 Research Article
Research Article
Quality of Life Improved by Adequate Physical Activity Levels Among University Employees
Gustavo Mendoza1 and Kenneth R Ecker²*
1Department of Health and Human Performance, University of Wisconsin River Falls, USA
2Department of Kinesiology, Pacific University, Forest Grove, USA
Kenneth R. Ecker, Ph.D., FACSM, Assistant Professor, Department of Kinesiology, Pacific University, Forest Grove, OR 97116, USA.
Received Date: March 02, 2021; Published Date:April 07, 2021
Abstract
Purpose: The purpose of this study was to determine if quality of life is affected through different levels of physical activity and compare those to the national norms. This study also looked at the number of ACSM cardiovascular disease risk factors and different physical activity levels among university faculty.
Methods: An SF-36, I-PAQ, and Health Questionnaire form was handed out to the participants to complete to the best of their knowledge.
Results: Comparative analysis showed that there was a significant positive difference among the participants who were HEPA active in general health (p = 0.0354) and the active group in comparing emotional well-being (p = 0.0346) and physical functioning (p = 0.0498) when using a oneway ANOVA. A Tukey-Kramer post hoc test was performed to prove honestly significant difference.
Conclusion: The results indicate that the university faculty had better quality of life scores in certain parts of the SF-36 sub-scales when being active and HEPA active. The university faculty seem to be at risk for some cardiovascular disease risk factors in which the need to reduce the risks by implementing an exercise-based health and wellness program within the university workplace should be considered in university workplace policies.
Keywords:Quality of life, Body mass index, Cardiovascular disease, Physical activity
Introduction
Physical activity is at the crux of many diseases and syndromes. Physical inactivity is one of the major risk factors for developing non-communicable diseases (NCDs), such as heart disease, stroke, cancer, and diabetes (1). The World Health Organization (WHO) states that a combination of environment, genetics, physiology, and behavior are factors in the development of NCDs, also known as chronic diseases [1]. Metabolic syndrome has also been mentioned as one of the key factors in developing non-communicable diseases [2]. NCDs account for 70% of all deaths globally, and 75% of those NCDs deaths occur in low- and middle-income countries [1]. One of the main reasons that cardiovascular disease is on the rise is because many of the risk factors for cardiovascular disease (CVD) are on the rise as well [1,3]. Some risk factors are outside of one’s control, such as age, gender, and genetics. However, many risk factors for the disease can be modified through lifestyle changes and medical intervention. Modifiable risk factors include physical activity level, hypertension, prediabetes, dyslipidemia, diet, and obesity. Physical activity has been shown to have an inverse relationship between premature mortality, CVD/CAD, hypertension, stroke, osteoporosis, Type 2 diabetes mellitus, metabolic syndrome, obesity, colon cancer, breast cancer, depression, functional health, falls, and cognitive function [3]. According to health.gov and the Office of Disease Prevention and Health Promotion, 500 to 1,000 MET-minutes a week should be done to get significant health benefits [4].
According the American College of Sports Medicine (ACSM), they recommend an exercise prescription of 150 total minutes a week of moderate intensity aerobic activity or 75 total minutes a week of vigorous intensity aerobic activity [3]. Some occupations have also reported a greater chance of developing CVD because of the time sitting and lack of physical activity [5]. Research shows that having a higher education level leads to improved quality of life through the understanding of the benefits of physical activity [6]. The problem comes when “white-collar” jobs, which often requires a higher education level, demand much of the workday sitting and requires less physical activity overall [7]. A major trend over the past five decades is a loss in occupation related energy expenditure [8]. Overall, measures should be taken to incorporate programs that aim to improve the health status of their workers, in order to reduce the risk factors for CVD.
The purpose of this study was to determine if there is a relationship between physical activity levels and a higher quality of life among university employees, and to compare these results with standardized norms based on age and gender. In addition, this study will also be used to help guide programmatic offerings in health and wellness within the university.
