 Case Report
Case Report
Aseptic Meningitis After Spinal Anesthesia
Mohamed Ibrahim*, Soham De and Ali Bilal
Department of Anesthesiology, University of Texas Medical Branch, USA
Mohamed Ibrahim, Department of Anesthesiology, University of Texas Medical Branch, 301 University Blvd, Galveston, TX, USA.
Received Date:July 21, 2021; Published Date:September 27, 2021
Abstract
Meningitis is a rare but serious complication of neuraxial anesthesia. Our team reports a case of a 32-year-old who developed aseptic meningitis following a spinal anesthetic for Caesarean delivery. She presented with fever, headache, and neck pain, suggesting meningitis. However, the workup was negative for any causative organism. Aseptic meningitis is a diagnosis of exclusion, secondary to chemical irritation of the meninges or drug hypersensitivity. Differentiation between bacterial and aseptic meningitis is important due the prompt need for antibiotic therapy and further lifethreatening complications.
Keywords:Spinal; Epidural; Anesthesia; Meningitis; Neuraxial; Obstetric; Caesarean section; Headache; Bacterial
Introduction
Neuraxial anesthesia techniques are among the most widely used techniques in the perioperative management of a wide variety of patient populations undergoing invasive procedures. Neuraxial anesthesia in the obstetric population involves injecting local anesthetic agents and opioid analgesics into the lumbar epidural/ subarachnoid space for motor and sensory blockade of the lower thoracic, lumbar, and sacral nerve roots during obstetric surgeries and all stages of fetal delivery [1]. Due to the invasive nature of the procedure, the clinician must be mindful that there are numerous, but rare, serious complications that can occur.
Absolute contraindications to neuraxial anesthesia include patient refusal, sepsis, localized skin infection/active viral lesions at the site of puncture, thrombocytopenia, therapeutic anticoagulation, pre-existing CNS disorders, preload dependent states, and increased intracranial pressure [2]. Common complications include LAST (local anesthetic systemic toxicity), TNS (transient neurological symptoms), the formation of hematoma, epidural abscess, postdural puncture headache, and meningitis [3,4]. The prevalence of meningitis after spinal anesthesia is a rare but severe complication with different etiologies. Because of the potential risk of bacterial introduction into the intrathecal space secondary to extrinsic contamination, both the American Society of Regional Anesthesia and Pain Medicine and the Healthcare Infection Control Practices Advisory Committee (HICPAC) in June 2006 and 2007, respectively [5] recommended the mandatory use of surgical masks during these procedures. Despite safe practices, differing etiologies are proposed for meningitis following a lumbar puncture apart from the bacterial/viral infection that may also include a specific subset of meningitis termed “aseptic meningitis.” Aseptic meningitis can occur secondary to either disinfectant induced chemical irritation of the meninges or a hypersensitivity reaction to local anesthetic usage. Nonetheless, the differentiation between bacterial and aseptic/chemical meningitis in the clinical setting is critical. The latter is characterized by normal CSF glucose and negative bacterial cultures and remains a diagnosis of exclusion.
Case Presentation
We are presenting a case report of a 32-year-old primigravida who was admitted at 37 weeks of gestation for spontaneous rupture of membranes (SROM). Preoperative anesthesia evaluation had shown a past medical history of asthma, herpes labialis, liver nodules, migraine headaches in the second and third trimester, depression, and anxiety. The patient had no known drug allergies. The physical exam showed a BMI of 29.42 kg/m2, Mallampati score II, thyromental distance > 5 cm, and unremarkable neurologic, cardiac, and pulmonary physical exam findings. The patient requested delivery by primary cesarean section and was consented for spinal anesthesia. The patient was positioned in a sitting position. Sterile gloves and a surgical face mask were donned prior to and during the entire procedure. The back was sterilely cleaned with povidone-iodine solution, allowed to dry for 3 minutes, and a sterile drape was applied over the back. At the L3-4 level, we used a 25-gauge pencil-point spinal needle to inject a mixture of 12 mg of 0.75% bupivacaine with 8.25% dextrose, 150 mcg of preservativefree morphine, and 15 mcg of fentanyl into the subarachnoid space. No complications or difficulty with the neuraxial procedure were noted. The patient underwent an uncomplicated cesarean delivery with an estimated blood loss of 700 ml. The infant was delivered with Apgar scores of 8 and 9 at 1 and 5 minutes, respectively. No other complications were noted during the intra-operative course.
