 Research Article
Research Article
Blood Morphology and Antioxidant Status of Broiler Fed Rhizopus Stolonifer-Detheobromized Cocoa Pod Husk Meal
Olawale Mojeed Akanb*, Johnson Olusola Agbede, Akinlolu Olufemi Ayeni, Muyiwa Adegbenro and Olayemi Aanuoluwapo Olugosi
The Federal University of Technology, Nigeria
Olawale Mojeed Akanbi, The Federal University of Technology, Akura, Nigeria.
Received Date: January 15, 2020; Published Date: February 04, 2020
Abstract
This study was carried out to access the blood morphology and antioxidant status of Arbor acre broiler. Four diets were formulated using fermented cocoa pod husk meal at 0, 5, 10 and 15% inclusion levels and labelled as D1, D2, D3 and D4 respectively. Ninety- six day old of Arbor acre broiler consisting of twenty-four birds per treatment of 8 birds per replicate diet group was set up in a Completely Randomized Design (CRD). 5% and 10% fermentation of cocoa pod husk meal (FCPHM) were significantly (P<0.05) higher in PCV (28.33% and 28.67%), MCHC (32.87g/dl and 32.91g/dl), Hb (9.44g/dl and 9.58g/dl) and WBC (5.57 x109/l and 5.80 x 109/l), respectively. Significantly lower values were observed in D4 (15%) with 25.50%, 31.65g/dl, 8.47g/dl and 3.77 x109/l. WBC of 0% was 3.05 x109/l is not statistically differed from 15%.The serum indices showed no significant (p>0.05) differences in all parameters except cholesterol and low-density lipoprotein with highest statistical value recorded in 5% FCPHM. Similarly, all the parameters for antioxidant activities of the birds were significantly (p<0.05) affected dietary treatment. From the results, it was concluded conceivably that inclusion of above 10% fermentation of cocoa pod husk mel (FCPHM) in diets of Arbor acre broiler may have a detrimental effect on the health.
Keywords: Antioxidant; Cocoa pod husk; Hematology; Rhizopus stolonifera; Serum
Abbreviations: MFCSR: Microbial Fermented Cassava Starch Residue; PCV: Packed Cell Volume; WBC: White Blood Cell; LDL: Low-Density Lipoprotein; MCV: Mean Cell Volume; MCHC: Mean Corpuscular Haemoglobin Concentration
Introduction
The exploitation of agro by-products and farm wastes as substitute feed resources in livestock feeding trials has been the current development in animal production [1] Some of these by-products include wheat and rice offals, maize bran and brewers dried grains; which can switch conventional feed resources in animal diets without harmful effects [2,3]. However, the current prices of these by-products have increased as a result of high demand; thereby demanding the quest for further research into other alternative feed ingredients that are yet to be discovered.
One of such agro by-products that have been exploited in animal feeding trials is cocoa pod husk meal with minute success. Reports have shown that each ton of dry cocoa (beans) epitomizes ten (10) tonnes of cocoa pod husks [4]. Presently, cocoa pod husks are initiating environmental pollution problem in cocoa produc ing regions of the world. They serve as possible sources of disease transmission when used as mulch in cocoa farms. However, when perfectly processed to reduce the theobromine content and stimulate digestibility; the cocoa pod husk in meal form can be used in livestock feed formulation as a valuable ingredient.
Nutritional value of cocoa pod is relatively low as it is low in crude protein (9.14%) and high crude fibre (35.78%). It also contains anti-nutrients such as theobromine (2.64%), caffeine (1.14%) and tannin 0.917%) [5]. Several methods have been adopted in the treatment and processing of cocoa pod husk meal for the purpose of animal feed formulation. Some of these methods include hot-water treatment [6] alkali treatment [7]; enzyme (mannanase) treatment [8,9]; urea treatment [10,11]; fungal treatment [12], microbial detheobromination [13] and rumen-potash treatment [14].