Methods
Participants
In this study, there were 47 participants, 14 males and 33 females, ranging in age from 26 to 65 years. All participants were employed by the University of Wisconsin-River Falls in River Falls, Wisconsin, USA, and volunteered for participation in the study to gain a better understanding of their personal health. Volunteers filled out the IPAQ [9,10], SF-36 [11,12], and a medical questionnaire. One of the participants failed to return part of the paperwork. The final count on the sample size consisted of 13 males and 33 females with a mean age of 48 (+/-10.91) years.
IPAQ (International Physical Activity Questionnaire)
This is an instrument that is used to analyze physical activity data. It has been tested for use in adults ranging in age from 15- 69 years old [9,10]. The IPAQ assesses health-related physical activity through a set of four questionnaires. The IPAQ classifies the participant into one of three categories: inactive, minimally active, and HEPA (health enhancing physical activity) active. Category 1, inactive, is the very lowest level of physical activity and is considered “insufficiently active.” Category 2 is minimally active and are considered “sufficiently active.” To qualify for this category, the participant must meet one of these three criteria:
• 20 minutes per day of vigorous activity for 3 or more days OR.
• 30 minutes per day of moderate intensity activity or walking for 5 or more days OR.
• Achieving a minimum of at least 600 MET-min/week of any combination of vigorous intensity, moderate intensity, and walking for 5 or more days.
This category means that the participants were getting the minimum level of physical activity recommended by public health officials, but not enough for “Total Physical Activity (PA)” when all was considered. Category 3 is HEPA active, which means that these participants were more physically active, and surpassed the minimum amount of physical activity required to maintain a healthy lifestyle. There are two criteria to be classified as HEPA active:
• A minimum of at least 1500 MET-minutes/week of vigorous intensity on at least 3 days OR
• A minimum of at least 3000 MET-minutes/week of any combination of vigorous intensity, moderate intensity or walking on 7 or more days.
MET-min per week were calculated by: MET level x minutes of activity x events per week. Walking had a MET level of 3.3 METs. Moderate intensity had a MET level of 4.0 METS. Vigorous intensity had a MET level of 8.0 METs. Total MET-min/week = (Walk METs x min x days) + (Moderate METs x min x days) + (Vigorous METs x min x days).
SF-36
This is a health survey from the patient’s and/or client’s perspective [11,12]. It is composed of 36 questions to measure functional health and well-being. The scoring consists of 8 scales which consist of the weighted sums of the questions in each section. The scale is between 0-100 and the higher the number, the less disability, while the lower the number, the more disability. There are 8 categories that consist of: physical functioning, role limitations due to physical health, role limitations due to emotional problems, energy/fatigue, social functioning, pain, and general health. It is used to evaluate the participant’s health status.
Physical functioning measures limitations on ordinary physical activities such as climbing stairs, bending, lifting, or walking moderate distance. Role limitations due to physical health measures limitations caused by physical health problems on a person’s work function, while role limitations due to emotional stresses focuses solely on limitations from a person’s work functions due to mental health. Energy/fatigue includes energy and fatigue in measuring a person’s feeling of well-being. Emotional well-being assesses psychological well-being, loss of behavioral/emotional control, depression and anxiety. Social functioning measures the interaction with others and social relationships’ quality and quantity. Pain levels considers daily activities and the degree to which the severity of pain hinders daily activities. Lastly, general health assesses the current and prior physical health status.
Medical History Questionnaire
A medical questionnaire from the University of Wisconsin River Falls Fitness/Wellness Center was used to obtain the medical history, family history, and health history habits of each participant. The following items on the medical questionnaire were analyzed: age, family history, cigarette smoking, obesity, tension/anxiety, and sedentary lifestyle.
Body Composition & BMI
Body composition and BMI were measured via the COSMED Bod Pod (COSMED USA, Concord, CA). The Bod Pod was calibrated between participants fully and participants were instructed to wear tight fitting clothing in order to ensure accuracy.
Resting Blood Pressure
Blood pressure was taken using a standard sphygmomanometer and stethoscope per the guidelines established by the American College of Sports Medicine in 2018 [3].
