 Research Article
Research Article
Pre-Clinical Validation of Cannabinoid and Mushroom Preparations for the Treatment of Cancer
Herbert A Fritsche*, Jeff Nagel and Stephen D Barnhill
Apollon Formularies PLC, London England
Herbert A Fritsche, Chief Science Officer, Apollon Formularies PLC, London England.
Received Date:November 23, 2023; Published Date:January 30, 2024
Abstract
Cannabinoids and mushrooms offer great potential for cancer therapy. However, there is debate regarding the optimal compositions of cannabinoids and mushrooms that are the most effective for each of the various cancer types. We determined the cytotoxicity of THC, CBD, CBG, THCA, six proprietary cannabinoid mixtures, and eight proprietary mushroom preparations for ten organoid cancer cell cultures. The cannabinoid preparations demonstrated high cytotoxicity for all of the cancer organoids, but not all performed equally well. Only two of the mushroom preparations demonstrated direct cytotoxicity. In addition to the assessment of direct cytotoxicity, we used a Her-2 overexpressing breast cancer cell line to assess the mushroom preparations for enhancement of antibody-dependent cytotoxicity (ADCC)and phagocytosis (ADCP). The mushroom preparations significantly improved the anti-tumor response by both mechanisms of cellular immunity. Surprisingly, the treatment of BT-474 cells with selected mushroom preparations performed similarly to the widely used drug, Trastuzumab. Trastuzumab is a human monoclonal antibody directed to the membrane HER2 receptor, causing cell death. However, the cytotoxicity of BT-474 cells was significantly increased when Trastuzumab was used with mushrooms. The combined use of cannabinoids with mushrooms, in a sequential manner, worked synergistically, presumably due to the activation of three distinct mechanisms for cell death. Our data supports the combined use of cannabinoids and mushroom preparations for cancer therapy.
Keywords:THC; CBD; THCA; Cannabinoids; Mushroom extracts; Cytotoxicity; ADCC; ADCP; Organoid cell cultures; Trastuzumab
Introduction
Recent reports continue to demonstrate the potential use of cannabis and mushroom preparations as anti-cancer agents [1- 4]. However, for the cannabinoids, there are conflicting reports on the effectiveness of these agents for inducing cytotoxicity using either the semi-purified agents (THC, CBD, THCA) or the whole plant extract [5,6]. For whole plant extracts, there is debate on the optimal cannabinoid composition that should be used, and if the cannabinoid composition should be dose optimized for the specific cancer type and tissue site to be treated. For mushrooms, there is some published data reporting the antibody-mediated cytotoxicity of mushroom extracts [7], but there are no comparative studies of the effectiveness of mushrooms for inducing cancer cell death. One objective of this study was to determine the direct cell cytotoxicity of our proprietary cannabinoid and mushroom preparations. A second objective was to assess the potential of our mushroom preparations to enhance cell-mediated cytotoxicity.
We initiated our study with an exhaustive internet search of published information on the efficacy of cannabis treatments with a “web crawl”, searching for correlations between the use of cannabinoid preparations with specific diseases and symptoms. Over 500,000 references were found that reported the use of specific cannabis strains or cannabinoid preparations for the treatment of seizures, cancer, migraine, nausea, loss of appetite, sleep deprivation, anxiety, energy, inflammation, and pain. We used the “recommender” method based on single value decomposition (SVD) for data reduction [8], followed by a proprietary artificial intelligence technique (machine learning algorithm), to rank the cannabis strains for therapeutic efficacy for each of those medical conditions. We selected the top two cannabis strains that were identified by the artificial intelligence algorithms as the most useful for cancer therapy, and to guide in determining the compounds for use in our proprietary therapeutic preparations. We determined the cytotoxicity of our proprietary cannabinoid/terpene preparations and compared the cytotoxicity to that given by the semi-purified preparations of THC, CBD, CBG, and THCA.