Omoifo CO [15] reported that Rhizopus stolonifer, a Zygomycete has a filamentous growing pattern. Its filaments are coenocytic, that is, they are non-septate. It is the only fungus yet known to yield rhizoids which infiltrate the substratum in order to obtain nutrients. Furthermore, he reported that the rhizoids also serve as support. Opposite the rhizoids, a sporangiophore juts into the atmosphere and this lay off in a club-shaped column lumen closed within the sporangial wall. Between the column lumen and wall are several asexual reproductive structures known as sporangiospores [16]. This organism is also categorized by the presence of stolons, which connect rhizoid joints and it is conceivable that this organism can be used to ferment the cocoa pod husk with a resultant enhancement in the nutritional quality. Thus, cocoa pod husk meal was subjected to solid state fermentation using Rhizopus stolonifer fungi in a 14-day fermentation period, characterized chemically and used in formulating diets for broiler chickens to measure the effects of the diets on the blood morphology and antioxidant activity of the use of cocoa pod husk meal fermented with Rhizopus stolonifer. The study was geared to ascertain the optimum inclusion level as an ingredient of Rhizopus stolonifer- detheobromized cocoa pod husk meal in the diets of broiler chicken on the blood morphology and antioxidant status.
Materials and Methods
Experimental location
The feeding trial was carried out at the Poultry Unit of the Livestock Section, Teaching and Research Farm, The Federal University of Technology, Akure (FUTA). The University (FUTA) is geographically located between latitude 7° 5’N and longitude 5°15’E at an altitude of 370m above sea level [17].
Collection and processing of cocoa (Theobroma cacao) pod husks
Freshly broken composite cocoa pods were obtained from cocoa farm plantations located in Idanre, Ondo State. The broken cocoa pods were washed, milled and fermented in vitro using Rhizopus stolonifer fungi for 14 days under room temperature. Thereafter, the FCPHM was dried, milled and sample analyzed for proximate composition along with the unfermented sample using the AOAC (2012) procedures.
Cocoa pod husk fermentation method using a starter culture
Fermentation of cocoa pod husk meal (CPHM) was done to reduce the theobromine and fibre contents that could inhibit the utilization of the pod meal in the birds. CPHM was fermented through solid state fermentation during each period of fermentation after milling. Ten (10) grams of urea was dissolved in 100 liters of water which was used to moist the sterilized CPHM. One liter of the prepared inoculums of the starter culture of Rhizopus stolonifer was used to inoculate the urea treated CPHM and kept in a tray in an incubating chamber. The fermentation of the cocoa pod husk meal was completed on the 14th day, followed by sun drying the substrates for five to seven days to inactivate the microorganism.
Table 1: Gross composition of the experimental starter diets.
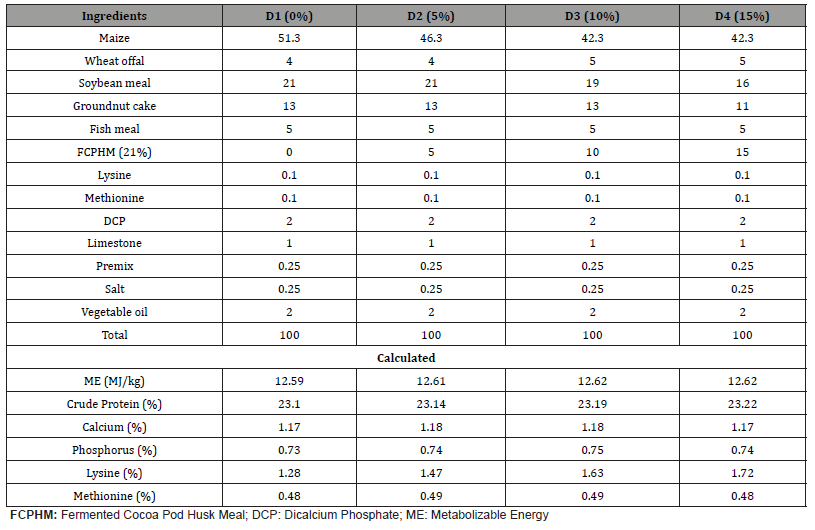
Table 2: Distribution of ever-married females by age group and gravida and para, Kampung Peninjau Lama, December 2013.
