 Review Article
Review Article
Could Probiotic Supplements Be an Effective Intervention to Reduce Hypertension? A Systematic Literature Review
Helen J Broomfield1, Miranda D Harris2* and Joanna L R Goldie3
1BSc (Hons), MSc Nutritional Therapy, PGCE, University of Worcester, England
2Senior Lecturer, MSc Nutritional Therapy, FHEA, University of Worcester, England
3Lecturer, MSc Nutritional Therapy, FHEA, University of Worcester, England
Miranda Harris, Senior Lecturer, MSc Nutritional Therapy, School of Allied Health and Community, University of Worcester, St John’s Campus, Henwick Grove, Worcester England.
Received Date:December 18, 2021; Published Date:March 18, 2022
Abstract
Introduction: Pathogenesis of high blood pressure or hypertension is associated with microbial imbalance or dysbiosis of the gut microbiome.
Previous research suggests probiotic consumption may reduce elevated blood pressure, possibly through manipulation of the gut microbiome, and
may offer a future potential therapy for hypertension.
Aim: The aim of this research was to critically evaluate current research evidence to assess whether probiotic supplements may reduce high
blood pressure and formulate recommendations regarding their use as an intervention to support hypertensive clients in a Nutritional Therapy
context. The objectives were to outline the possible association between gut dysbiosis and hypertension, and to explore possible mechanisms by
which probiotics may influence blood pressure.
Methods: A systematic review of the literature based upon PRISMA protocol was conducted. Four databases were searched: Cochrane Library
(Central), CINAHL, Medline and TRIP from January 2014 until July 2020. Five eligible randomised controlled trials, including 453 participants, were
identified and critically appraised to assess the quality of their evidence [1].
Results: Of the three highest quality studies, two supported probiotic supplements to be effective in reducing blood pressure, one study
reported no effect. The remaining two studies were appraised to be of lesser methodological quality so were given less weighting for quality of
evidence. This research study found moderate evidence that probiotic supplementation can significantly reduce blood pressure in individuals with
borderline hypertension. No effect was reported in normotensives.
Conclusion: Probiotic supplementation may offer a convenient and effective adjunct for hypertensives to reduce high blood pressure alongside
other dietary/lifestyle/medical interventions.
Recommendation: Further large-scale trials of longer duration on hypertensives are recommended to establish functional pathways, bacterial
strain, dosage and required timescale.
Keywords:Probiotic Supplement; Hypertension; Dysbiosis; Blood Pressure; Gut microbiome
Abbreviations:ACE: Angiotensin-converting Enzyme; ACEI: ACE inhibitor; BASE: Bielefeld Academic Search Engine; BP: Blood pressure; CA: Critical appraisal; CINAHL: Cumulative Index of Nursing and Allied Health Literature; CONSORT: Consolidated Standards of Reporting of Trials; CFU: Colony Forming Units; CVD: Cardiovascular disease; DASH: Dietary Approaches to Stop Hypertension; DBP: Diastolic Blood Pressure; EC: Exclusion criteria; F/B: Firmicutes/Bacteroidetes; FMT: Faecal microbiota transplantation; GF: Germ free; GM: Gut microbiome; GPCR: G protein-coupled receptors; GRADE: Grading of Recommendations, Assessment, Development and Evaluation; HBP: High Blood Pressure; HT: Hypertensive(s); HTN: Hypertension; IC: Inclusion criteria; ITT: Intention-to-treat; LR: Literature review; MA: Meta-analysis; MECIR: Methodological Expectations of Cochrane Intervention Reviews; MeSH: Medical Subject Heading; Met-S: Metabolic syndrome; mmHg: millimetres of Mercury; NHS: National Health Service; NICE: National Institute for Health and Care Excellence; NO: Nitric oxide; NS: Narrative synthesis; NT: Normotension/normotensive; Pre- HT: Pre-hypertensive; Pre-HTN: Pre-hypertension; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-analyses; RAAS: Renin- Angiotensin-Aldosterone System; RCT: Randomised controlled trial; RO: Research objective; RoB: Risk of bias; RQ: Research question; SBP: Systolic Blood Pressure; SCFA: Short-chain fatty acid; SHR: Spontaneously hypertensive rat(s); SR: Systematic review; SNS: Sympathetic nervous system; T2DM: Type 2 Diabetes Mellitus; WHO: World Health Organization