Aqueous preparations of mushrooms have also been shown to possess cytotoxic activity on cancer cell lines [9,10]. The direct cytotoxicity of mushrooms has been attributed to the presence of water-soluble terpenoids [11]. In addition, some mushrooms have been implicated in enhancing immune-related toxicity [12,13]. We created eight mushroom preparations for the determination of their direct cytotoxicity and immune-mediated cytotoxicity. Direct cell cytotoxicity of the cannabinoid and mushroom preparations was determined for ten organoid cancer cell cultures that represented the major cancer sites. Dose-response curves enabled the determination of EC50 and IC50 values. Cell-mediated cytotoxicity was determined with assays for antibody-mediated cell cytotoxicity (ADCC) and antibody-dependent cell phagocytosis (ADCP).
Materials and Methods
Cannabis Preparations
Full spectrum, whole plant tinctures, were prepared by alcohol extraction (APOLC1) or CO2 extraction (APOLC2 and APOLC3) obtained by ethanol extraction, using a Waters SFE 5L CO2 Extractor. Extract distillates were obtained with a Pope Wiped-Film, 2-inch Distillation Unit. The APOLC1 and APOLC2 distillates were mixed with sunflower lecithin (Lekithos, Beach County FL), heated to 50° C and mixed with organic MCT oil derived from coconuts (Nutiva, Richmond CA). The mixtures were then homogenized with a Kinematica Polytron dispersing aggregate style high shear homogenizer (Bohemia NY). APOLC3 distillate was processed in a similar fashion, but not mixed with sunflower lecithin. APOLC4, APOLC5 and APOLM1 were mixtures of APOLC1 and C2 with either a cannabinoid blend or terpene blend.
Semi-purified isolates of THC, CBD and CBG were purchased from Covalent (Las Vegas NV) and THCA was a gift from Voyage Labs (Los Angeles CA). Cannabinoid emulsions of the distillates and isolates were made by adding five times the volume of PS80 polysorbate surfactant to one volume of the cannabinoid oil and 10 volumes of warm distilled water. A Sonomechanics Nano Optimizer (Miami FL) was used to sonicate the solutions The amplitude of sonication was 90 microns. Particle size analysis was performed by PTL Labs (Downers Grove IL 60515) using the Malvern Zetasizer. The particle size of the cannabinoid emulsions ranged from 1.0 to 5.0 microns. The emulsified solutions were stored at 4° C for up to 7 days before use. The composition of the cannabinoid and terpene preparations was determined by HPLC analysis at Niva Labs (Sylmar, CA 91342).
Mushroom Preparations
The following mushroom fungal preparations were made from dried mushrooms purchased from in nature Health (Chicago, IL): Turkey Tail, Reishi, Shiitake, Cordyceps, Maitake, Lions Mane. Chaga mushroom was obtained from Chagit (Camarillo CA) and Reishi triterpene powder from Navi Organics (United Kingdom). For each mushroom, 45.0 g of powder was added to 90 ml of warm water, sonicated at 50% amplitude, and filtered. The particle size of each mushroom extract ranged from 20.0 to 90.0 microns.
Cell Lines Used for Organoid Cell Cultures
All cancer cell lines that were used to make organoid cultures were obtained from the repository of cell lines maintained at the BIOENSIS Laboratory. Two prostate cancer cell lines were used, the 22RV1 line is androgen sensitive, while the PC3 line is androgen independent. The three cell lines used for breast cancer were: BT474 is ER positive, PR positive, Her2 positive; MDA MB231 is ER negative, PR negative, and Her2 positive; T47D is Her2 negative. The other cancer cell lines were for: Lung, A549 and NCI 460; Colon, HT 29; Skin, A431; and Bladder, T24.