Blood Lipid and Glucose Profile
Blood lipid and glucose profiles were measured using an Alere Cholestech LDX machine (Abbott Diagnostics, Livermore, CA). To maintain accuracy in the results, subjects were required to fast 12 hours prior to administration of the test but were instructed to consume ample water in order to maintain hydration.
Data Analysis
The investigation was a descriptive study where mean and standard deviation values for male and female cardiovascular disease risk factors, SF-36 scores and IPAQ physical activity levels were assessed. The data then compared specific standardized norms using one-way ANOVA. The Shapiro-Wilks test was run for normality while a Tukey-Kramer post hoc test was performed to assess honestly significant difference (HSD). An alpha value of .05 was used to determine significance.
The statistical analysis, JMP 13.1, SAS Institute Inc., was used. The National Health and Nutrition Examination Survey (NHANES) [13], 2013- 2014 data was used for comparing the university employee physiologic data to that of the general public. The U.S Department of Health and Human Services and U.S Department of Agriculture (USDA) recommendations were used for dietary recommendations [14].
Result
Participants
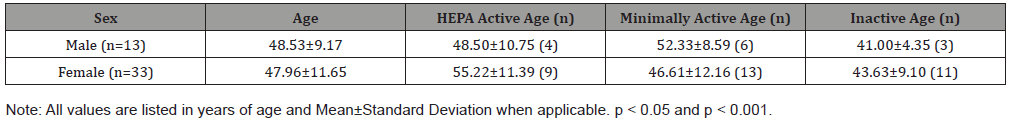
A sample of university employees was used for the purpose of this study. The sample consisted of 47 participants, 14 males (29.7%) and 33 females (70.3%), ranging in age from 26 to 65 years of age with a mean age of 46.15 + 11.17. All participants were employed by the University of Wisconsin-River Falls (UWRF) in River Falls, Wisconsin, USA. Out of the 47 subjects, 46 subjects turned in their health, SF-36, and IPAQ questionnaires (Table 1). Table 1shows that the participants were divided into three different categories depending on their IPAQ scores that determined their physical activity level.
Table 1:
SF-36
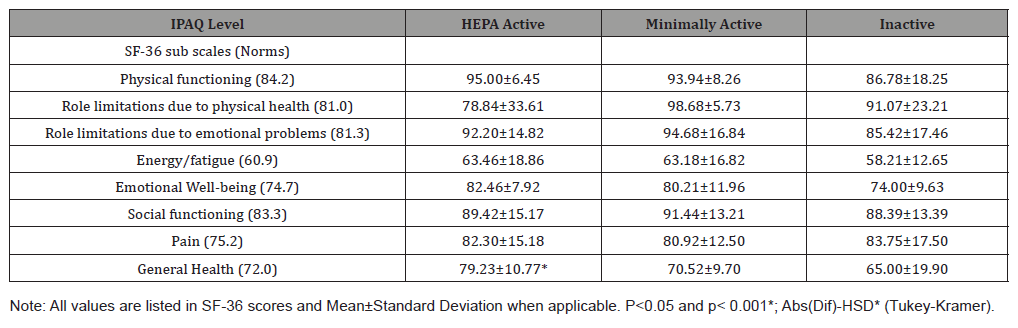
Table 2 represents the participants’ mean and standard deviation scores from their SF-36 test. The participants were divided into the three different levels of physical activity level according to their IPAQ scores. The study compared the participants’ SF-36 scores in the eight health categories: physical functioning, role limitations due to physical health, role limitations due to emotional problems, energy/fatigue, emotional well-being, social functioning, pain, and general health to the population norms. As a whole, the university employees scored higher in every health category than the population norms, except general health. There was a statistically significant difference between the HEPA active group and the minimally and inactive group when scoring general health (p = 0.0354). There was also statistically significant difference when combining HEPA and the minimally active group and then compared to the inactive group in emotional well-being (p = 0.0346) and physical functioning (p = 0.0498). It was trending in a positive direction that the HEPA active scored close to statistically significant when comparing role limitations due to physical health (p = 0.0558) (Table 2).