Dietary treatment
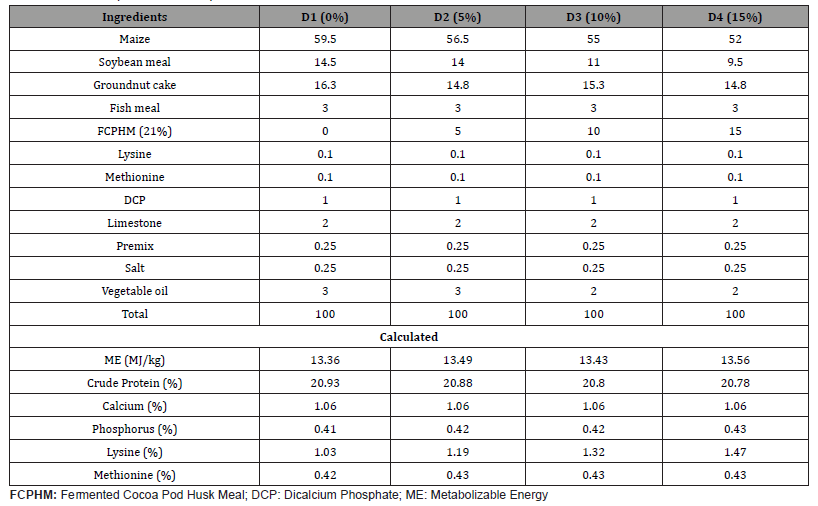
One basal diet was formulated to meet the nutrient requirement of broiler chicks according to NRC (1994) recommendation. The fermented cocoa pod husks (FCPH) meal was included as an independent ingredient at 0%, 5%, 10% and 15% levels in the diets and were designated as D1, D2, D3 and D4, respectively. The dietary formulae were balanced in such a way that the four diets were isonitrogenous and isocaloric. The formulae for both starter and finisher diets are presented in Tables 1 & 2, respectively.
Chicks arrangement and management
One hundred (100) day-old Arbor Acre broiler chicks were procured from Fidan farms in Ibadan, Oyo State, Nigeria out of which ninety- six (96) were assigned to four (4) dietary treatments of three (3) replicates of eight (8) chicks per replicate. The design of the experiment was a Completely Randomized Design.
Birds slaughtering and blood collection
The birds were fastened 12 hours before the collection of blood early in the morning. Six birds were randomly selected per treatment to determine the carcass and relative organ characteristics, haematological parameters, serum parameters and antioxidants activities. The birds were stunned before slaughtering in compliance with World Poultry Association guidelines. They were bled by severing the jugular vein and carotid artery and blood samples were collected during slaughtering at the end of the experimental period. The blood samples for haematological studies were collected in ethylenediaminetetraacetic acid (EDTA) bottles and blood meant for serum biochemical indices were collected in a test tube without anticoagulants and placed in the slanted form. The blood collected into bottles containing lithium- heparin from each treatment groups were analyzed for oxidative metabolites to ascertain the oxidative stress levels of the experimental birds.
Statistical Analysis
All data that were collected were subjected to One Way Analysis of Variance (ANOVA) of SPSS version 23. Significant treatment means were compared using New Duncan’s Multiple Range Test of the same package. The statistical model adopted is as shown below:
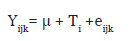
Where Yijk = any observation considered, μ= population mean, Ti = effect of FCPHM level,
eijk = experimental error (assumed to be identical, independently and normally distributed with zero mean and constant variance).