Protocol for Cytotoxicity Assessment
Organoid cell cultures were made by BIOENSIS Laboratories (Bothwell WA 98011). The testing protocol consisted of seeding 1,000 cells per well in appropriate growth media in 384-well Corning Spheroid microplates. The microplates were incubated at 37° C in a 5% CO2 incubator for spheroid formation. Aliquots of the sample to be tested were vortexed and centrifuged at 800 X g for 5 minutes at room temperature. The stock solutions were diluted in 0.2% DMSO to make 500-fold dilutions. The preparations were stored at 4° C for up to 3 days before use. On the day of the assay, nine half-log dilutions of the stock were prepared in 0.2% DMSO ranging from one-fold to 1e-04 fold concentration. Assay plates were incubated for 5 days at 37° C and in 5% CO2. Cell viability was determined at 5 days after addition of the test sample. 3-D-Cell Titer Glow reagent (Promega, Madison WI) was added to the wells of the assay plates, and the plates were shaken until the spheroids were lysed. Luminescence measurements were made at room temperature to determine the number of viable cells. Each test sample was assayed in 4 replicates at each of the nine concentrations. Dose response curves were made and the EC50 of each test sample was calculated. Data was analyzed using R statistical software, and EC50 values were calculated using nonlinear regression to fit data to a sigmoidal 4 parameter log-logistic dose response model. Curve fitting and EC 50 calculations were performed using the R statistical software package with R’s drc library (www.r-project.org). The EC50 is the concentration in ug/ml that produced a response half-way between the maximal and baseline values.
ADCC Assay Protocol
Assessment of antibody-dependent cell cytotoxicity of the mushroom extracts was performed with Her2-expressing BT474 cells. The cells were labeled with Cell Brite green dye reagent (Biotium) according to the manufacturer’s instructions. Aliquots of the 500-fold dilutions were centrifuged at 800xG for 5 minutes at room temperature. The supernatant stock solutions were stored under sterile conditions at 4° C until use. On the day of the assay, a 6-point dose-response study was performed. Half-log dilutions were prepared and added to wells of assay plates containing fluorescentlabeled BT474 target cells with or without 20.0 ng/ml trastuzumab or isotype IgG in RPMI 1640 media for one hour. PBMC effector cells were added at a 1:9 ratio. The assay plates were incubated overnight at 37° C in a 5.0% CO2 incubator. After incubation, a dead-cell dye reagent (Biotium) was added according to the manufacturer’s recommendations. After a two-hour incubation, the cells were fixed with 2% formalin for 10 minutes, washed with PBS buffer, and read with a Lionheart automated microscope. The green labeled BT474 cells co-localized with red dye were identified, and the percent of ADCC was calculated. All tests were performed in triplicate. After correction for background, the results were reported as %ADCC mean value +/- the standard deviation.
ADCP Assay Protocol
Human monocytes were obtained from plasma using negative selection with magnetic beads, and were differentiated into primary monocyte-derived macrophage effector cells, after 6 days of incubation in 10% FBS RPMI 1640 media containing 50.0 ng/ml hM-CSF at 37° C, in a 5% CO2 incubator. Her2-expressing BT474 target cells were labeled with pHRhodo red dye (Thermo fisher Scientific). The monocyte-derived macrophage effector cells were labeled with Cell Trace Violet reagent (Thermo fisher Scientific) according to the manufacturer’s recommendations. On the day of the assay, a 6-point dose response study was performed. Stock solutions of the mushroom extracts were serially diluted, half-log, and added to assay plates containing the fluorescently labeled BT474 cells with or without 50.0 ng/ml trastuzumab or isotype IgG control in RPMI 1640 media for one hour. The labeled macrophages were added at a 1:2 ratio. The assay plates were incubated for 2 hours at 37° C in a 5% CO2 incubator. The cells were fixed with 2.0% for 10 minutes, washed with PBS buffer, and read with a Lionheart automated microscope. Phagocytized BT474 cells (red) were co-localized with macrophages (blue). The percent of colocalized cells was determined, corrected for background, and reported as a phagocytosis index. All tests were performed in triplicate and reported as % phagocytosis index mean value +/- standard deviation.