On postoperative day (POD) 1, the patient started complaining of a non-radiating frontal headache with a severity of 10/10. The headache did not improve or worsen with the change of position, and was described as penetrating, pressure, and sharp in nature. The patient denied fever, back pain, nausea, vomiting, or photophobia. No alleviating or aggravating factors were noted. The anesthesia team evaluated the patient giving initial recommendations for bed rest, hydration, and butalbital-acetaminophen-caffeine (Esgic) administration. Later during the day, the pain started radiating behind the patients’ eyes. The obstetrics team consulted neurology, and a CT scan of the head without contrast showed no acute intracranial hemorrhages or mass effect. The neurology team’s preliminary diagnosis was status migrainosus secondary to hormonal changes, for which that patient was started on sumatriptan. On POD 3, the patient reported improvement in her headache, stating a pain score of 2/10 down from 10/10. The patient was discharged from the hospital with instructions to continue sumatriptan, Esgic, and hydrocodone-acetaminophen as needed for 7 days and to follow up in the outpatient neurology clinic.
On POD 4, the patient returned to the hospital with a persistent headache in addition to a fever of 102 °F at home and “fever blisters” on her lips. Her headache at the time was described as temporal and located behind her eyes, non-positional, aggravated by movement, associated with nausea and photophobia, and with radiation down her neck, back, and bilateral legs causing difficulty with ambulation. The anesthesia and neurology teams evaluated the patient at this time and determined that the patient’s headache symptoms were unlikely to be a post dural punctural headache (PDPH). Based on the clinical manifestations, the onset of fever, nuchal rigidity, and an elevated white blood cell count at 20x103/mm3, the patient was diagnosed with meningitis. Empiric piperacillin-tazobactam and valacyclovir were started. A CT scan of the head with contrast showed no acute intracranial abnormalities. On POD 5, MRI of the brain revealed diffusion restriction within the lateral ventricles, suggesting possible early changes of meningitis or blood from recent neuraxial anesthesia. A lumbar puncture was performed by interventional radiology (Figure 1).
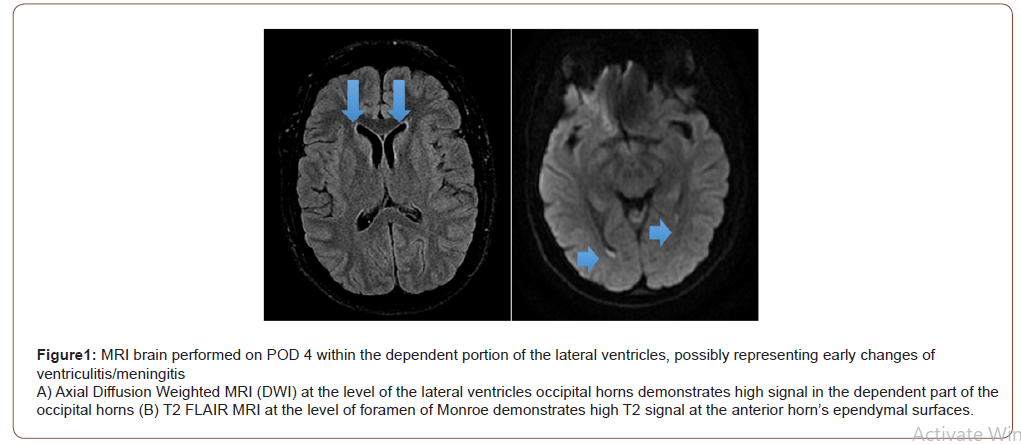
The cerebrospinal fluid (CSF) analysis revealed decreased glucose at 23 mg/dL, increased protein levels at 197 mg/dL, and elevated WBC count at 386//mm3, suggestive of bacterial meningitis. The CSF, blood, and urine cultures were negative for bacterial organisms, viruses, or fungi. On POD 6, the infectious diseases service was consulted and recommended changing antibiotics to vancomycin and cefepime. On POD 7, the patient reported no improvement in her headache and neck pain, but the fever has resolved. On POD 8, amphotericin B was added for empiric coverage of fungal organisms. A peripherally inserted central catheter was placed for long term antibiotic administration. On POD 9, a repeat MRI brain was obtained, which showed no significant change from the previous MRI. Additionally, the leukocytosis was resolved with a WBC count of 10x103/mm3.
On POD 10, despite broad-spectrum antibiotics, antifungal, and antiviral medications, the patient’s headache and neck stiffness persisted. Her headache was being treated with morphine, Esgic, and ketorolac. A repeat lumbar puncture was performed by interventional radiology, and the second CSF analysis showed a similar elevation in protein (114 mg/dL), a decrease in glucose (38 mg/dL), and an increased WBC count (174/mm3) with negative CSF culture growths for bacteria, viral, or fungal organisms. On POD 11 and 12, the neurology team added gabapentin, cyclobenzaprine, and topiramate to her regimen with the patient reporting some improvement in her headache and neck pain. With improvement in her symptom, the patient was discharged from the hospital on POD 13 with a PICC line to continue antibiotics as an outpatient for 14 days. The patient followed up with her obstetrician on POD 16; she reported significant improvement of her headache with no residual neurologic deficits.