Table 2:
ACSM CVD Risk Factors
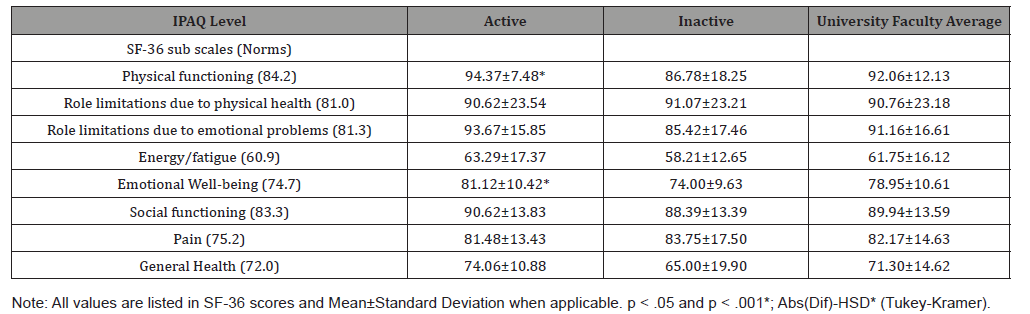
Table 3 shows the three different IPAQ levels and the mean number of ACSM CVD risk factors. There was no statistically significant difference between HEPA active (p = 0.1551) and the other IPAQ groups even when the HEPA active and minimally active groups were combined (p = 0.0530) for number of CVD risk factors. There were favorable trends with HEPA active in BMI scores (p = 0.0907) when compared to the other groups. When combining HEPA active and minimally active and comparing them to the inactive group, the active group had statistically significant difference in BMI scores (p = 0.0282) and had a favorable trend in body fat percentage (p = 0.0820) (Table 3).
Table 3:
IPAQ
Table 4 shows the mean and standard deviation on sitting time (hours). Research has shown that sitting time for “white-collar” work was around 6 hours a day, which follows the university employees average sitting time of 6.32 hours a day (60). Other research has shown that the average U.S. citizen is engaged in sedentary behaviors ≥9 hours of each day (61). The data shows sitting time had statistically significant difference between the HEPA active group when compared to the minimally active group and inactive group (p = 0.0111). The active group, HEPA and minimally active groups, was statistically significant different when comparing sitting time (hours) (p =0.0421) to the inactive group (Table 4).
Table 4:

MET-min/week levels
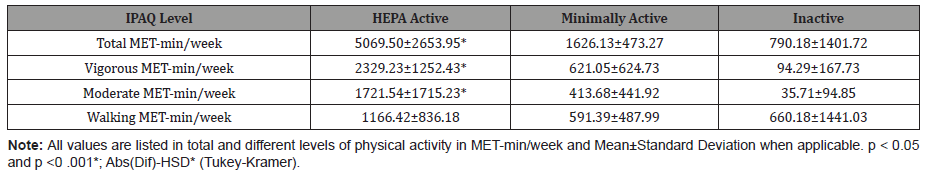
Table 5 shows the breakdown of Total MET-min/week among physical activity levels. The 2008 Physical Activity Guidelines for Americans state that 500-1,000 MET-min/week are needed to yield significant health benefits [10]. The data showed HEPA active group was significantly different among the inactive group and the minimally active group in Vigorous MET-min/week (p = 0.0001), Moderate MET-min/week (p = 0.0001), and Total MET-min/week (p = 0.0001) while walking showed no significant difference (p = 0.2288). The active group, HEPA and minimally active groups combined, were statistically significant different when compared to the inactive group for Vigorous MET-min/week (p = 0.0008), Moderate MET-min/week (p = 0.00124), and Total MET-min/week (p = 0.0024) while walking showed no significant difference (p = 0.6022). Table 6 represents this (Table 5,6).
Table 5:

Table 6:
Discussion
The purpose of this study was to assess the differences among physical activity levels to see if it affects quality of life and then compare them to national norms. This study also compared the different physical activity levels to the total number of CVD risk factors, according to ACSM criteria, and sitting time.