Results
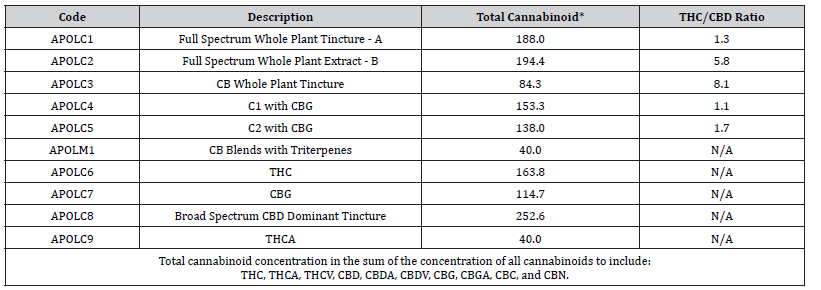
Composition of the cannabinoid preparations
Table 1 describes the cannabinoid and mushroom preparations that were used in this study, and lists the total cannabinoid concentration and the THC/CBD ratio for each preparation. APOLC1, APOLC2, APOLC3, APOLC4, and APOLC5 were proprietary cannabinoid preparations containing different ratios of CBD:THC, with and without CBG or THCA, along with varying amounts of CBDA, CBC, CBDV, and CBGA. APOLC1, APOLC2, and APOLC3 were full spectrum tinctures; APOLC4 and APOLC5 were modifications of APOLC1 and APOLC2 with added cannabinoids. APOLC8 was a CDB-dominant broad-spectrum tincture. Preparations APOLC6 (THC), APOLC7 (CBG), and APOLC9 (THCA) are semi-purified isolates with greater than 95% purity for the specified cannabinoid.
Table 1: Composition of cannabinoid preparations.
EC50 Values for Cannabinoid Preparations
Table 2 lists the EC50 values (10-6 /L) for all the cannabinoid preparations. All but one (HT29 for APOLC7) of the dose response curves showed 100% cell kill in a dose-related manner. Preparation APOLC8 broad spectrum CBD dominant (THC-free) was the most cytotoxic for a majority of the organoid cell cultures with EC50 values in the concentration range of 5.0 to 17.0 micromolar. The three breast cancer cell lines, BT474, MB231 and T47D, were especially sensitive to the broad-spectrum CBD dominant distillate (EC50 values of 1.6 micromolar). However, the colon cancer HT29 cell line was most sensitive to full spectrum distillate (APOLC1) which had a THC/CBD ratio of 1:1. HT29 was also sensitive to APOLC4 and APOLC5, both of which had THC and CBD concentrations less than that of APOLC1.
Table 2: EC50 values for cannabinoid preparations.
APOLC6 was somewhat less effective than APOLC8 for all of the cell lines, but it was more effective than THCA. APOLC3 performed particularly well on the androgen sensitive prostate cancer cell line (22Rv-1), and moderately well for all of the other cell lines. APOLC9 performed less well than APOLC6, but better than APOLC7. APOLC7 was the least effective cytotoxic agent for these cell lines.
APOLC1 and APOLC2 were two proprietary full spectrum distillates, with APOLC1 having a THC:CBD ratio of about 1:1 and APOLC2 a ratio of about 1:5, both with additional cannabinoid and terpene compounds added. The APOLC1 and APOLC2 EC50 values for all of the organoid cell lines were similar, with the APOLC1 containing an equivalent percentage of CBD, being just slightly less effective in most cases. APOLC4 and APOLC5 were blends of APOLC1 and APOLC2 with lower concentrations of THC and CBD but were supplemented with CBG and THCA. It is interesting to note that the APOLC4 and APOLC5 blends performed as well as the APOLC1 and APOLC2 blends, suggesting a role for CBG and THCA to effectively reduce the concentration of THC in a presumed entourage effect. However, pure preparations of APOLC7 and APOLC9 showed the least cytotoxic activity, when used alone. The androgen sensitive prostate cancer cell line (22Rv1) was more sensitive to all of the cannabinoid preparations than was the androgen resistant cell line (PC3). The bladder organoid line was the most resistant to cytotoxicity for all of the preparations.