Discussion
In our case, the patient was a 32-year-old primigravida with a pertinent past medical history of herpes labialis and migraine headaches who underwent spinal neuraxial anesthesia. Routine stringent aseptic techniques are followed to protect against nosocomial bacterial infection; sterile disposable syringes, needles, and local anesthetics obtained from an unopened, undamaged spinal kit are placed onto the sterile field and the procedure is performed with sterile gloves, face mask, and hat.
On post-op day 1, the patient started complaining of an initial non-radiating non-positional frontal headache with no aggravating or alleviating factors, without any signs of systemic symptoms or focal neurological deficits to suggest bacterial etiology. Furthermore, CT imaging was negative for any signs of acute intracranial abnormalities. The post-dural puncture headache was considered in the differential diagnosis and was initially treated with conservative measures. Although the patient developed an acute onset headache, she denied any change in the severity of the headache with changes in position. Furthermore, the needle gauge seems to be the most important precipitating factor for PDPH; Turnbull DK, et al. [7], reasoned that with the introduction of finegauge pencil-point needles in 1951, the incidence of PDPH has significantly decreased.
Additionally, status migrainosus was considered given the patient’s past medical history of migraines, it’s critical to recognize the increased incidence of postpartum headaches (PPH) in this patient population. Several studies, such as Goldszmidt E, et al. [8] and Stein G, et al. [12] have found that primary PPH (i.e., tension and migrainous) in the first few days after delivery is more common in those with a previous history of headaches; estrogen has been elucidated as a common factor, as the postpartum migraine may be related to falling estrogen levels [9]. On POD 4, the onset of new constitutional symptoms suggested meningeal irritation. The patient was acutely febrile and had a headache associated with photophobia radiating down her neck, back, and bilateral legs causing difficulty with ambulation. In addition, she had orolabial blisters; in light of these dermatological findings, this suggested reactivation of HSV-1. The patient was diagnosed with meningitis following her clinical presentation, leukocytosis, and brain imaging; she was started on empiric antibiotics for bacterial meningitis and valacyclovir prophylaxis. Regarding the use of neuraxial anesthesia for cesarean section in patients with a history of HSV-1 infection, there have been concerns regarding its safety. This is partly due to the possible risk of the central nervous system dissemination following reactivation of the latent virus (from the trigeminal ganglia), especially in viremia cases, and is caused by intrathecal morphine use demonstrated in a study by Davies PW, et al. [11] and pregnancy-associated depression of cell-mediated immunity [10].
Aseptic meningitis remains a diagnosis of exclusion. Several case reports on aseptic meningitis attribute it to potentially several causes, including contamination from detergent used to sterilize the spinal needle, hypersensitivity reaction to spinal medication, direct chemical irritation of the meninges, and systemic immunologic hypersensitivity to drugs such as non-steroidal anti-inflammatories (NSAIDS), intravenous immunoglobulins, and antibiotics [13,14,16]. With regards to the neuraxial technique, previous case reports have suggested bupivacaine-induced aseptic meningitis. The mechanism for drug induced meningitis after bupivacaine is not well defined similar to other drugs that are implicated, however the fact that there are several reports suggests that chemical meningitis is a rare potential side effect of the medication itself. Bupivacaine, like other local anesthetics, have several transient neurologic symptoms such as tinnitus, dizziness, and altered vision [13,14,17].
A review article from Baer ET, et al. [15], analyzed several possible causes for bacterial post-dural puncture meningitis. A reported 179 cases of post-dural puncture meningitis, with 114 having an organism have been identified. Of these 114 cases, 76% were caused by mouth commensal organisms, 23% were caused by organisms found on the skin, and 1% with endogenous origin. This possibly suggests droplets from medical personnel as a source of contamination. Additionally, there currently are no guidelines regarding the necessity for maximal sterile barrier precautions (face mask, cap, sterile gloves and gown, large sterile drape) during neuraxial placement. A central venous line placement showed several studies that reduced the rate of infection when maximal sterile technique is followed, but similar infection control studies for neuraxial placement have not been performed [15].
Overall, meningitis after neuraxial block is a rare but serious condition. CSF culture is critical to determining the source and guide antimicrobial therapy. In most cases, the risk of not treating a bacterial meningitis would have devastating consequences, thus the need for empiric antibiotics administration while the workup is performed is essential. In our case, we suspect bupivacaine induced chemical meningitis, however the patient’s history of migraines and herpes simplex virus infection may have contributing factors as well. The post-dural puncture headache has a broad differential, and it is important to consider meningitis as an early possibility.