Quality of Life
The participants’ quality of life was measured using the SF-36, which is a 36-question test on one’s overall health status. It is a generic measure of health status which contains eight sub-scales ranging from physical capabilities to mental health.
For physical activity research, the SF-36 is the most widely used tool to measure health related quality of life [12]. A major public health service goal is to monitor the improvement of quality of life, according to the publication titled “Healthy People 2020” published by the United States Department of Health and Human Services [15]. A study by Hart and Kang [12]found that even across a wide variety of physical activity studies, the quality of life sub-scales remained reliable.
Kurklu S, et al. [16] measured different levels of physical activity on quality of life among health care workers in a training and research hospital. They used the IPAQ to determine levels of physical activity in the 120 hospital workers and found that the high physical activity group had statistically higher scores in total physical and mental health score (p < 0.001). Although not statistically significant, the researchers also found that the emotional well-being, pain levels, and energy/fatigue were higher in the high physical activity groups. Our study did not find the same statistically significant differences when comparing the IPAQ levels to SF-36 scores, but the results did follow a similar trend. Our study found that the active group (HEPA and minimally active groups combined) was statistically significantly higher when compared to the inactive group in emotional well-being (p = 0.0346). This trend could be attributed to the population having a better understanding that consistent physical activity not only improves physical health, but also can improve mental health.
Another investigation by Shibata Ai, et al. [17] looked at different levels of physical activity according to the IPAQ and compared that to the SF-36 scores. The researchers used 1,211 men and women who were middle-aged Japanese. They found that the recommended group was statistically significant higher for physical functioning (p < 0.05) when compared to the inactive group. The recommended group also had higher scores in general health (p <0.05) than the insufficient and inactive groups. These results were found to be similar to the current investigation in that the active group was statistically significantly higher in physical functioning (p = 0.0498) when compared to the inactive group. This trend could be explained through the physiological benefits that occur through physical activity. The active group could be benefiting from improved strength, endurance, and flexibility, which aid in physical functioning. Our study also showed that when scoring general health (p = 0.0354), the HEPA group scored statistically significantly higher when compared to the minimally and inactive groups. This trend could be explained through the improved overall health when an individual participates in regular physical activity. The benefits of exercise are even enhanced due to the consistency factor for the HEPA group.
In another study involving IPAQ and physical activity levels, Farid M, et al. [18] evaluated 360 Iranian women divided into the different IPAQ physical activity levels. Results showed that the high physical activity group had higher vitality scores (p = 0.01) and lower role limitations due to physical health scores (p = 0.02). They also found that the high physical activity group had higher scores in general health (p = 0.06) and mental well-being (p = 0.1). Our study followed this trend, but the results were not statistically significant. The active group scored favorably higher when compared to the inactive group in general health (p = 0.0520). The HEPA group had a lower score for role limitations due to physical health (p = 0.0558) when compared to the minimally and inactive groups. These results might possibly be explained by the faculty being able to move easier because they were consistently more physically active, and their bodies were more accustomed to the exercises.
CVD Risk Factors
The CVD risk factors, according to ACSM criteria are age, family history, cigarette smoking, sedentary lifestyle, obesity, hypertension, dyslipidemia, prediabetes, and HDL [3]. The studies used to guide this research used different criteria for CVD risk factors from other organizations, but they all were almost exact to the ACSM CVD risk factors definitions [3].
Ford E, et al. [19] looked at the occurrence of metabolic syndrome in the U.S. in an effort to provide treatment to prevent CVD. This study used the Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III) to define metabolic syndrome [20]. They define metabolic syndrome as three or more of these criteria: waist circumference greater than 102 cm (40 in) for men and 88 cm (35 in) for women, triglycerides ≥150 mg/dL-1, HDL less than 40 mg/dL-1 in men and 50 mg/dL-1 in women, glucose ≥110 mg/dL-1, and blood pressure greater than 130/85 mm Hg [20]. This study also used the Third National Health and Nutrition Examination Survey on 8,814 men and women aged 20 years and older. The results indicated that the prevalence of metabolic syndrome increased 6.7% among participants aged 20- 29 years old and 42% among participants aged 60-69 years old [19]. They concluded that about 47 million U.S. citizens have metabolic syndrome, which indicated there is a high prevalence of metabolic syndrome among U.S. adults [19]. A systematic review done by Saboya P, et al. [21] looked at the metabolic syndrome and quality of life. This review involved 30 studies and 62,063 participants, and it was found that the presence of metabolic syndrome is significantly related with lower quality of life [21].