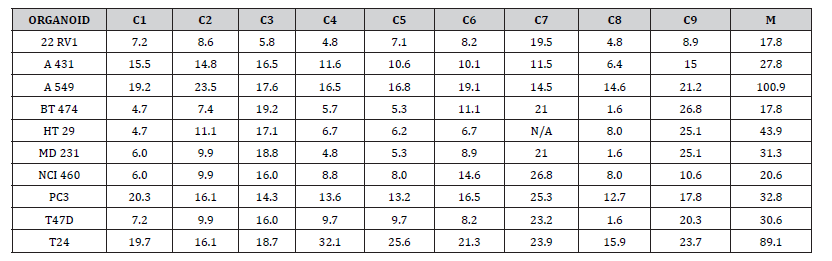
Relative IC50 Values of Mushroom Preparations
IC50 values were calculated for the mushroom preparations instead of EC50 since they did not achieve 100% cell death. The relative IC50 values for the mushroom preparations are expressed in units of micrograms of mushroom powder/ml. Since all mushroom preparations had stock concentrations of 40.0 mg/100ml, the relative IC50 values can be used to compare the cytotoxic activity to the other mushroom preparations, but they cannot be compared to the EC50 values that were determined for the cannabinoid preparations. APOLF1 and APOLF6 mushroom preparations did not achieve 50% reduction in cell number and thus IC50 values were not determined. APOLF2 and APOLF3 had IC50 values only for 22RV1, these were 0.88 and 0.92, respectively. APOLF4 and APOLF5 were cytotoxic to A431 skin cancer organoid at IC values of 0.61 and 0.19, respectively. IC50 values for the other organoids could not be calculated. The APOLF7 Chaga based mushroom preparation was cytotoxic to A431 (IC50=0.06); A549 (IC50=0.20); HT29, MD231 and NCI460 (IC50 = 0.40); and PC3 (IC50=0.61).
Since the direct cytotoxicity of mushrooms has been attributed to water soluble triterpenes, we assessed the cytotoxicity of APOLF8, a Reishi-based triterpene preparation (40.0 mg/100ml) containing cannabinoids. All of the organoids showed cytotoxicity to the APOLF8 triterpene preparation with IC 50 values ranging from 0.17 to 0.95. The IC50 values for MB231, NCI460, PC3 were similar to those for the APOLF7 Chaga based mushroom-based preparation, but were higher than the APOLF7 Chaga values for the other cell lines. It seems that the triterpene concentration in most of our mushroom extracts were not adequate to achieve a 50% cell kill. Since APOLF7 Chaga based triterpene preparation showed cytotoxicity to some of the organoid cultures, the triterpene concentration of Chaga is presumed to be higher than for the other mushrooms.
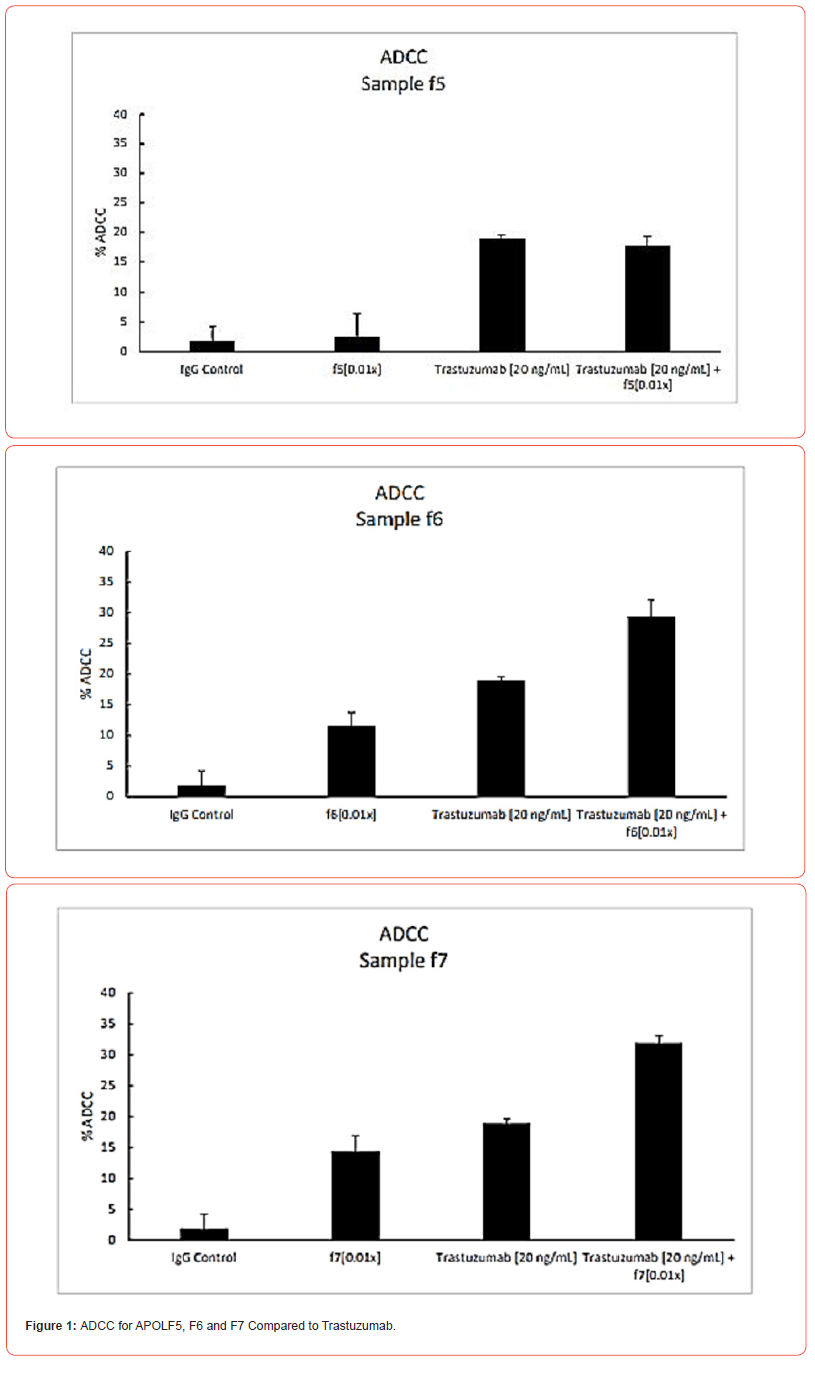
ADCC of mushroom extracts