Acknowledgement
None.
Conflict of Interest
The authors declare no financial conflicts of interest exist.
References
- Ohgaki H, Kleihues P (2005) Epidemiology and etiology of gliomas. Acta Neuropathol 109(1): 93-108.
- Maher EA, Brennan C, Wen PY, Durso L, Ligon KL, et al. (2006) Marked genomic differences characterize primary and secondary glioblastoma subtypes and identify two distinct molecular and clinical secondary glioblastoma entities. Cancer Res 66(23): 11502-11513.
- Cohen MH, Johnson JR, Pazdur R (2005) Food and Drug Administration Drug approval summary: temozolomide plus radiation therapy for the treatment of newly diagnosed glioblastoma multiforme. Clin Cancer Res 11(19 Pt 1): 6767-6771.
- Sun T, Warrington NM, Luo J, Brooks MD, Dahiya S, et al. (2014) Sexually dimorphic RB inactivation underlies mesenchymal glioblastoma prevalence in males. J Clin Invest 124(9): 4123-4133.
- Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, et al. (2016) Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 66(4): 271-289.
- Stupp R, Pavlidis N, Jelic S, Guidelines Task Force (2005) ESMO Minimum Clinical Recommendations for diagnosis, treatment and follow-up of malignant glioma. Ann Oncol 16(1) i64-i65.
- Schreck KC, Grossman SA (2018) Role of Temozolomide in the Treatment of Cancers Involving the Central Nervous System. Oncology (Williston Park) 32(11): 555-560.
- Nikolova T, Roos WP, Krämer OH, Strik HM, Kaina B (2017) Chloroethylating nitrosoureas in cancer therapy: DNA damage, repair and cell death signaling. Biochim Biophys Acta Rev Cancer 1868(1): 29-39.
- Ashby LS, Smith KA, Stea B (2016) Gliadel wafer implantation combined with standard radiotherapy and concurrent followed by adjuvant temozolomide for treatment of newly diagnosed high-grade glioma: a systematic literature review. World J Surg Oncol 14(1): 225.
- Champeaux C, Weller J (2020) Implantation of carmustine wafers (Gliadel®) for high-grade glioma treatment. A 9-year nationwide retrospective study. J Neurooncol 147(1): 159-169.
- Mao Z, Shen K, Zhu L, Xu M, Yu F, et al. (2019) Comparisons of cardiotoxicity and efficacy of anthracycline-based therapies in breast cancer: A network meta-analysis of randomized clinical trials. Oncol Res Treat 42(7-8): 405-413.
- Mao Z, Shen K, Zhu L, Xu M, Yu F, et al. (2019) Comparisons of cardiotoxicity and efficacy of anthracycline-based therapies in breast cancer: A network meta-analysis of randomized clinical trials. Oncol Res Treat 42(7-8): 405-413.
- Chamberlain FE, Jones RL, Chawla SP (2019) Aldoxorubicin in soft tissue sarcomas. Future Oncol 15(13): 1429-1435.
- Groves MD, Portnow J, Boulmay BC, Chawla SP, Dinh H, et al. (2016) Phase 2 study of aldoxorubicin in relapsed glioblastoma. Journal of Clinical Oncology 34(15 Suppl): 2027-2027.
- Carter TC, Medina-Flores R, Lawler BE (2018) Glioblastoma Treatment with Temozolomide and Bevacizumab and Overall Survival in a Rural Tertiary Healthcare Practice. Biomed Res Int 2018: 6204676.
- Seystahl K, Hentschel B, Loew S, Gramatzki D, Felsberg J, et al. (2020) Bevacizumab versus alkylating chemotherapy in recurrent glioblastoma. J Cancer Res Clin Oncol 146(3): 659-670.
- Schreck KC, Grossman SA (2018) Role of Temozolomide in the Treatment of Cancers Involving the Central Nervous System. Oncology (Williston Park) 32(11): 555-560.
- Zhang J, Stevens MF, Bradshaw TD (2012) Temozolomide: mechanisms of action, repair and resistance. Curr Mol Pharmacol 5(1): 102-114.
- Sedgwick B, Bates PA, Paik J, Jacobs SC, Lindahl T (2007) Repair of alkylated DNA: recent advances. DNA Repair (Amst) 6(4): 429-442.
- D'Atri S, Tentori L, Lacal PM, Graziani G, Pagani, E et al. (1998) Involvement of the mismatch repair system in temozolomide induced apoptosis. Mol Pharmacol 54(2): 334-341.