Ko K, et al. [22] also found a connection between low physical activity and a greater chance for the development of metabolic syndrome. They examined 385 white-collar male workers and divided them into low, moderate, and high physical activity and used the U.S. national Cholesterol Education Program’s standard measure metabolic syndrome [20]. The results of this study found that the low physical activity group had significantly higher waist circumference and triglycerides than the moderate and high activity groups. The low physical activity group also had lower HDL than the other active groups. They concluded that there was a 2.03 higher chance for metabolic syndrome among the low physical activity groups as compared to the moderate and higher physical activity groups. It was concluded that among physically inactive whitecollar workers, they were more at risk for developing metabolic syndrome.
Our study found that there was no statistically significant difference in the number of CVD risk factors (p = 0.1551) between the participants when they were divided into HEPA active, minimally active, and inactive groups. However, there was a favorable trend (p = 0.0530) for fewer number of CVD risk factors for the active group (combining the HEPA and minimally active group), when compared to the inactive group. This study used the ACSM CVD risk factor criteria and compared the number of risk factors against IPAQ physical activity level scoring [3]. Although the designs from the other studies were different from ours, the trend still appeared. Our study followed the same tendency of university employees and white-collar workers having a risk of metabolic disease, which leads to CVD, and that the amount of physical activity can influence the number of CVD risk factors.
Sitting Time
A study by Bauman A, et al. [23] investigated the prevalence of sitting time of people across twenty countries using the IPAQ to measure sitting. This study involving 49,493 adults found that those who were classified as low physical activity were three times more likely to have higher sitting times. The same study also found that individuals with post-secondary education had higher sitting times than those with a high school diploma or less. Our study found a similar relationship when looking at the HEPA and active groups. The HEPA group, when compared to the minimally and inactive groups, had a greater statistically significant difference in sitting time (p = 0.0111). When comparing the active group to the inactive group, the active group had a greater statistical difference in sitting time (p = 0.0421). This could be attributed to the physically active subjects having built a routine of exercising, and therefore, had less overall sitting time.
Another study looking at the association between physical activity, sitting time and risk factors was performed by Chue and May [24]. The study with 686 Malay men and women were asked to fill out the fill out the IPAQ. The criteria used to determine metabolic disease was from the NCEP ATP III (Third Report of the National Cholesterol Education Program Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults) [20]. This study revealed that the odds for metabolic risk factors among middle-aged adults in Malaysia increased with the higher sitting times and physical inactivity [24].
BMI
Ul-Haq Z et al. [25] did a meta-analysis on the association between obesity and quality of life using the SF-36. The researchers using 8 studies with 43,086 participants reported that adults with higher than normal BMIs had significantly lower quality of life scores. They saw that any obese participant had lower physical and mental quality of life scores [25]. Consequently, this study did not find any statistically significant difference in the SF-36 scores between the obese and non-obese, according to ACSM (≥30 BMI), however, there were some favorable positive trends in physical functioning (p = 0.0579) and general health (p = 0.1147) for the non-obese.
In a study looking at whether BMI is associated with quality of life, Derraik J, et al. [26] looked at 38 men from New Zealand who were between the ages of 45.9±5.4 and with BMI’s between 25-30 kg/m2, and used the SF-36 to measure quality of life. The participants filled out the SF-36 at baseline, 12, and 30 weeks, and were told to make no changes to their daily lives in terms of diet and activity. The results showed that an increasing BMI led to lower scores in general health (p = 0.036), physical functioning (p = 0.024), and bodily pain (p = 0.030). The researchers also found lower scores in physical functioning (p = 0.040), bodily pain (p = 0.044, and general health (p = 0.073) in the participants who were overweight (n = 19; BMI 27.5-30 kg/m2), compared to those who were less overweight (n = 19; BMI 25-27 kg/m2). This investigation concluded that increasing BMI is associated with a gradual reduction in quality of life [26]. This could possibly be attributed to individuals increasing fat mass which lead to health complications and limited mobility, which lowered the overall quality of life score in the SF-36.