Results
Table 3 shows the haematological parameters of broiler fed varied inclusion levels of fermented cocoa pod husk meal (FCPHM). The dietary treatment recorded significant (p<0.05) differences in PCV, MCHC, Hb and WBC. RBC, MCV and MCH were not significantly (p>0.05) affected with the inclusion levels. D2 and D3 were significantly (p<0.05) higher in PCV (28.33% and 28.67%), MCHC (32.87g/dl and 32.91g/dl), Hb (9.44g/dl and 9.58g/dl), WBC (5.87 x109/l and 5.80 x109/l), respectively.
Table 3: Haematological parameters of broiler fed varying levels of fermented cocoa pod husk meal.
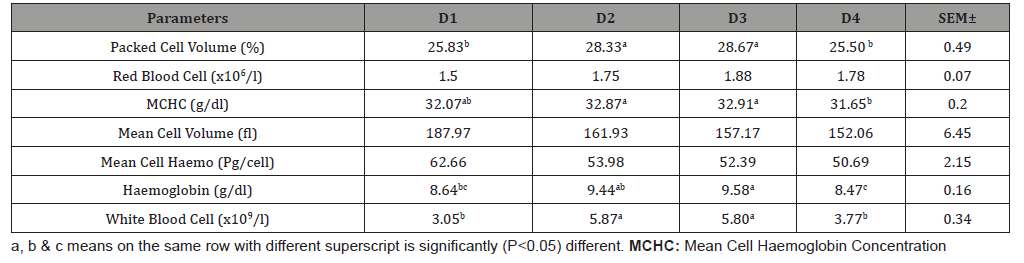
Significantly lower values were observed in D4 with 25.50%, 31.65g/dl, 8.47g/dl and 3.77 x 109/l respectively. WBC of D1 (3.05 x109/l) is not statistically differed from D4.
Serum biochemical parameters as shown in Table 4revealed show no significant (p>0.05) differences in all parameters except cholesterol and LDL. D2 had the highest statistical values of 3.25Mmol/l and 1.49Mmol/l compared to other dietary treatments. HDL, total protein, albumin and AST differed numerically with D1 recording the highest values of 2.95 Mmol/l, 44.07g/l, 12.23g/l and 186.13ui/l respectively. D3 recorded the highest numerical value in globulin with 35.98g/l while D4 also had the highest numerical value of 40.05ui/l in ALT.
Table 4: Serum biochemical parameters of broiler fed varying levels of fermented cocoa pod husk meal.
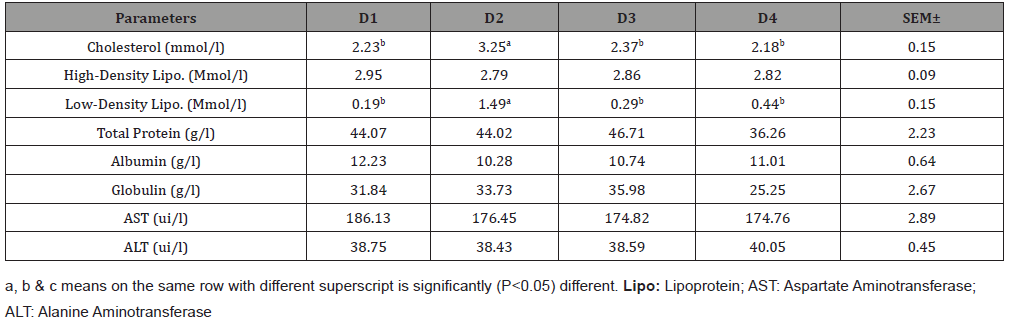
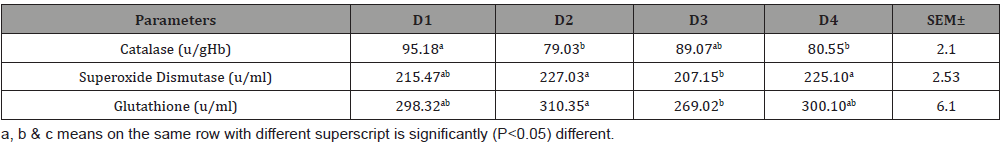
Table 5: Antioxidant activities of broiler fed varying levels of fermented cocoa pod husk meal.