Two mushroom preparations (APOLF6 and F7) showed significant antibody dependent cytotoxicity activity. The ADCC response given by one non-active preparation (APOLF5) and the two active mushroom preparations are shown in Figure 1. The ADCC activity of both F6 and F7 were only slightly less than that given by the 20.0 ng/ml dose of Trastuzumab. But in both cases, the mix of either mushroom with Trastuzumab gave the highest level of ADCC. Trastuzumab is a human monoclonal antibody directed to the membrane HER2 receptor, causing cell death. These findings suggest that APOLF6 and F7 mushroom preparations could be considered for treatment of Her2-positive cancer patients. Since Her2 antibody treatment is associated with a cardiotoxic side effect, the ApolF6 and F7 mushroom preparations might offer a safer treatment option than the current standard of care. In any case, our data suggest that supplementation of Trastuzumab with mushrooms could improve the treatment response, or perhaps, allow for dose reduction of Trastuzumab.
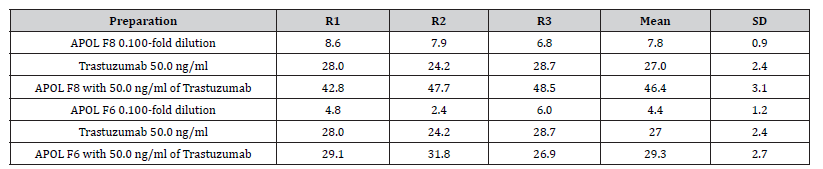
ADCP of Mushroom Preparations
The phagocytosis index of the mushroom preparations was determined in an ADCP assay utilizing a 50.0 ng/ml dose of trastuzumab. The %phagocytosis index for trastuzumab at 50.0 ng/ ml is 27%, calculated from the dose response curve. Dose response curves of the mushroom preparations at concentrations ranging from 0.24 to 80.0 ug mushroom powder/ml, were obtained in the presence and absence of 50.0 ng/ml trastuzumab. The phagocytosis indices obtained for APOLF1, APOLF2, APOLF3, APOLF4, APOLF5, APOLF6 were not greater than the control and did not improve ADCP over that produced by trastuzumab. However as shown in Table 3, APOLF8 showed significant ADCP_activity in the absence of trastuzumab. In addition, the ADCP produced by APOLF8 (index = 27.0) appeared to be additive to that given by the trastuzumab (index= 27.0), resulting in a total %phagocytosis index of 46.4. This remarkable increase in phagocytosis was not observed for the other mushroom preparations as represented in Table 3 by the APOLF6 response.