Introduction
Hypertension: High blood pressure (HBP) or hypertension (HTN) is a major cause of premature death with around 10.4 million deaths worldwide annually attributed to HTN [2] and around 75,000 deaths in England in 2015 [3]. Moreover, there are an estimated 1.13 billion hypertensives (HT) [4] and the Global Burden of Disease (2015) study recognises HTN as the second largest known global risk factor for disease after poor diet [5] presenting an important global health challenge. Defined as a systolic pressure ≥140mmHg and/or a diastolic pressure of ≥90mmHg on two different days [6], HTN is a recognised major risk factor for a number of pathophysiologies [2] including coronary heart disease, stroke and ischaemic heart disease as well as other complications such as renal impairment, visual impairment and peripheral vascular disease [4]. HTN in early adulthood increases an individual’s risk of cardiovascular disease (CVD) [7]. An increase of 1.56 billion adults with HTN is forecasted by 2025 [8], with the growing burden of disease shifting to low income and developing countries [9], exacting a large public health burden on these countries.
Notwithstanding increased awareness, monitoring and a plethora of hypotensive pharmacotherapies, fewer than one in five people have their HTN under control and, with a global target to reduce its prevalence by 25% by 2025 [6], which is unlikely to be met [10], reduction of HTN presents a critical public health challenge. Collectively these statistics indicate a need for early intervention to prevent or ameliorate HTN.
Influences upon blood pressure: Influences upon BP are multifactorial and include lifestyle, environmental and genetic factors [11]. Targeted interventions to support healthy BP include eating plans such as the high-fibre, low-fat DASH (Dietary Approaches to Stop Hypertension) diet [12]. However, a significant focus has turned to the gut microbial population and their possible role in both BP maintenance and HTN development [13-15], with speculation that modification of the gut microbiota may offer novel therapeutic potential [16].
Gut microbiome: Although several hundred bacterial species reside in the gut, the predominant phyla, Firmicutes and Bacteroidetes compose over 90%, with a lower Firmicutes to Bacteroidetes (F/B) ratio generally considered a measure of good health [17]. Microbial diversity has been found to be inversely associated with increasing BP [18,19]. Moreover, an increase in the F/B ratio appears to correlate with increasing BP [20] implicating a disrupted gut microbial profile, known as dysbiosis, in HTN pathogenesis and suggests the GM may present a future area of focus in BP management.
Probiotics
Probiotics are defined by the Food and Agricultural Organization (FAO) of the United Nations and World Health Organization (WHO) as ‘Live microorganisms, which, when administered in adequate amounts, confer a health benefit on the host’ [21], and are found naturally in foods such as yoghurt. Findings from a seminal systematic review (SR) and meta-analysis (MA) indicated an antihypertensive effect of probiotics [22,23]. Further evidence has accumulated in recent years to support beneficial effects of probiotics upon health, including HTN [24-26], although precise mechanisms remain unclear.
The overall aim of this research was to critically review more
recent published studies to determine whether probiotics in
supplement form may present a novel therapeutic tool to reduce
HTN and, if so, formulate a probiotic supplement protocol to
support hypertensive clients in a Nutritional Therapy setting. The
research objectives were specifically
• To outline the possible association between gut dysbiosis
and HTN from the existing evidence base.
• To explore possible mechanisms by which probiotics may
influence blood pressure.
• To critically evaluate whether current research supports
the use of probiotic supplements to manage HTN.