Discussion
Haematological parameters have been linked with health indices and are of diagnostic significance in the routine clinical assessment of the state of health [18]. Reports also stated that PCV, HB and MCH were major keys for estimating circulating avian erythrocytes and were very significant in the identification of anaemia and also served as useful parameters of bone marrow capacity to yield red blood cells as in mammals [19].
Haematological parameters have been linked with health indices and are of diagnostic significance in the routine clinical assessment of the state of health [18]. Reports also stated that PCV, HB and MCH were major keys for estimating circulating avian erythrocytes and were very significant in the identification of anaemia and also served as useful parameters of bone marrow capacity to yield red blood cells as in mammals [19]. anti-nutrients contained in FCPHM at higher levels. Similarly, the findings of [25] using microbial fermented cassava starch residue (MFCSR) in broilers were closely in agreement with the result of this study.
Neufingerl N, et al. [26] reported that to evaluate the metabolic status of an animal, the serum metabolites levels are vital indicators to verify. In this study, all the serum metabolites measured were not statistically influenced by FCPHM except cholesterol and low-density lipoprotein. Cholesterol, a high molecular weight sterol is used in the body as raw material for a therapeutic process useful in the normal role of the brain and it is an essential constituent of the cell membrane with organelles inside the cell [27]. The present study shows the effect of FCPHM inclusion levels on cholesterol level, though falling within the normal range for healthy chicken did not follow a well- defined trend. Globulin is an important information of blood protein which when present in very low concentration could result in high mortality rate [28]. Sekine S, et al. [29] reported that albumin concentration in serum is established on their factors that are self-determining of nutrition such as infections, liver function, kidney disease, trauma and hydration status which the result of this study clearly shows that none of these extra- nutritional factors had considerable effects on the birds as indicated in the serum antioxidants status of the birds as supported by [25,27] who used MFCSR. 0%, 10% and 15% FCPHM inclusion in arbor acre broiler lessen significantly compared to 5% FCPHM which signifies the possible presence of some bioactive mixtures in FCPHM which impaired fat absorption and consequent fat reduction. The reduced HDL (good cholesterol) and increased LDL (bad Cholesterol) as determined in the result supported the claimed of impaired fat absorption and are also of health aids to the consumers, especially those predisposed to heart diseases. The inclusion of FCPHM in the diet vis- a- vis decreased uptake of cholesterol or improved loss or catabolism of cholesterol [30] ALT and AST are significant in the diagnosis of heart liver diseases and also the transamination in the metabolism of precise amino acids. The enzyme (ALT) results in this study were affected by the level of FCPHM inclusion in the diets and did not follow a definite form of effect. This findings is in consistent with [16] when Fungi treated cocoa pod husk meal was tested in the performance and health implication of marshall broilers. The report by [25] findings using MFCSR in broiler birds is in agreement with this present study.
One of the key causes of retarded growth in broiler is oxidative stress under a set of imposed environments. The birds were in very good conditions with physical examination; good toes, well-formed faecal droppings, active behaviour, bright eyes, clean and glossy feathers. Afolabi AB & Oloyede OI [32] reported that the use of herbal established antioxidants to improve the stress is becoming popular and antioxidants significantly delay or prevent oxidation of carbohydrates, Deoxyribonucleic acid (DNA), proteins and lipids. For instance, an antioxidants enzyme like glutathione (GSH), superoxide dismutase (SOD) and catalase can prevent oxidation both by calming transition metal radicals such as Fe2+ or Cu+ or by foraging instigated free radicals such as superoxide and hydrogen peroxide, the best reactive free radical in- vivo [32]. In this study, the inclusion FCPHM did not trail a particular movement in the serum antioxidants. This implies that higher serum antioxidants concentration values recorded for the birds fed diet containing FCPHM had more free radicals- mediated cell damage. The statistically higher results in 5 - 15% FCPHM could be due to the high inclusion level of theobromine content contained in FCPHM. It is, however, noteworthy that the birds fed a diet containing 0% FCPHM had no deleterious effect and less subjected to free radicals- mediated cell damage and oxidative stress. The results obtained in this study are supported by findings of [33-44].