Onge St, et al. [27] also studied BMI and its possible link with metabolic syndrome. The research team used the Third National Health and Nutrition Examination Survey and the National Cholesterol Education Program Adult Treatment Panel III criteria for the definition of metabolic disease in their study of 7,602 participants [13,20]. Their results showed that the incidence of metabolic syndrome increased with higher BMI’s. Participants near or over the BMI range had a greater chance of having metabolic syndrome. This study found that when dividing the participants into obese and non-obese categories according to ACSM (>30 BMI), the non-obese group had a statistically significant difference in fewer number of total CVD risk factors (p = 0.0005). Our study using ACSM criteria to define obesity, also showed results similar to St-Onge et al in that the non-obese group had fewer CVD risk factors, resulting in a decreased chance of developing CVD. This could be attributed to the understanding that excess weight is the first indicator to developing NCD. Maintaining a healthy body weight can decrease the chances of an individual developing hypertension and diabetes.
Conclusion
While some of the variables in this study appeared to be significantly under control within the sample’s current lifestyles, some fell short. These short comings may be due to lifestyle or genetic factors. In future investigations, it would be informative to study lifestyle choices via survey to determine the reason behind these results. In addition, it would be of interest to survey for past medical history, tobacco use, and physical activity level to more fully determine total risk for cardiovascular disease among this population.
Acknowledgement
No.
Conflict of Interest
Authors declare no conflict of interest.
References
- (2017) Non communicable Diseases and their Risk Factors. World Health Organization.
- Lars Bo Andersen, Jorge Mota, Loretta Di Pietro (2016) Update on the Global Pandemic of Physical Inactivity. Lancet 388(10051): 1255-1256.
- (2018) ACSM's Guidelines for Exercise Testing and Prescription, (10th Edn). In: Wolters Kluwer/Lippincott Williams & Wilkins Health, Philadelphia, pp. 309.
- (2017) Translating Scientific Evidence About Total Amount and Intensity of Physical Activity into Guidelines. health.gov.
- MA Pereira, AM Kriska, VR Collins, GK Dowse, J Tuomilehto, et al. (1998) Occupational status and cardiovascular disease risk factors in the rapidly developing, high-risk population of Mauritius. Am J Epidemiol 148(2): 148-159.
- Einar Thornór Thornórarinsson, Thornórður Harðarson, Helgi Sigvaldason, Nikulás Sigfússon (2002) The relationship between educational level, physical activity and mortality. Læknablađiđ 88(6): 497-502.
- Corneel Vandelanotte, Mitch J Duncan, Camille Short, Matthew Rockloff, Kevin Ronan, et al. (2013) Associations between occupational indicators and total, work-based and leisure-time sitting: A cross-sectional study. BMC Public Health 13: 1110.
- Timothy S Church, Diana M Thomas, Catrine Tudor-Locke, Peter T Katzmarzyk, Conrad P Earnest, et al. (2011) Trends over 5 Decades in U.S. Occupation-Related Physical Activity and Their Associations with Obesity. PLoS One 6(5): e19657.
- Maria Hagströmer 1, Pekka Oja, Michael Sjöström (2006) The International Physical Activity Questionnaire (IPAQ): A study of concurrent and construct validity. Public Health Nutr 9(6): 755-762.
- Cora L Craig, Alison L Marshall, Michael Sjöström, Adrian E Bauman, Michael L Booth, et al. (2003) International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35(8): 1381-1395.
- J E Ware Jr, B Gandek (1998) Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J Clin Epidemiol 51(11): 903-912.
