 Research Article
Research Article
Drug-Drug Interactions in Common Mental Disorders Patients in Primary Health Care
Tatiana Longo Borges1*, Adriana Inocenti Miasso2, Kelly Graziani Giacchero Vedana2, Ellen Cristina Dias Castilho2 and Leonardo Moscovici3
1Postdoctoral Fellowship at Escola de Enfermagem de Ribeirão Preto da Universidade de São Paulo, Brazil
2Doctor Professor at Escola de Enfermagem de Ribeirão Preto da Universidade de São Paulo, Brazil
3Family Doctor and associate professor at Centro Universitário Estácio de Ribeirão Preto, Brazil
Tatiana Longo Borges, University of São Paulo at Ribeirão Preto College of Nursing, Avenida Bandeirantes 3900, Monte Alegre, Ribeirão Preto, São Paulo, Brazil.
Received Date:October 17, 2022; Published Date:February 14, 2023
Abstract
Objectives: to compare the frequency and characterization of potential drug-drug interactions among patients with and without common mental disorders, to verify whether there is correlation of the CMD with potential DDI, demographic, use of psychoactive drugs, number of medications prescribed and severity of potential DDIs. Quantitative, cross-sectional and descriptive correlational study carried primary health care services in Brazil. Among 430 patients interviewed, 190 had more than two medications prescribed. Univariate and multivariate analysis having CMD as dependent variable. 58.4% had DDI and 53.1% had CMD. Associations were found between age, monthly income psychotropic medication and severity of DDI with CMD.
Keywords:Drug interactions; Mental health; Patient safety; Primary health care
Background
Prevalence studies in Brazil show that around 50% of people attended in Primary Health Care (PHC) have Common Mental Disorders (CMD) [1,2]. CMD include patients with anxious, depressive or somatoform symptoms that do not reach diagnostic criteria for mental disorder. They show a high prevalence in the adult population worldwide, particularly in primary care [3]. It is important to highlight that in Brazil PHC is responsible to deal with emotional complaints, including CMD.
The high prevalence of CMD in PHC leads not only to psychotropic prescription, but also to other medications in general [1]. CMD patients have more medications prescribed than those with no mental disorder [1], increasing the chance of potential drug-drug interactions (DDI). According to the World Health Organization, DDI refers to the interference of one drug in the action of another or of a drug in relation to a nutrient [4].
Particularly interesting to this study, DDI occur when the effects of one drug are modified by the concomitant or subsequent administration of another drug [5]. Such interactions are risk factors for the occurrence of adverse drug reactions, which affect patient safety in treatment [6]. Moreover, DDI are a significant cause of medication-related problems [7]. They are also associated to higher length of stay and cost of hospitalization [8]. High-risk interactions occur mostly with drugs with a narrow therapeutic index, which includes many psychotropic medications [9]. Therefore, PHC plays an important role in Brazilian health assistance to CMD patients. However, there are few studies regarding DDI in CMD patients in PHC settings [1,2].
Considering the relevance of such context to patient safety and nursing practice, the objectives of this study were: to compare the frequency and characterization of potential DDI among patients with and without CMD, and to verify whether there is correlation of CMD with potential DDI, sociodemographic characteristics, use of psychoactive drugs, number of medications prescribed and severity of potential DDI.
Method
Design
This is a cross-sectional and descriptive correlational study carried out in five PHC services in the city of Ribeirão Preto, an urban centre in the state of São Paulo, Brazil. The health units with largest number of people in each health sector of the city were selected (the city is subdivided by the Municipal Health Department in five health sectors). PHC services in Brazil are the gateway for patients in the Unified Health System (UHS - in portuguese Sistema Único de Saúde - SUS), a public health system used by the majority of the Brazilian population. Medications are also distributed by the UHS. For this, there is a range of standardized medicines that may be prescribed for patients [10]. Inclusion criteria were: i) 18 years of age or older; ii) being able to communicate verbally in Portuguese, and iii) to have had a medical appointment in the health services during data collection period.
Sample
We established a stratified sampling plan with proportional allocation by strata. Each stratum resembles to one of the selected health services. For sample calculation we used an estimated prevalence of 50% of CMD in order to assure the largest sample size to include the estimation of DDI, (since prevalence observed in literature is less than 50%). The tolerable sampling error was 5% and the significance level was 5%. A non-response rate of 15% was added, resulting in a sample size of 430 subjects.