Table 3: Phagocytosis index for APOL F6 and F8 compared to Trastuzumab.
Discussion
The cell cytotoxicity of purified single cannabinoid agents and whole plant extracts on various cancer cell lines has been reported to occur at concentrations ranging from 2.0 to 40.0 Micromoles/ Liter [2,14]. The EC50 values determined in this report ranged from 1.6 to 32.0 and are consistent with earlier reports. Also, we agree with in most reports in demonstrating the cytotoxic activity of CBD to be greater than for THC, and for both of these single agents to have greater cytotoxic activity than the other cannabinoids. The potency of a cannabinoid preparation is defined by the type and concentration of the cannabinoid receptor on the cancer cell. THC acts through CB1 and CB2 receptors, while CBD and other cannabinoids have multiple mechanisms of action [15]. Thus, the cytotoxicity of cannabinoid mixtures varies for specific cell lines and is seemingly dependent upon the biochemical composition of the cannabinoid mixture, as well as the type and concentration of the receptor proteins on the target cells. Thus, it is likely that the cannabinoid composition of preparations to be used for the treatment of human cancers will need to be optimized on the basis of tumor cell type and tissue site. The direct cell toxicity of mushrooms appears to be low for most mushrooms and may be due to the low concentration of soluble terpenoids. However, certain mushrooms demonstrate the capability for enhancing cell death by antibody-dependent cytotoxicity (ADCC) and phagocytosis (ADCP).
We believe this report to be the first comprehensive study of the cytotoxicity of cannabinoids, terpenes and mushroom preparations, used separately and together, for ten organoid cancer cell cultures. For the cannabinoids, the whole plant tincture with high CBD content was in, general, the most cytotoxic agent for the cell lines. The EC50 data derived from dose response measurements demonstrate that our proprietary cannabinoid-based formulations effectively kill cancer cells in-vitro. Baram et al [15], came to a similar conclusion regarding the use of cannabinoids in their study using conventional cancer cell cultures. However, in order to achieve maximum cytotoxic responses, it may be advisable to include terpenoids with the cannabis extracts [16].
Our mushroom preparations demonstrated immune-related cytotoxicity by enhancing antibody dependent T-cell cytotoxicity and phagocytosis. Different formulations of cannabinoids and mushroom formulations showed dose-dependent responses in different cancer cell organoids demonstrating that both the direct cytotoxic effect and immune-stimulated cytotoxic response were both formulation and dose-dependent for the individual cancer cell types. A recent review article has focused on the multiple ways that mushroom polysaccharides can induce immune stimulation [17]. Our data supports the combined use of cannabinoid and mushroom preparations as a stand-alone therapy, or in combination with monoclonal antibody-based treatments, such as Trastuzumab.
Acknowledgement
The authors wish to acknowledge the support provided by Bioensis Laboratories, for performing the dose response experiments on the organoid cell cultures, under the direction of Gonzalo Castillo, PhD.
Patents
The findings reported in this manuscript are protected by patent applications which have now reached national filings in numerous countries around the world.
Author Contributions
Conceptualization and study design, S.B. and H.F.; methodology, J.N.; data analysis, S.B, H.F. and J.N.; writing—original draft preparation, H.F., writing—review and editing, S.B.; and J.N.; project administration, S.B.; Y.Y. All authors have read and agreed to the published version of the manuscript.”
Institutional Review Board
This study did not require ethical approval, as it did not involve humans or animals.
Informed Consent Statement
Informed consent was not applicable, as human subjects were not involved in the study.
Conflict of Interest
The authors of this manuscript are employees of Apollon Formularies, and were responsible for study design, interpretation of the data and writing the manuscript. This study was funded by Apollon Formularies. However, the all of the data generated for this report was obtained in a blinded manner by Bioensis Lab, as an independent third-party.