Conclusion
Within the limit of this study, the inclusion of FCPHM above 10% may have a detrimental effect on the haematological, serum biochemical indices and serum antioxidant activities of the broiler upon subjection to stress. Additional research is needed to find further techniques of processing cocoa pod husk so that its theobromine content could be further reduced.
Acknowledgement
I hereby appreciate Prof. J. O. Agbede for designing the work and for thorough supervision during the course of the field work. The efforts of Drs. A. O. Ayeni and M. Adegbenro is also acknowledged during the supervision.
Availability of Data and Materials
It is available from the corresponding author on reasonable request.
Author’s Contributions
JOA designed the study. All authors managed the activities of the experiment and interpreted the data collectively. AOM and MO prepared the proposal of the study. AOM and AOA prepared the first draft of the manuscript. JOA reviewed the first draft. AOA prepared the second draft. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
References
- Ozung PO, Kennedy OO, Agiang EA (2016) Chemical composition of differently treated forms of cocoa pod husk meal (CPHM). Asian Journal of Agricultural Sciences 8(2): 5-9.
- Atteh JO, Opawande FE (2000) Replacement of brewer’s dry grain for groundnut cake in broiler finisher diets. Proceedings of the 25th Annual Conference of the Nigerian Society for Animal Production. Nigeria, 165-168.
- Awesu RJ, Bamgbose AM, Oduguwa OO, Fanimo AO, Oguntona EB (2000) Utilization of rice milling waste in diets for cockerel finishers. Proceedings of the 25th Annual Conference of the Nigerian Society for Animal Production. Nigeria, 201-204.
- Ozung PO, Kennedy OO, Agiang EA, Eburu PO, Evans EI, et al. (2016) Growth performance and apparent nutrient digestibility co-efficients of weaned rabbits fed diets containing different forms of cocoa pod husk meal. Agriculture and Food Science Research 4(1): 8-19.
- Adeyeye SA, Agbede JO, Aletor VA, Oloruntola OD, Ayodele SO, et al. (2016) Effects of rumen liquor fermentation on the proximate composition and antinutritional factors of ash- extract treated cocoa. (Theobroma cocoa) pod husks 1: 52-55.
- Adegbola AA, Omole TA (1973) A simple technique for preparing cocoa discarded bean-meal for use as livestock feed. Nigeria Agricultural Journal 10(1): 72-81.
- Isika MA, Nsa EE, Ozung PO (2012) Replacement value of processed cocoa bean meal for groundnut cake in rations for fryer rabbits. Journal of Sustainable Technology 3(1): 118-127.
- Bedford M (2000) Removal of antibiotic growth promoters from poultry diets: Implications and strategies to minimise subsequent problems. World Poultry Science Journal 56: 347-367.
- Zakaria HAH, Jalal MAR, Jabarin AS (2008) Effect of exogenous enzymes on the growing performance of broiler chickens fed regular corn soybean-based diets and the economics of enzyme supplementation. Pakistan Journal of Nutrition 7(4): 534-539.
- Olubamiwa O, Akinwale TO (2000) Partial placement of maize with cocoa husks meals in layers mash: An on- farm experience. Journal for Food Technology of Africa, 5(2): 62-63.
- Iyayi EA, Olubamiwa O, Ayuk A, Orowvegodo S, Ogunaike EF (2001) Utilization of urea treated and untreated cocoa pod husk-based diets by growing pigs: An on-farm study. Tropiculture 19(3): 101-104.
- Adamafio NA, Ayombil F, Tano Debrah K (2011) Microbial detheobromination of cocoa (Theobroma cacao) pod husk Journal of Biochemistry 6(2): 200-207.