- Aasland D, Götzinger L, Hauck L, Berte N, Meyer J, et al. (2019) Temozolomide Induces Senescence and Repression of DNA Repair Pathways in Glioblastoma Cells via Activation of ATR-CHK1, p21, and NF-κ Cancer Res 79(1): 99-113.
- Baer JC, Freeman AA, Newlands ES, Watson AJ, Rafferty JA, et al. (1993) Depletion of O6-alkylguanine-DNA alkyltransferase correlates with potentiation of temozolomide and CCNU toxicity in human tumour cells. Br J Cancer 67(6): 1299-1302.
- Kanzawa T, Bedwell J, Kondo Y, Kondo S, Germano IM (2003) Inhibition of DNA repair for sensitizing resistant glioma cells to temozolomide. J Neurosurg 99(6): 1047-1052.
- Agnihotri S, Gajadhar AS, Ternamian C, Gorlia T, Diefes KL, et al. (2012) Alkylpurine-DNA-N-glycosylase confers resistance to temozolomide in xenograft models of glioblastoma multiforme and is associated with poor survival in patients. J Clin Invest 122(1): 253-266.
- Loeb LA, Preston BD (1986) Mutagenesis by purinic/apyrimidinic sites. Annu Rev Genet 20: 201-230.
- Berdis AJ (2001) Dynamics of translesion DNA synthesis catalyzed by the bacteriophage T4 exonuclease-deficient DNA polymerase. Biochemistry 40(24): 7180-7191.
- Sabouri N, Johansson E (2009) Translesion synthesis of abasic sites by yeast DNA polymerase epsilon. J Biol Chem 284(46): 31555-31563.
- Wilson DM, Barsky D (2001) The major human abasic endonuclease: formation, consequences and repair of abasic lesions in DNA. Mutat Res 485(4): 283-307.
- Roos WP, Kaina B (2013) DNA damage-induced cell death: from specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett 332(2): 237-248.
- Quiros S, Roos WP, Kaina B (2010) Processing of O6-methylguanine into DNA double-strand breaks requires two rounds of replication whereas apoptosis is also induced in subsequent cell cycles. Cell Cycle 9(1): 168-178.
- Hazra TK, Roy R, Biswas T, Grabowski DT, Pegg AE, et al. (1997) Specific recognition of O6-methylguanine in DNA by active site mutants of human O6-methylguanine-DNA methyltransferase. Biochemistry 36(19): 5769-5776.
- Gouws C, Pretorius PJ (2011) O6-methylguanine-DNA methyltransferase (MGMT): can function explain a suicidal mechanism? Med Hypotheses 77(5): 857-860.
- Dolan ME, Pegg AE, Dumenco LL, Moschel RC, Gerson SL (1991) Comparison of the inactivation of mammalian and bacterial O6-alkylguanine-DNA alkyltransferases by O6-benzylguanine and O6-methylguanine. Carcinogenesis 12(12): 2305-2309.
- Schold SC Jr, Kokkinakis DM, Rudy JL, Moschel RC, Pegg AE (1996) Treatment of human brain tumor xenografts with O6-benzyl-2'-deoxyguanosine and BCNU. Cancer Res 56(9): 2076-2081.
- Cabrini G, Fabbri E, Lo Nigro C, Dechecchi MC, Gambari R (2015) Regulation of expression of O6-methylguanine-DNA methyltransferase and the treatment of glioblastoma. Int J Oncol 47(2): 417-428.
- Weller M, Tabatabai G, Kästner B, Felsberg J, Steinbach JP, et al. (2015) MGMT Promoter Methylation Is a Strong Prognostic Biomarker for Benefit from Dose-Intensified Temozolomide Rechallenge in Progressive Glioblastoma: The DIRECTOR Trial. Clin Cancer Res 21(9): 2057-2064.
- Binabaj MM, Bahrami A, ShahidSales S, Joodi M, Joudi Mashhad M, et al. (2018) The prognostic value of MGMT promoter methylation in glioblastoma: A meta-analysis of clinical trials. J Cell Physiol 233(1): 378-386.
- Martinez, R, Schackert G, Yaya-Tur R, Rojas-Marcos I, Herman JG, et al. (2007) Frequent hypermethylation of the DNA repair gene MGMT in long-term survivors of glioblastoma multiforme. J Neurooncol 83(1): 91-93.
- Hegi ME, Liu L, Herman JG, Stupp R, Wick W, et al. (2008) Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol 26(25): 4189-4199.
- Sasmita AO, Wong YP, Ling APK (2018) Biomarkers and therapeutic advances in glioblastoma multiforme. Asia Pac J Clin Oncol 14(1): 40-51.