The variables selected for this study are those that may be related to the presence of CMD and DDI, according to available literature [8]. The presence of potential CMD was considered as a dependent variable. The independent variables were those related to the sociodemographic data (age, sex, marital status, income and occupation), pharmacotherapeutic profile (number and type of medications) and use of psychotropic medication and DDI.
Compliance with Ethical Standards
Disclosure of potential conflicts of interest: the authors report no conflicts of interest in this work.
Research involving Human Participants and/or Animals: the present study follows Brazilian guidance for research with human beings and had approval of the Research Ethics Committee of Ribeirão Preto Nursing College – University of São Paulo (Protocol number: 1474/2011). Informed consent: participants were asked to sign the informed consent term(s), in accordance with Resolution 466/12 of the National Health Council of Brazil.
Procedures
For data collection researchers stayed in the health services during all day (5 days a week), so it was possible to comprise the units’ operating hours. Data collection was guided by a structured questionnaire with questions about sociodemographic profile, pharmacotherapeutics and presence of clinical disease. Subsequently, the patient records were checked. It was performed a pretest to verify if contents and the form of the questionnaire would reach the desired objectives. The patients interviewed were not part of the sample. The questionnaire addressed patient’s medication(s) prescription and its classification, potential DDI and its severity.
In order to the medication classification, it was consulted the drug database, available on the National Health Surveillance Agency website. The classification of drugs was drawn up as a polytomous categorical variable, i.e., referred to the anatomical and therapeutic groups classification anatomical therapeutic chemical – ATC [11]. To confirm potential occurrence of DDI it was utilized the Drug- Reax Thompson Healthcare System program. Drug-Reax System has been seen as adequate to detect DDI and is useful in scientific research and care practice [12].
The Drug-Reax and Martindale are part of the Micromedex Healthcare Series edited by Thompson Healthcare, whose access is available in the journal´s portal of Coordination for the Improvement of Higher Education Personnel– CAPES. To estimate CMD we used the validated Brazilian version of the Self-Reporting Questionnaire–20 (SRQ-20). Cut-off points recommended by the SRQ-20 validation were female patients eight or more questions answered with ‘yes’; and males six or more questions “yes” (Mari & Williams, 1986).
Statistics
A quantitative approach was applied through the Statistical Package for the Social Sciences (SPSS, version 17.0). Statistical associations were investigated between the dependent variable or response (CMD) and the independent variables using the chisquare test. The association hypothesis was accepted when p value was less than or equal to 0.05. Then logistic regression models were generated in order to verify the impact of the independent variables on the dependent variable (CMD). We included in the models all explanatory variables with univariate analysis p <0.05 and variables that were strongly correlated to the dependent variables, according to the literature. Multicollinearity tests were performed and variables with problems on this were not included. The adequacy of the models was verified by the Hosmer–Lemeshow test.
Results
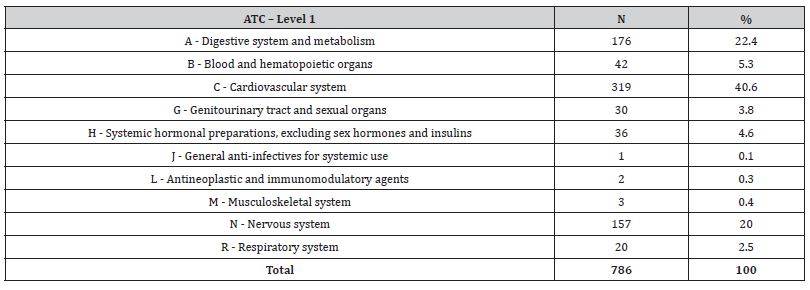
Of all the participants interviewed 283, medication used for the cardiovascular system (ATC C) made up 40.6% of the total drugs prescribed, followed by medication for the digestive system and metabolism (ATC A – 22.4%) and medication for the nervous system, i.e., psychotropic, (ATC N – 20%). For prevalence of the type of medication according to ATC classification please refer to Table [1].