• To formulate recommendations as to the use of probiotic
supplements as an intervention to support hypertensive clients
in a Nutritional Therapy setting.
Methods
Study design
Placebo-controlled randomised controlled trials evaluating the effect of probiotic supplements on blood pressure were identified through a systematic literature review based on Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [27] and Methodological Expectations of Cochrane Intervention reviews (MECIR) [28].
Data collection
Predetermined search terms and inclusion/exclusion criteria were used to search for relevant journals in the databases below (Table 1). The search was conducted from January 2014 to ensure some overlap with [22] as the intention of this study was to look at emerging evidence since this seminal SR. Final search were carried out up to July 2020 resulting in a total of 1075 studies identified.
Table 1:Results of final search showing final search string used and number of studies identified in each database. For a more detailed final search, see Appendix 1.
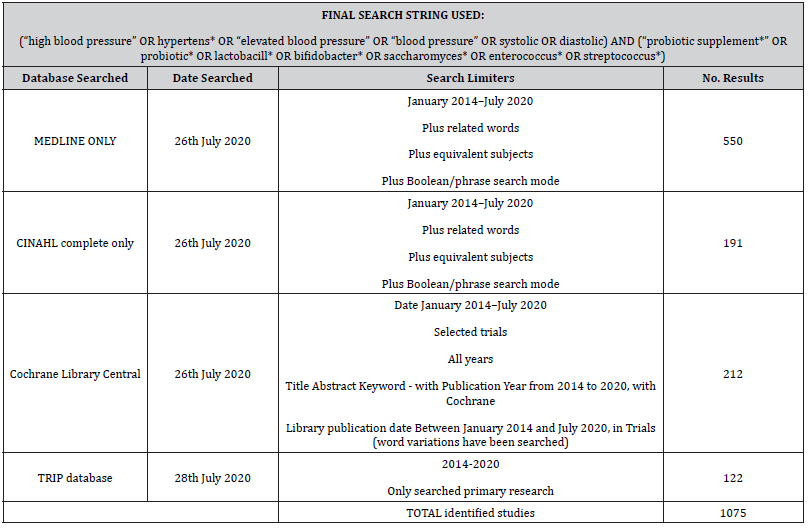
Databases searched
To conduct a comprehensive search, databases selected were those that focused on healthcare and scientific trials; namely CINAHL, Medline, Cochrane Library Central and TRIP.
Inclusion and exclusion criteria (IC & EC) and screening of studies
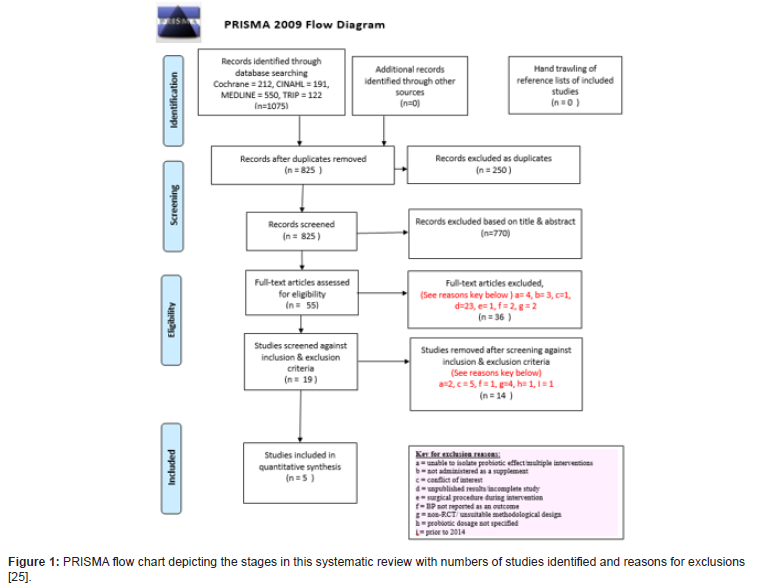
BP is often measured as a secondary outcome where participants have cardiovascular health issues such as hypercholesterolemia, so these studies were included, however those with other potential confounding conditions such as diabetes or pregnancy were excluded. Since the review is intended to inform clinical practice, strain and dosage of probiotic were criteria to be included. To minimize bias any declared conflict of interest resulted in exclusion. Identified studies were initially screened manually based on title and abstract. Duplicate studies were removed and remaining relevant full text studies were screened against pre-defined eligibility criteria (Table 2) as described below using a customized version of a Cochrane study eligibility form [29]. Unfinished/not yet published trials were excluded upon screening. The refined searches were summarised in a PRISMA flowchart [30,31] (Figure 1). Reference lists of selected studies were hand trawled to yield any further relevant studies [32].