- Mazzafera P (2002) Degradation of caffeine by microorganisms and potential use of decaffeinated coffee husk and pulp in animal feeding. Science Agricultural 59(4): 815-821.
- Adeyeye SA, Ayodele SO, Oloruntola OD, Agbede JO, (2019) Processed cocoa pod husk dietary inclusion: effects on the performance, carcass, haematogram, biochemical indices, antioxidants enzyme and histology of the liver and kidney in broiler chicken. Bulletin of the National Research Centre 43(54): 1-9.
- Omoifo CO (2011) Rhizopus stolonifer exhibits dimorphism. African Journal of Biotechnology 10(20): 4269-4275.
- Akanbi OM (2019b) Performance and health implication of feeding Fungi treated cocoa pod husk meal on broiler. Bulletin of the National Research Centre 43(55): 1-8.
- Oyinloye MA (2013) Monitoring spatial growth of educational institution using geographical information system: A Focus on Federal University of Technology, Nigeria. American Journal of Humanities and Social Sciences 1(3): 163- 173.
- Saliu JA, Elekofehinti OO, Komolafe K, Oboh G (2012) Effects of some green leafy vegetables on the Hematological parameters of Diabetic Rats. Scholars Research Library. Journal of Natural Product and Plant Resources 2(4): 7-15.
- Chineke CA, Ologun AG, Ikeobi CON (2006) Haematological parameters in rabbit breeds and crosses in humid tropics. Pakistan Journal of Biological Sciences 9(11): 2102-2106.
- Akanbi OM (2019a) Effect of Telfaira occidentalis leaf extract on the growth and health implications of Japanese quails. Discovery Nature 13: 29-35.
- Swenson MJ (1977) Dukes physiology of domestic animals (9th edn) p.14-34 Cornstock Publishing Associates, Cornell University Press, London.
- Oyewo EB, Adetutu A, Ayoade A, Akanni MA (2013) Repeated oral administration of aqueous leaf extract of Moringa oleifera modulated immunoactivities in Wister rats. Journal of National Science Research 3(6): 100-109.
- Faluyi OB, Agbede JO (2018) Immunostimulatory activity of aqueous leaf extract of Moringa oleifera in broiler chickens. International Journal of Environment, Agriculture and Biotechnology 3(1): 49-54.
- Mitruka BM, Rawnsley HM (1977) Clinical biochemical and haematological references values in normal experimental animals. Masson Publishing, USA, 88: 46-47.
- Aro SO, Agbede JO, Dairo OO, Ogunsote E, Aletor VA (2012) Evaluation of fermented cassava tuber wastes in broiler chickens feeding. Archiva Zootechnica. 15(3): 49-60.
- Neufingerl N, Zebregs YE, Schuring EAH, Traubwein EA (2013) Effects of cocoa and theobromine consumption on serum HDL- cholesterol concentrations: A randomized controlled trial. American Journal of Clinical Nutrition 97(6): 1201-1209.
- Oloruntola OD, Agbede JO, Onibi GE, Igbasan FA, Ogunsipe MH, et al. (2018b) Rabbits fed fermented cassava starch residue II: Enzyme supplementation influence on performance and Health status. Archivos de Zootechnica 67(260): 588-595.
- Adegbenro M, Agbede JO, Onibi GE, Aletor VA (2016) Composite leaf meal: Effects on haematology and biochemical indices of growing pigs. Archiva Zootechnica 19(2): 65-69.
- Sekine S, Terada S, Aoyama T (2013) Medium chain triacylglycerol suppresses the decrease of plasma albumin level through the insulin-Akt-mTOR pathway in the livers of malnourished rats. J Nutr Sci Vitaminol (Tokyo) 59(2): 123-128.
- Peter ML, Susan CEF (1999) Interpretation of laboratory results, Australian Veterinary Practice. 21(4): 188-193.