Table 1: Distribution of drugs prescribed for study participants (N = 786), according to the Anatomical Classification Therapeutic Chemical (ATC).
The sample considered for the analysis of DDI was 190, since this was the number of users with two or more types of medication. In this sample Table [2]. CMD prevalence was 53.15% and DDI prevalence was 58.4%. The sample was composed mainly by women (80.53%), aged between 41 and 59 years (47.37%), up to four years of schooling (65.79%), monthly income of up to three minimum wages (59.47%), and using psychoactive drugs (52.63%). It is worth to note that 92.45% of patients who make use of five or more types of medication had DDI in their prescriptions.
Table 2: Distribution of medication users (n = 190) treated at primary health care according to sociodemographic variables, number of medication classes, drug-drug interaction and results of the Self-Report Questionnaire-20 (SRQ-20).
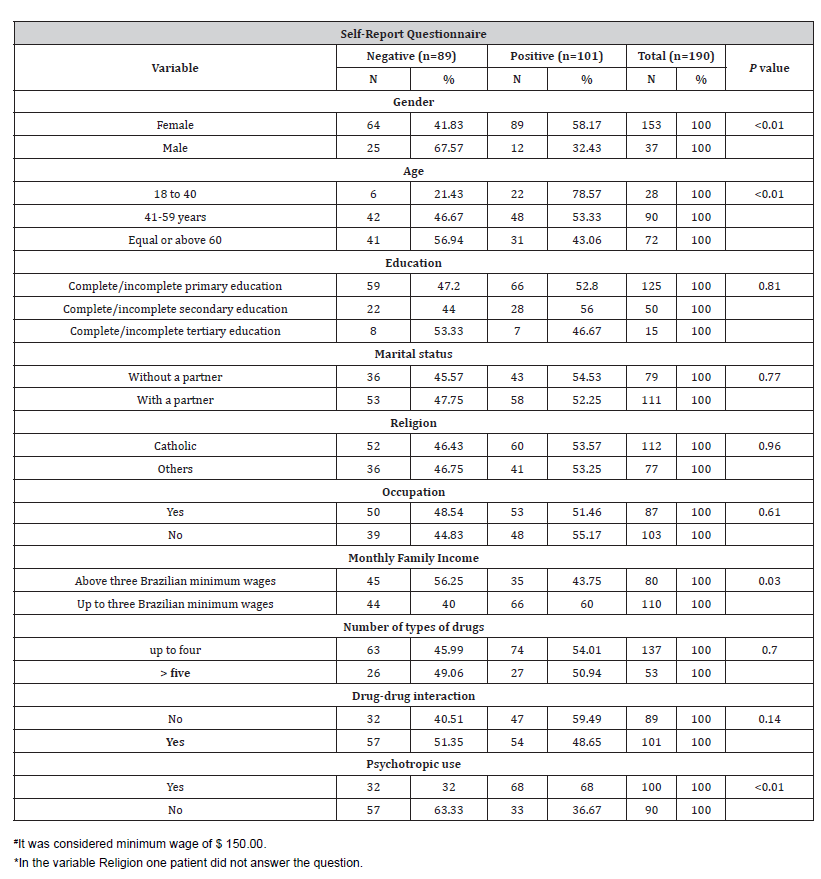
The factors associated with CMD in the univariate analysis were gender (p<0.01), where 58.17% of the women respondents were positive to CMD; age (p<0.01); monthly income (p=0.03) and psychotropic use (P<0.01).
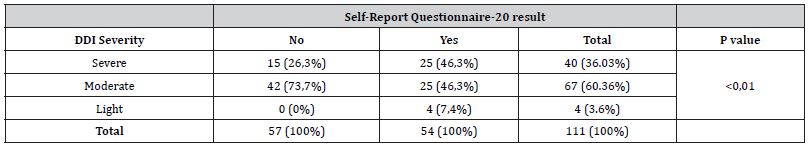
Among the patients evaluated 36% had severe DDI in their prescription. Severity of DDI was associated in the univariate analysis to CMD, 60.36% moderate. Patients positive to CMD presented more severe DDI (46.3%) in comparison to those negative (26.3%) (p<0.01) Table [3].