Table 2:Inclusion and exclusion criteria applied to identified studies.
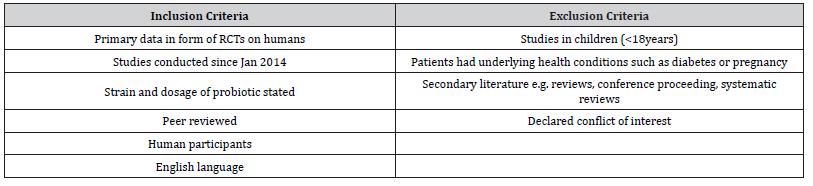
Ethics
This study involved no direct contact with human participants, however in accordance with the University of Worcester Ethics policy, all studies included had been ethically approved and informed consent of participants was acknowledged in the study details.
Data extraction and critical appraisal of selected studies
A customised data extraction form was used based on a Cochrane tool [33,34] and data including patient demographics, sample size, probiotic strain and dosage, setting, baseline and post-intervention blood pressure were tabulated in the results section. Each publication was critically appraised using the following tools: The Cochrane Risk of Bias (RoB2) tool and Joanna Briggs Institute (JBI) Critical Appraisal tool. Once reviewed, methodological quality and risk of bias assessment were graded to produce an evidence profile (EP) displayed as a stellar chart. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) system [35] was used as a basis for an evidence statement.
Results
The final literature search from January 2014 to July 2020 identified 1075 studies. Following removal of duplicates and screening against eligibility criteria, five RCTs, with 453 participants in total, were included in the final analysis (Figure 1).
Study characteristics
The main characteristics of the included studies are summarised below (Table 3). For a more detailed table of key characteristics see Appendix 2. Forthwith, studies will be referenced according to their number in the table below:
Table 3:Summary of main characteristics of included studies.
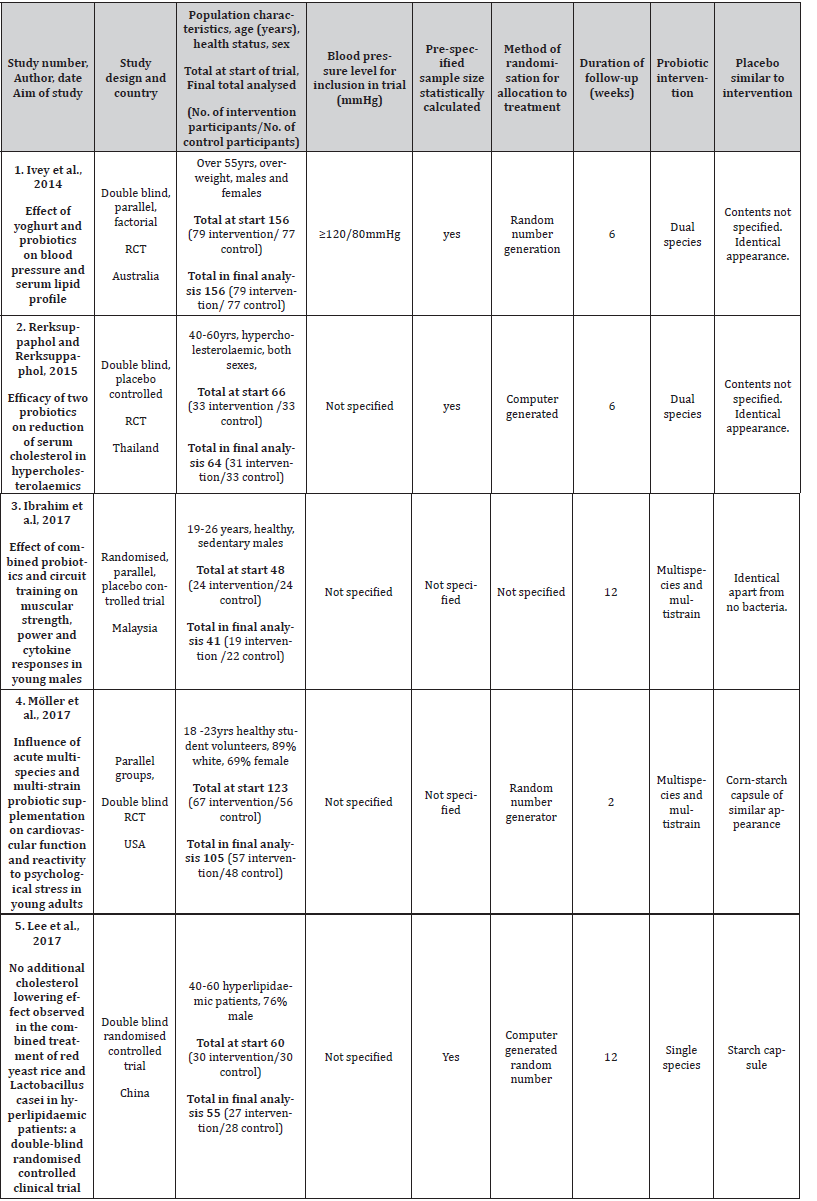
Overview of included studies
Included studies were RCTs with four (1, 2, 4 & 5) reporting a double-blind design, with detailing in studies 2, 4 and 5. Two studies (2 & 4) measured the effect of the probiotic supplement (intervention) upon a number of outcomes including BP. The other three (1, 3 & 5) had a factorial design investigating other interventions alongside probiotic supplementation, but required data was extracted without contamination from the total data. Study 5 did not report final BP measurements but did include change in BP (Table 4). Sample size ranged from 48 (study 3) to 156 (study 1) with studies 3 and 4 experiencing greatest loss to follow up. Trial duration ranged from two to 12 weeks. Two studies (3 & 4) recruited young (18-26 years) volunteers, whilst the remaining studies (1, 2 & 5) used participants aged over 40 years. Studies 2 and 5 recruited hypercholesterolemia/hyperlipidemic participants from hospital clinic settings respectively, whereas two other studies (3 & 4) recruited from a university setting. Study 1 recruited randomly from the electoral roll. Baseline characteristics were well matched for each cohort in each study. All studies reported use of a placebo identical in appearance to the probiotic.
Table 4:Details of intervention and placebo, BP measurements pre- and post-intervention with findings.