- Sledenski P, Nowack-Terpiloska A, Zeyland J (2021) Cannabinoids in Medicine: Cancer, Immunity and Microbial Diseases. Int J Mol Sci 22(1): 263.
- Nahler G (2022) Cannabidiol and other Phytocannabinoids as Cancer Therapeutics. Pharmaceut Med 36(2): 99-129.
- Hinz D, Ramer R (2022) Cannabinoids as anticancer drugs: current status of preclinical research. Br J Cancer 127(1): 1-13.
- Park, Hye-Jin (2022) Current Use of Mushrooms for Cancer Treatment and Their Mechanisms of Action. Int J of Mol Sci 23(18): 10502.
- Selzer ES, Watters AK, Mackenzie D, Granat LM, et al. (2020) Cannabidiol as a Promising Anti-cancer drug. Cancers (Basel) 12(11): 3203.
- Petrocellis LD, Ligresti A, Moriello AS, Iapelli M, Verde R, et al. (2013) Non-THC Cannabinoids Inhibit Prostate Cancer in vitro and in vivo: Pro-apoptotic effects and Underlying Mechanisms. Br J Pharmacol 168(1): 79-102.
- Arora S, Tandon S (2015) Mushroom Extracts Induce Human Colon Cancer Cell Line (COLO-205) Death by Triggering the Mitochondrial Apoptosis Pathway and Go/G1 Phase Cell Cycle Arrest. Arch Iran Med 18(5): 284-295.
- Zhou X, He J, Huang G, Zhang Y (2015) SVD-Based Incremental Approaches for Recommender Systems. J Comp System Sci 81(4): 717-733.
- Muzinic NR, Bratancevic MJ, Grubic M, Matas RF, Cagalj M, et al. (2023) Golden Chanterelle or a Gold Mine? Metabolites from Aqueous Extracts of Golden Chanterelle (Cantharellus cibarius) and Their Antioxidant and Cytotoxic Activities. Molecules 28(5): 2110.
- Gery A, Dubreule C, Bouchart V, Heutte N, Andre V, et al. (2018) Chaga (Inonotus obliquus), a Future Potential Medicinal Fungus in Oncology? A Chemical Study and a Comparison of the Cytotoxicity Against Human Lung Adenocarcinoma Cells (A549) and Human Bronchial Epithelial Cells (BEAS-2B). Integr Cancer Ther 17(3): 832-843.
- Lysakowska P, Sobota A, Workijowska A (2023) Medical Mushrooms: Their Bioactive Components, Nutritional Value and Applications in Funtional Food Production-A Review. Molecules 28(14): 5393.
- Guggenheim AG, Wright K, Zwickey HL (2014) Immune Modulation from Five Mushrooms: Application to Integrative Oncology. Integr Med (Encinitas) 13(1): 32-44.
- Mallard B, Leach D, Wohlmuth H, Tiralonfo J (2019) Synergistic Immuno-modulatory Activity in Human Macrophages of a Medicinal Mushroom Formulation Consisting of Reishi, Shiitake and Maitake. PLoS One 14(11): e0224740.
- Baram L, Peled E, Berman P, Yellen B, Besser E, et al. (2019) The Heterogeneity and Complexity of Cannabis Extracts as Antitumor Agents. Oncotarget 10(41): 4091-4106.
- Velasco G, Hernandez-Tiedra S, Davila D, Lorente M (2016) The Use of Cannabinoids as Anticancer agents. Prog Neuropsychopharmacol Biol Psychiatry 64: 259-266.
- Namdar D, Voet H, Ajjampura V, Nadarajan S, Mayzlish-Gati E, et al. Terpenoids and Phytocannabinoids Co-Produced in Cannabis Sativa Strains Show Specific Interaction for Cell Cytotoxic Activity. Molecules 24(17): 3031.
- Chakrabarty N, Banerjee A, Sarkar A, Ghosh S, Archaya K (2021) Mushroom Polysaccharides: A Potent Immune Modulator. Biointerface Res Applied Chem 11(2): 8915-8930.