Table 3: Distribution of patients treated at PHC according to presenting potential DDI classified for severity according to Self-Report Questionnaire-20 result (n = 190).
Potential for severe DDI with a frequency of more than or equal to two can be seen in Table [4]. Nine types of severe DDI were identified as being present in 32 prescriptions. It is worth noting that most of them involved psychotropic drugs (71.9%) and most of the severe DDI were in CMD positive patients (62.5%).
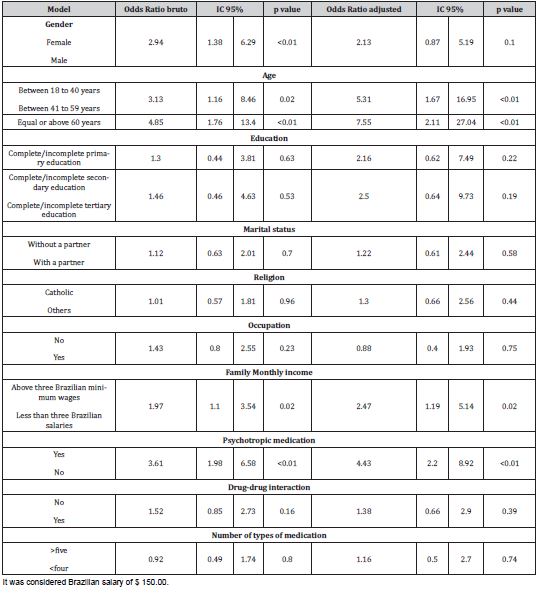
In Table [5] we show final logistic regression model for predicting potential DDI in the sample. Only the variable number of types of drugs contributed significantly to the model (p<0.05).
Table 4: Distribution of potentially severe drug interactions with greater absolute frequency equal or more than two, detected in patients (n = 190) treated in primary health care according to Common Mental Disorders.
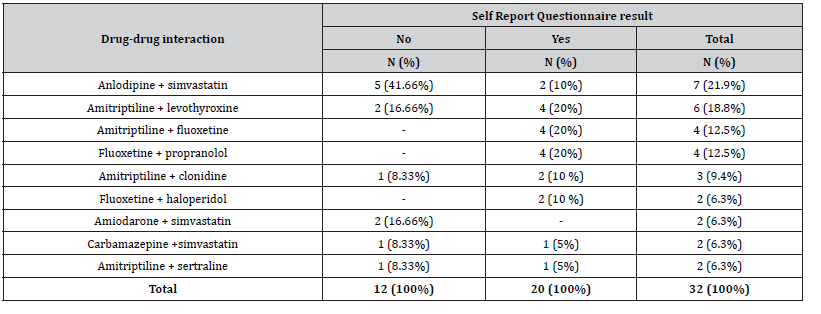
Table 5: Final model of logistic regression for occurrence of CMD in medication patients treated in PHC (n = 190).
Discussion
Regarding CMD prevalence, 53.15% of the individuals were positive. The prevalence rates of CMD observed in this study are similar to those found in Brazil and worldwide, which ranges from 15% to 50.3% [13-18]. It is important to acknowledge these high rates of mental health issues, especially as more countries are moving to PHC as the entry into the health system [19,20].
In this sense, several authors propose that there is a relation between CMD and sociodemographic factors, since these factors would act as stress inductors, increasing chances of psychological vulnerability [15]. In developing countries, including Brazil, it is especially relevant, since the population is more vulnerable to economic and social instability [21]. This supposition is supported by the results found in the univariate analyses of this study, which it found an association between CMD and gender, age and monthly income. However, in the multivariate analysis, only age remained as an associated factor to CMD.
Mental health may correspond up to half of the workload in PHC. Therefore, innovative models of care, such as shared care or collaborative care or matrix support, should be developed to deal with these figures.
Regarding DDI, main objective of this study, a prevalence of 58.4% was found. Determining prevalence of DDI in PHC is important, since literature has few studies on this topic. Moreover, it is crucial to recognize DDI in CMD patients. In Brazil, evidence shows prevalence of DDI varies from 22 to 37% in hospital settings [8]. Interestingly - but not a surprise - it seems prevalence of DDI in PHC of developing countries tends to be higher. For instance, research from Saudi Arabia found a prevalence of 52.5%, similar to the present study [22].