- Rao AM, Quddusi A, Shamim MS (2018) The significance of MGMT methylation in Glioblastoma Multiforme prognosis. J Pak Med Assoc 68(7): 1137-1139.
- Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, et al. (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352(10): 997-1003.
- Morandi L, Franceschi E, de Biase D, Marucci G, Tosoni A, et al. (2010) Promoter methylation analysis of O6-methylguanine-DNA methyltransferase in glioblastoma: detection by locked nucleic acid based quantitative PCR using an imprinted gene (SNURF) as a reference. BMC Cancer 10: 48.
- Kim DC, Kim KU, Kim YZ (2016) Prognostic Role of Methylation Status of the MGMT Promoter Determined Quantitatively by Pyrosequencing in Glioblastoma Patients. J Korean Neurosurg Soc 59(1): 26-36.
- Mansouri A, Hachem LD, Mansouri S, Nassiri F, Laperriere NJ, et al. (2019) MGMT promoter methylation status testing to guide therapy for glioblastoma: refining the approach based on emerging evidence and current challenges. Neuro Oncol 21(2): 167-178.
- Thomas A, Tanaka M, Trepel J, Reinhold WC, Rajapakse VN, et al. (2017) Temozolomide in the Era of Precision Medicine. Cancer Res 77(4): 823-826.
- Baer JC, Freeman AA, Newlands ES, Watson AJ, Rafferty JA, et al. (1993) Depletion of O6-alkylguanine-DNA alkyltransferase correlates with potentiation of temozolomide and CCNU toxicity in human tumour cells. Br J Cancer 67(6): 1299-1302.
- Kanzawa T, Bedwell J, Kondo Y, Kondo S, Germano IM (2003) Inhibition of DNA repair for sensitizing resistant glioma cells to temozolomide. J Neurosurg 99(6): 1047-1052.
- Agnihotri S, Gajadhar AS, Ternamian C, Gorlia T, Diefes KL, et al. (2012) Alkylpurine-DNA-N-glycosylase confers resistance to temozolomide in xenograft models of glioblastoma multiforme and is associated with poor survival in patients. J Clin Invest 122(1): 253-266.
- Schold SC Jr, Kokkinakis DM, Chang SM, Berger MS, Hess KR, et al. (2004) O6-benzylguanine suppression of O6-alkylguanine-DNA alkyltransferase in anaplastic gliomas. Neuro Oncol 6(1): 28-32.
- Friedman HS, Dolan ME, Moschel RC, Pegg AE, Felker GM, et al. (2002) Enhancement of nitrosourea activity in medulloblastoma and glioblastoma multiforme. J Natl Cancer Inst 84(24): 1926-1931.
- Wedge SR, Newlands ES (1996) O6-benzylguanine enhances the sensitivity of a glioma xenograft with low O6-alkylguanine-DNA alkyltransferase activity to temozolomide and BCNU. Br J Cancer 73(9): 1049-1052.
- Quinn JA, Jiang SX, Carter J, Reardon DA, Desjardins A, et al. (2009) Phase II trial of Gliadel plus O6-benzylguanine in adults with recurrent glioblastoma multiforme. Clin Cancer Res 15(3): 1064-1068.
- Kunkel TA, Erie DA (2005) DNA mismatch repair. Annu Rev Biochem 74: 681-710.
- Lenhart JS, Pillon MC, Guarné A, Biteen JS, Simmons LA (2016) Mismatch repair in Gram-positive bacteria. Res Microbiol 167(1): 4-12.
- Schofield MJ, Hsieh P (2003) DNA mismatch repair: molecular mechanisms and biological function. Annu Rev Microbiol 57: 579-608.
- Guarné A, Charbonnier JB (2015) Insights from a decade of biophysical studies on MutL: Roles in strand discrimination and mismatch removal. Prog Biophys Mol Biol 117(2-3): 149-156.
- Masih PJ, Kunnev D, Melendy T (2008) Mismatch Repair proteins are recruited to replicating DNA through interaction with Proliferating Cell Nuclear Antigen (PCNA). Nucleic Acids Res 36(1): 67-75.
- Surtees JA, Alani E (2004) Replication factors license exonuclease I in mismatch repair. Mol Cell 15(2): 164-166.
- Lancey C, Tehseen M, Raducanu VS, Rashid F, Merino N, et al. (2020) Structure of the processive human Pol δ Nat Commun 11(1): 1109.
- Bowen N, Kolodner RD (2017) Reconstitution of Saccharomyces cerevisiae DNA polymerase ε-dependent mismatch repair with purified proteins. Proc Natl Acad Sci USA 114: 3607-3612.
- Yi GZ, Huang G, Guo M, Zhang X, Wang H, et al. (2019) Acquired temozolomide resistance in MGMT-deficient glioblastoma cells is associated with regulation of DNA repair by DHC2. Brain 142(8): 2352-2366.
