) Effects of plant extracts on salivary gland chromosomes of house fly (Musca domestica L). Life Science Journal 9: 1930-1935.
- Nworu CS, AKah PA (2015) Inflammation as common soil of multifactorial diseases. Autoimmunity Reviews 10(7): 369-374.
- Adeyemi OO, Okpo SO, Ogunti OO (2002) Analgesic and anti-inflammatory effects of the aqueous extract of leaves of Persea americana Mill Lauraceae. Fitoterapia 73: 375-380.
- Kumaran T, Citarasu (2015) Ethnopharmacological investigation and antibacterial evaluation of the methanolic extract of Asparagus racemosus (Shatavari). Tropical Plant Research 2: 175-179.
- Aiyeloja AA, Bello OA (2006) Ethnobotanical potentials of common herbs in Nigeria: A case study of Enugu state. Educ Res Rev 1(1): 16-22.
- Bako SP, Bakfur MJ, John I, Bala EI (2005) Ethnomedicinal and Phytochemical profile of some savanna plant species in Nigeria. Int J Bot 1(2): 147-150.
- Cohen ML (1992) Epidemiology of drug resistance: implications for a post antimicrobial era. Science 257(5073): 1050-1055.
- Edeoga HO, Omosun G, Uche LC (2006) Chemical composition of Hyptis suaveolens and Ocimum gratissimum hybrids from Nigeria. Afr J Biotech 5(10): 892-895.
- Elujoba AA (1997) The role of pharmacognosy in phytotherapy the challenges of our time. Nigerian J Nat Prod 2: 34-36.
- Nayak BS, Sandiford S, Maxwell A (2009) Evaluation of the wound-healing activity of ethanolic extract of Morinda citrifola leaf. Evidence-based complementary and alternative medicine 6(3): 351-356.
- Ramos Jerz MDR (2007) Phytochemical analysis of avocado seeds (Persea americana Mill., c.v. Hass) Cuvillier: Gottingen.
- Sorathia KD (2014) Ethnomedicinal explorations for certain Convolvulaceae members of Anjar Taluka. Life Sciences Leaflets 49: 28-33.
-
Frank Adusei Mensah*, Jussi Kauhanen and Carina Tikkanen Kaukanen. The Need for a Paradigm Shift in the Existing Strategies for Effective COVID-19 Control. On J Complement & Alt Med. 8(1): 2022. OJCAM.MS.ID.000680.
-
COVID-19; Coronavirus; Therapies; Natural products; Acute respiratory illness; Ginseng; Functional foods; Existing Strategy; Shots vaccines; Vaccine triggered immunity; Secondary infection; Viral influenza
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