Regarding the nursing practice, 28% of the patients presented severe potential DDI in their prescription. Moderate DDI was observed in 62.9% of all prescriptions. Prevalence found in the present research was higher than those reported in the literature of hospital settings [23,24]. Studies involving DDI in PHC are scarce. However, previous research in Brazil showed a 3.2% prevalence of incidents related to severe DDI [25]. Thus, this study represents a pioneer contribution about DDI.
Considering the objectives of this study, CMD patients showed more psychotropic medication prescribed compared to patients without CMD, which was confirmed through univariate and multivariate analysis. In this sense, studies have reported the high use of psychotropic medications among CMD patients in PHC [26]. Also, we found 71.9% of DDI involving psychotropic medication. This fact strengthens the proposition that these medications are usually overprescribed in PHC. However, these prescriptions seem not connected to the substantial necessities of patients [27,28].
There are few health services specialized in mental health, and they are aimed to severe patients. CMD patients are supposed to have their problem dealt in PHC, that is, usually without any other kind of mental health intervention. Psychotropic prescription remains the most important therapetics. In fact, literature evidence shows inconsistency in psychotropic prescription in PHC. A few factors seem to influence it, such as media influence, limited access to other therapeutic alternatives, and lack of updated knowledge and on medication [27].
However, in respect to the association between CMD and DDI, the hypothesis of this study, was not supported by our findings. On the other hand, and hugely relevant, it is the fact that severity of DDI was associated with CMD in the univariate analysis, showing that, although in this sample CMD patients did not present more DDI than CMD negative patients, they are more exposed to risk. The severity of DDI could not have odds ratios calculated because of monotonous likelihood function problems, complete and nearcomplete separation cases [29].
In this context, reducing psychotropic prescription would be important to ensure medication safety, since it would decrease chances of DDI. Nurses as health professionals, together with family doctors and general practitioners, should balance the benefits and harms of psychotropic medication. Also taking into account the patient’s preferences [6]. Another alternative could be to set psychoeducation groups or brief psychological approaches [2].
Regarding the subclasses of psychotropic prescribed, it is worth bringing up some considerations. The widely held of DDI involving psychotropics had amitriptyline, a tricyclic antidepressant that interacts with many kinds of medications. Brazilian evidence observed that this is one of the most common DDI with high clinical relevance [30].
Levothyroxine can potentiate the antidepressant effect of amitriptyline. It is known that this effect may be helpful in thyroid diseases, since these patients may be resistant to therapeutic action of amitriptyline (Cooper & Lerer, 2004). But it may contribute to adverse events, such as central nervous system depression, dizziness, gastrointestinal symptoms, etc. [31]. Clonidine prescribed together with amitriptyline could lead to decrease sensitivity of adrenoreceptors [32], influencing the effect of clonidine on blood pressure [31].
The results highlight the risk for DDI in patients with CMD. They emphasize the urgent need of investigation and implementation of strategies aiming this particular population. Some actions may include computerized resources [33], clinical pharmacist interventions [34-37] and improvement of professional training.
Although the results identified in this study have important relevance to CMD patients and medication in PHC, there is an inherent limitation, due to the transversal nature of this study. It was possible to identify potential DDI. However, we were unable to verify whether these DDIs resulted in adverse events in all patients exposed. The software used in this study to detect potential DDI is widely utilized and validated. Despite that, the present study is pioneer to contribute to knowledge of DDI in CMD patients in the PHC setting.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgment
None.
- Borges TL, Miasso AI, Vedana KGG, Telles-Filho PCP, Hegadoren KM, et al. (2015) Prevalence in the use of psychotropics and associated factors in primary health care. Acta Paulista Enferm 28(4): 344-349.
- Fortes S, Lopes CS, Villano LAB, Campos MR, Gonçalves DA, et al. (2011) Common mental disorders in Petrópolis-RJ: A challenge to integrate mental health into primary care strategies. Rev Bras Psiquiatr 33(2): 150-156.
- Bener A, Dafeeah EE, Chaturvedi SK, Bhugra D (2013) Somatic symptoms in primary care and psychological comorbidities in Qatar: Neglected burden of disease. Int Rev Psychiatry 25(1): 100-106.