- Perazzoli G, Prados J, Ortiz R, Caba O, Cabeza L, et al. (2015) Temozolomide Resistance in Glioblastoma Cell Lines: Implication of MGMT, MMR, P-Glycoprotein and CD133 Expression. PLoS One 10(10): e0140131.
- Friedman HS, McLendon RE, Kerby T, Dugan M, Bigner SH, et al. (1998) DNA mismatch repair and O6-alkylguanine-DNA alkyltransferase analysis andresponse to Temodal in newly diagnosed malignant glioma. J Clin Oncol 16(2): 3851-3857.
- Stark AM, Doukas A, Hugo HH, Hedderich J, Hattermann K, et al. (2015) Expression of DNA mismatch repair proteins MLH1, MSH2, and MSH6 in recurrent glioblastoma. Neurol Res 37(2): 95-105.
- Indraccolo S, Lombardi G, Fassan M, Pasqualini L, Giunco S, et al. (2019) Genetic, Epigenetic, and Immunologic Profiling of MMR-Deficient Relapsed Glioblastoma. Clin Cancer Res 25(6): 1828-1837.
- Struve N, Binder ZA, Stead LF, Brend T, Bagley SJ, et al. (2020) EGFRvIII upregulates DNA mismatch repair resulting in increased temozolomide sensitivity of MGMT promoter methylated glioblastoma. Oncogene 39(15): 3041-3055.
- Nagel ZD, Kitange GJ, Gupta SK, Joughin BA, Chaim IA, et al. (2017) DNA Repair Capacity in Multiple Pathways Predicts Chemoresistance in Glioblastoma Multiforme. Cancer Res 77(1): 198-206.
- Sedgwick B, Bates PA, Paik J, Jacobs SC, Lindahl T (2007) Repair of alkylated DNA: recent advances. DNA Repair (Amst) 6(4): 429-442.
- Plosky BS, Frank EG, Berry DA, Vennall GP, McDonald JP, et al. (2008) Eukaryotic Y-family polymerases bypass a 3-methyl-2'-deoxyadenosine analog in vitro and methyl methane sulfonate-induced DNA damage in vivo. Nucleic Acids Res 36(7): 2152-2162.
- Myers K, Gagou ME, Zuazua-Villar P, Rodriguez R, Meuth M (2009) ATR and Chk1 suppress a caspase-3-dependent apoptotic response following DNA replication stress. PLoS Genet 5(1): e1000324.
- Loeb LA, Preston BD, Snow ET, Schaaper RM (1986) Apurinic sites as common intermediates in mutagenesis. Basic Life Sci 38: 341-347.
- Rubinson EH, Christov PP, Eichman BF (2013) Depurination of N7-methylguanine by DNA glycosylase AlkD is dependent on the DNA backbone. Biochemistry 52(42): 7363-7365.
- Taylor JS (2002) New structural and mechanistic insight into the A-rule and the instructional and non-instructional behavior of DNA photoproducts and other lesions. Mutat Res 510(1-2): 55-70.
- Sabouri N, Johansson E (2009) Translesion synthesis of abasic sites by yeast DNA polymerase epsilon. J Biol Chem 284(46): 31555-31563.
- Yang Z, Price NE, Johnson KM, Wang Y, Gates KS (2017) Interstrand cross-links arising from strand breaks at true abasic sites in duplex DNA. Nucleic Acids Res 45(11): 6275-6283.
- Robertson AB, Klungland A, Rognes T, Leiros I (2009) DNA repair in mammalian cells: Base excision repair: the long and short of it. Cell Mol Life Sci 66(6): 981-993.
- Lindahl T (2000) Suppression of spontaneous mutagenesis in human cells by DNA base excision-repair. Mutat Res 462(2-3): 129-35.
- Goellner EM, Grimme B, Brown AR, Lin YC, Wang XH, et al. (2011) Overcoming temozolomide resistance in lioblastoma via dual inhibition of NAD+ biosynthesis and base excision repair. Cancer Res 71(6): 2308-2317.
- Shi J, Dong B, Zhou P, Guan W, Peng Y (2017) Functional network analysis of gene phenotype connectivity associated with temozolomide. Oncotarget 8(50): 87554-87567.
- Bobola MS, Kolstoe DD, Blank A, Chamberlain MC, Silber JR (2012) Repair of 3-methyladenine and abasic sites by base excision repair mediates glioblastoma resistance to temozolomide. Front Oncol 2: 176.