- Hart PD, Kang M (2015) Reliability of the Short-Form Health Survey (SF-36) in Physical Activity Research Using Meta-Analysis. World Journal of Preventive Medicine 3(2): 17-23.
- (2016) The National Center for Health Statistics, Division of Health and Nutrition Examination Surveys, and Centers for Disease Control and Prevention. NHANES. Centers for Disease Control and Prevention.
- (2015) US Department of Health and Human Services and U.S. Department of Agriculture. In: 2015-2020 Dietary Guidelines for Americans (8th Edn).
- (2011) United States Department of Health & Human Services.
- Kurklu S, Babayigit MA, Oysul FG, Aktas AM (2015) Examination of Possible Effects of Physical Activity Level (IPAQ) on Quality of Life (SF-36) in Health Care Workers Who Employed in a Training and Research Hospital. SM Journal of Public Health & Epidemiology, 1(2): 01.
- Ai Shibata, Koichiro Oka, Yoshio Nakamura, Isao Muraoka (2007) Recommended Level of Physical Activity and Health-related Quality of Life among Japanese Adults. Health Quality Life Outcomes 5: 64.
- Maliheh Farid, Soheila Dabiran (2012) Health-related Quality of Life in Iranian Women with Different Levels of Physical Activity. Asian J Sports Med 3(3): 203-207.
- Earl S Ford, Wayne H Giles, William H Dietz (2002) Prevalence of the Metabolic Syndrome among US Adults: Findings from the Third National Health and Nutrition Examination Survey. JAMA 287(3): 356-359.
- (2013) Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III Final Report). National Cholesterol Education Program (NCEP), National Institute of Health.
- Saboya Patrícia Pozas, Luiz Carlos Bodanese, Paulo Roberto Zimmermann, Andréia Da Silva Gustavo, Caroline Melo Assumpção, et al. (2016) Metabolic Syndrome and Quality of Life: A Systematic Review. Rev Lat Am De Enfermgem 24: e2848.
- Ko Kwang Jun, Eon Ho Kim, Un Hyo Baek, Zhao Gang, Seol Jung Kang (2016) The Relationship between Physical Activity Levels and Metabolic Syndrome in Male White-collar Workers. J Phys Ther Sci 28(11): 3041-3046.
- Adrian Bauman, Barbara E Ainsworth, James F Sallis, Maria Hagströmer, Cora L Craig, et al. (2011) The Descriptive Epidemiology of Sitting: A 20-Country Comparison Using the International Physical Activity Questionnaire (IPAQ). Am J Prev Med 41(2): 228-325.
- Anne H Y Chu, Foong Ming Moy (2013) Joint Association of Sitting Time and Physical Activity with Metabolic Risk Factors among Middle-Aged Malays in a Developing Country: A Cross-Sectional Study. PLoS One 8(4): e61723.
- Zia Ul-Haq, Daniel F Mackay, Elisabeth Fenwick, Jill P Pell (2013) Meta-Analysis of the Association Between Body Mass Index and Health-Related Quality of Life Among Adults Assessed by the SF-36. Obesity (Silver Spring) 21(3): E322-327.
- José G B Derraik, Martin de Bock, Paul L Hofman, Wayne S Cutfield (2014) Increasing BMI Is Associated with a Progressive Reduction in Physical Quality of Life among Overweight Middle-aged Men. Sci Rep 4: 3677.
- Marie-Pierre St-Onge, Ian Janssen, Steven B Heymsfield (2004) Metabolic Syndrome in Normal-weight Americans: New Definition of the Metabolically Obese, Normal-weight Individual. Diabetes Care 27(9): 2222-2228.
-
Gustavo Mendoza, Kenneth R Ecker. Quality of Life Improved by Adequate Physical Activity Levels Among University Employees. On J Complement & Alt Med. 6(2): 2021. OJCAM.MS.ID.000634.
-
Quality of life, Body mass index, Cardiovascular disease, Physical activity, Emotional well-being, Physiology, Chronic diseases, Hypertension, Prediabetes, Dyslipidemia, Diet Obesity
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