- Oloruntola OD, Agbede JO, Ayoade SO, Ayedun ES, Daramola OT, et al. (2018a) Gliricidia leaf meal and multi-enzyme in Rabbits diets: Effect on performance, blood indices, serum metabolites and antioxidants status. J Anim Sci Technol 60(24): 1-8.
- Afolabi AB, Oloyede OI (2014) Antioxidants properties of the extracts of Talinum triangulare and its effects on antioxidants enzymes in tissue homogenate of the Swiss albino rat. Toxicol Int 21(3): 307-313.
- Faine LA, Rodrigues HG, Galhardi CM, Ebaid GM, Diniz YS, et al. (2006) Effects of olive oil and its minor constituents on serum lipids, oxidative stress and energy metabolism in cardiac muscle. Can J Physiol Pharmacol. 84(2): 239- 245.
- Rubio LO, Seria A, Chen CY, Macià A, Romero MP, (2014) Effect of the co-occurring components from olive oil and thyme extracts on the antioxidant’s status and its bioavailability in acute ingestion in rats. Food and Function 5(4): 740- 747.
- Gerasopoulous K, Stagos D, Kokass S, Petrotos K, Kantas D, et al. (2015) Feed supplemented with by-products from olive oil mill wastewater processing increases antioxidants capacity in broiler chickens. Food and Chemical Toxicology 82: 42-49.
- Amorin GM, Oliveira AC, Gutarra MLE, Godoy MG, Freire DMG (2017) Solid state fermentation as a tool for methylxanthine reduction and simultaneous xylanase production in cocoa meal. Biocatalyst and Agricultural Biotechnology 11: 34-41.
- AOAC (2012) Association of analytical chemists. Official methods of analysis (17th edn) Washington DC, USA.
- Bentil JA, Dzogbefia VP, Alemawor F (2015) Enhancement of the nutritive value of cocoa (Theobroma cacao) bean shells for the use as fed for animals through a two-stage solid state fermentation with Plerotus osteatus and Aspergilus niger. International Journal of Applied Microbiology and Biotechnology Research 3(2): 20-30.
- Gilson G (2016) The impact of phytate as an anti-nutrients in poultry diets. International poultry production 22(3): 15-17.
- National Research Council (1994) Nutrients requirements of poultry (9th Edn) Washington DC, USA.
- Nortey TN, Ewusi I, Kpogo LA, Oddoye EOK, Naazie A (2015) Cocoa pod husk with enzyme supplementation is a potential feed ingredient in broiler diets. Livestock Research for Rural Development. 27(5): 8-13.
- Oduro Mensah D, Ocloo A, Lowor ST, Bonney EY, Okine L, et al. (2018) Bio-detheobromination of cocoa pod husks: reduction of ochratoxin a content without change in nutrient profile. Microbial Cell factory 17: 79-88.
- SPSS (version 23.0) SPSS Software products, Marketing Department, SPSS Inc. Chicago, USA.
- Adeyeye SA, Agbede JO, Aletor VA, Oloruntola OD (2017) Processed cocoa (Theobroma cacao) pod husk in rabbit’s diets: Effects on haematological and serum biochemical indices. Asian Journal of Advanced Agricultural Research 2(4): 1-9.
-
Olawale Mojeed Akanb, Johnson Olusola Agbede, Akinlolu Olufemi Ayeni, Muyiwa Adegbenro, Olayemi Aanuoluwapo Olugosi. Blood Morphology and Antioxidant Status of Broiler Fed Rhizopus Stolonifer-Detheobromized Cocoa Pod Husk Meal. On J Complement & Alt Med. 3(3): 2020. OJCAM.MS.ID.000561.
-
Antioxidant, Cocoa pod husk, Haematology, Rhizopus stolonifer, Serum, Theobromine, Zygomycete, Atmosphere, Anti-nutrients, Alkali Treatment, Fermentation, Isonitrogenous, Ethylenediaminetetraacetic Acid, Haematological Parameters, Oxidative Metabolites
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