- (2006) World Health Organization. WHO Model Formulary 2006. Geneva: World Health Organization.
- Sinclair LI, Davies SJ, Parton G, Potokar JP (2010) Drug–drug interactions in general hospital and psychiatric hospital in-patients prescribed psychotropic medications. Int J Psychiatry Clin Pract 14(3): 212-219.
- Marengoni A, Onder G (2015) Guidelines, polypharmacy, and drug–drug interactions in patients with multimorbidity. BMJ 350: h1059.
- Sánchez-Fidalgo S, Guzmán-Ramos MI, Galván-Banqueri M, Bernabeu-Wittel M, Santos-Ramos B, et al. (2017) Prevalence of drug interactions in elderly patients with multimorbidity in primary care. Int J Clin Pharm 39(2): 343-353.
- Moura C, Acurcio F, Belo N (2009) Drug-drug interactions associated with length of stay and cost of hospitalization. J Pharm Pharmaceut Sci 12(3): 266-272.
- Moura CS, Ribeiro AQ, Magalhães SMM (2007) Avaliação de interações medicamentosas potenciais em prescrições médicas do Hospital das Clínicas da Universidade Federal de Minas Gerais (Brasil). Lat Am J Pharm 26(4): 596-601.
- Paim J, Travassos C, Almeida C, Bahia L, Macinko J, et al. (2011) The Brazilian health system: history, advances, and challenges. The Lancet – Series: Health in Brazil 377(9779): 1778-1797.
- (2015) World Health Organization. Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC classification and DDD assignment: 2016. Oslo.
- Oesterhus R, Aarsland D, Soennesyn H, Rongve A, Selbaek G, et al. (2017) Potentially inappropriate medications and drug–drug interactions in home-dwelling people with mil dementia. International Journal of Geriatric Psychiatry 32(2): 183-192.
- Telles Filho PP, Borges TL, Pereira Júnior AC, Vedana KGG, Shasanmi RO, et al. (2017) Factors Associated with Psychotropic Medication Use in Individuals in Brazilian Primary Health Care Units: A Descriptive, Cross-Sectional Study. Journal of Psychosocial Nursing and Mental Health Services 55(3): 38-45.
- Aillon JL, Ndetei DM, Khasakhala L, Ngari WN, Achola HO, et al. (2014) Prevalence, types and comorbidity of mental disorders in a Kenyan primary health centre. Soc Psychiatry Psychiatr Epidemiol 49(8): 1257-1268.
- Ghuloum S, Bener A, Abou-Saleh MT (2011) Prevalence of mental disorders in adult population attending primary health care setting in Qatari population. J Pak Med Assoc 61(3): 216-221.
- Kasckow JW, Karp JF, Whyte E, Butters M, Brown C, Begley A, et al. (2013) Subsyndromal depression and anxiety in older adults: health related, functional, cognitive and diagnostic implications. J Psychiatr Res 47(5): 599-603.
- Kroenke K, Outcalt S, Krebs E, Bair MJ, Wu J, et al. (2013) Association between anxiety, health-related quality of life and functional impairment in primary care patients with chronic pain. Gen Hosp Psychiatry 35(4): 359-365.
- Löwe B, Spitzer RL, Williams JB, Mussell M, Schellberg D, et al. (2008) Depression, anxiety and somatization in primary care: syndrome overlap and functional impairment. Gen Hosp Psychiatry 30(3): 191-199.
- Moffitt TE, Caspi A, Taylor A, Kokaua J, Milne BJ, Polanczyk G, et al. (2010) How common are common mental disorders? Evidence that lifetime prevalence rates are doubled by prospective versus retrospective ascertainment. Psychol Med 40(6): 899-909.
- Strachan J, Yellowlees G, Quigley A (2015) General practitioners’ assessment of, and treatment decisions regarding, common mental disorder in older adults: thematic analysis of interview data. Ageing and Society 35(1): 150-68.
- Patel V, Eaton J (2010) Principles to guide mental health policies in low- and middle-income countries. Revista Brasileira de Psiquiatria 32(4): 345-346.