- Higuchi F, Nagashima H, Ning J, Koerner MVA, Wakimoto H, et al. (2020) Restoration of Temozolomide Sensitivity by PARP Inhibitors in Mismatch Repair Deficient Glioblastoma is Independent of Base Excision Repair. Clin Cancer Res 26(7): 1690-1699.
- Tang JB, Svilar D, Trivedi RN, Wang XH, Goellner EM, et al. (2011) N-methylpurine DNA glycosylase and DNA polymerase beta modulate BER inhibitor potentiation of glioma cells to temozolomide. Neuro Oncol 13(5): 471-486.
- Agnihotri S, Burrell K, Buczkowicz P, Remke M, Golbourn B, et al. (2014) ATM regulates 3-methylpurine-DNA glycosylase and promotes therapeutic resistance to alkylating agents. Cancer Discov 4(10): 1198-1213.
- Thanasupawat T, Natarajan S, Rommel A, Glogowska A, Bergen H, et al. (2017) Dovitinib enhances temozolomide efficacy in glioblastoma cells. Mol Oncol 11(8): 1078-1098.
- Parsons JL, Dianova I, Allinson, SL, Dianov GL (2005) Poly (ADP-ribose) polymerase-1 protects excessive DNA strand breaks from deterioration during repair in human cell extracts. FEBS J 272(8): 2012-2021.
- Johnson BE, Mazor T, Hong C, Barnes M, Aihara K, et al. (2014) Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science 343(6167): 189-193.
- Reha-Krantz LJ, Nonay RL, Day RS, Wilson SH (1996) Replication of O6-methylguanine-containing DNA by repair and replicative DNA polymerases. J Biol Chem 271(33): 20088-20095.
- Chavarria D, Ramos-Serrano A, Hirao I, Berdis AJ (2011) Exploring the roles of nucleobase desolvation and shape complementarity during the misreplication of O(6)-methylguanine. J Mol Biol 412(3): 325-339.
- Jałoszyński P, Ohashi E, Ohmori H, Nishimura S (2005) Error-prone and inefficient replication across 8-hydroxyguanine (8-oxoguanine) in human and mouse ras gene fragments by DNA polymerase kappa. Genes Cells 10(6): 543-550.
- Nick McElhinny SA, Havener JM, Garcia-Diaz M, Juárez R, Bebenek K, et al. (2005) A gradient of template dependence defines distinct biological roles for family X polymerases in nonhomologous end joining. Mol Cell 19(3): 357-366.
- Bertocci B, De Smet A, Weill JC, Reynaud CA (2006) Nonoverlapping functions of DNA polymerases mu, lambda, and terminal deoxynucleotidyltransferase during immunoglobulin V(D)J recombination in vivo. Immunity 25(1): 31-41.
- Wang H, Wu W, Wang HW, Wang S, Chen Y, et al. (2010) Analysis of specialized DNA polymerases expression in human gliomas: association with prognostic significance. Neuro Oncol 12(7): 679-686.
- Peng C, Chen Z, Wang S, Wang HW, Qiu W, et al. (2015) The Error-Prone DNA Polymerase κ Promotes Temozolomide Resistance in Glioblastoma through Rad17-Dependent Activation of ATR-Chk1 Signaling. Cancer Res 76(8): 2340-2353.
- Bostian AC, Maddukuri L, Reed MR, Savenka T, Hartman JH, et al. (2016) Kynurenine Signaling Increases DNA Polymerase Kappa Expression and Promotes Genomic Instability in Glioblastoma Cells. Chem Res Toxicol 29(1): 101-108.
- Campbell BB, Light N, Fabrizio D, Zatzman M, Fuligni F, et al. (2017) Comprehensive Analysis of Hypermutation in Human Cancer. Cell 171(5): 1042-1056.
- Choi JS, Kim S, Motea E, Berdis A (2017) Inhibiting translesion DNA synthesis as an approach to combat drug resistance to DNA damaging agents. Oncotarget 8(25): 40804-40816.
- Reineks EZ, Berdis AJ (2004) Evaluating the contribution of base stacking during trans lesion DNA replication. Biochemistry 43(2): 393-404.
- Choi JS, Kim CS, Berdis A (2018) Inhibition of Translesion DNA Synthesis as a Novel Therapeutic Strategy to Treat Brain Cancer. Cancer Res 78(4): 1083-1096.
-
Mohamed Ibrahim, Soham De, Ali Bilal. Aseptic Meningitis After Spinal Anesthesia. On J Complement & Alt Med. 6(5): 2021. OJCAM.MS.ID.000650.
-
Spinal; Epidural; Anesthesia; Meningitis; Neuraxial; Obstetric; Caesarean section; Headache; Bacterial; Aseptic meningitis; Lower thoracic; Obstetric surgeries
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