- Odhayani AA, Tourkmani A, Alshehri M, Alqahtani H, Mishriky A, et al. (2017) Potentially inappropriate medications prescribed for elderly patients through family physicians. Saudi Journal of Biological Sciences 24(1): 200-207.
- Gotardelo DR, Fonseca LS, Masson ER, Lopes LN, Toledo VN, et al. (2014) Prevalence and factors associated with potential drug interactions among elderly in a population-based study. Rev Bras Med Família Comunidade 9: 111-118.
- Juárez-Cedillo T, Martinez-Hernández C, Hérnandez-Constatino A, Garcia-Cruz JC, Avalos-Mejia AM, et al. (2016) Clinical weighting of drug–drug interactions in hospitalized elderly. Basic & Clinical Pharmacology & Toxicology 118(4): 298-305.
- Leão DF, Moura CS, Medeiros DS (2014) Evaluation of potential drug interactions in primary health care prescriptions in Vitória da Conquista, Bahia (Brazil). Ciência & Saúde Coletiva 19(1): 311-318.
- Gonçalves DA, Mari JJ, Bower P, Gask L, Dowrick C, et al. (2014) Brazilian multicentre study of common mental disorders in primary care: Rates and related social and demographic factors. Cad Saúde Pública 30(3): 623-632.
- Hedenrud TM, Svensson SA, Wallerstedt SM (2013) ‘Psychiatry is not a science like others’: A focus group study on psychotropic prescribing in primary care. BMC Family Practice 14: 115.
- Sirdifield C, Anthierens S, Creupelandt H, Chipchase SY, Christiaens T, et al. (2013) General practitioners’ experiences and perceptions of benzodiazepine prescribing: Systematic review and meta-synthesis. BMC Fam Pract 14: 191.
- Albert A, Anderson JÁ (1984) On the existence of maximum likelihood estimates in logistic regression models. Biometrika 71:1-10.
- Neto PRO, Nobili A, Marusic S, Pilger D, Guidoni CM, et al. (2012) Prevalence and predictors of potential drug–drug interactions in the elderly: A cross-sectional study in the Brazilian primary public health system. J Pharm Pharmaceut Sci 15(2): 344-354.
- Andrade MF, Andrade RCG, Santos V (2004) Psychotropic prescription: the evaluation of related directions and notifications. Rev Bras Cienc Farm 4(40): 471-79.
- Dilsaver SC, Davidson R, Majchrzak M (1989) Chronic treatment with amitriptyline produces sub sensitivity to the hypothermic effects of clonidine. Prog Neuropsychopharmacol Biol Psychiatry 13(1-2): 297-302.
- Missiakos O, Baysari MT, Day RO (2015) Identifying effective computerized strategies to prevent drug-drug interactions in hospital: A user-centered approach. Int J Med Inform 84(8): 595-600.
- C Lopez-Martin, Garrido Siles M, Alcaide-Garcia J, Faus Felipe V (2014) Role of clinical pharmacists to prevent drug interactions in cancer outpatients: a single-centre experience. Int J Clin Pharm 36(6):1251-1259.
- Lao CK, Ho SC, Chan KK, Tou CF, Tong HH, et al. (2013) Potentially inappropriate prescribing and drug–drug interactions among elderly Chinese nursing home residents in Macao. International Journal of Clinical Pharmacy 35(5): 805-812.
- Lea M, Rognan SE, Koristovic R, Wyller TB, Molden E, et al. (2013) Severity and management of drug–drug interactions in acute geriatric patients. Drugs & Aging 30(9): 721-727.
- Teixeira JJV, Crozatti MTL, Santos CA, Romano-Lieber NS (2012) Potential drug–drug interactions in prescriptions to patients over 45 years of age in primary care, Southern Brazil. PLoS One 7(10):
-
Tatiana Longo Borges*, Adriana Inocenti Miasso, Kelly Graziani Giacchero Vedana, Ellen Cristina Dias Castilho and Leonardo Moscovici. Drug-Drug Interactions in Common Mental Disorders Patients in Primary Health Care. On J Complement & Alt Med. 8(2): 2023. OJCAM.MS.ID.000683.
-
Drug interactions, Mental health, Patient safety, Primary health care; Mental Disorders Patients; Psychoactive drugs; Psychotropic medication; Mental disorder
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