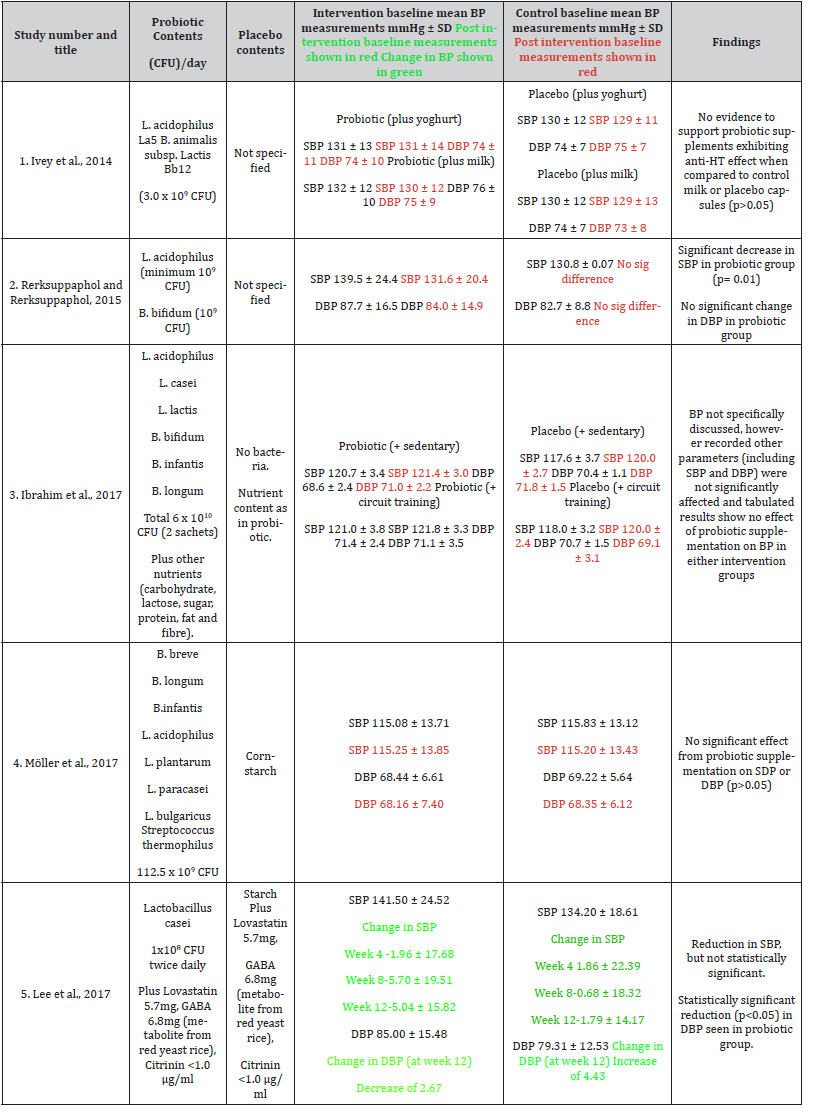
Key findings
The table above (Table 4) presents details of intervention, placebo and key findings of each included study
Studies 2 and 5 report a clinically significant decrease in either SBP (study 2) or DBP (study 5) in the probiotic cohort. It is important to note that the intervention cohort in both of these studies (2 & 5) had elevated baseline BP (study 2: SBP 139.5 ± 24.4 mmHg, DBP 87.7 ± 16.5 mmHg; study 5: SBP 141.50 ± 24.52 mmHg, DBP 85.00 ± 15.48 mmHg) compared to the placebo group (study 2: SBP 130.8 ± 0.07 mmHg, DBP 82.7 ± 8.8 mmHg; study 5: SBP 134.20 ± 18.61 mmHg, DBP 79.31 ± 12.53 mmHg) placing the intervention cohorts in the high pre-HT range [36] and, interestingly, only the probiotic groups in these studies (2 & 5) reported a significant decrease in BP. Contrastingly three studies (1,3 & 4) reported no significant effect upon BP following probiotic supplementation. Study 1 found no effect of the probiotic upon BP and reported mean baseline SBP as 131 (±12) mmHg placing it in the mid pre-HT range, however mean baseline DBP at 74 (±9) mmHg was normotensive. Studies 3 & 4 both had normotensive cohorts at baseline. The possible implication of this is baseline BP may be an important consideration. Findings are discussed as a critical analysis of the studies and the weighting given to their evidence.
Methodological quality and risk of bias assessment
Results of CA and RoB assessment are summarised below (Tables 5 & 6).
Table 5:Critical appraisal of included studies.
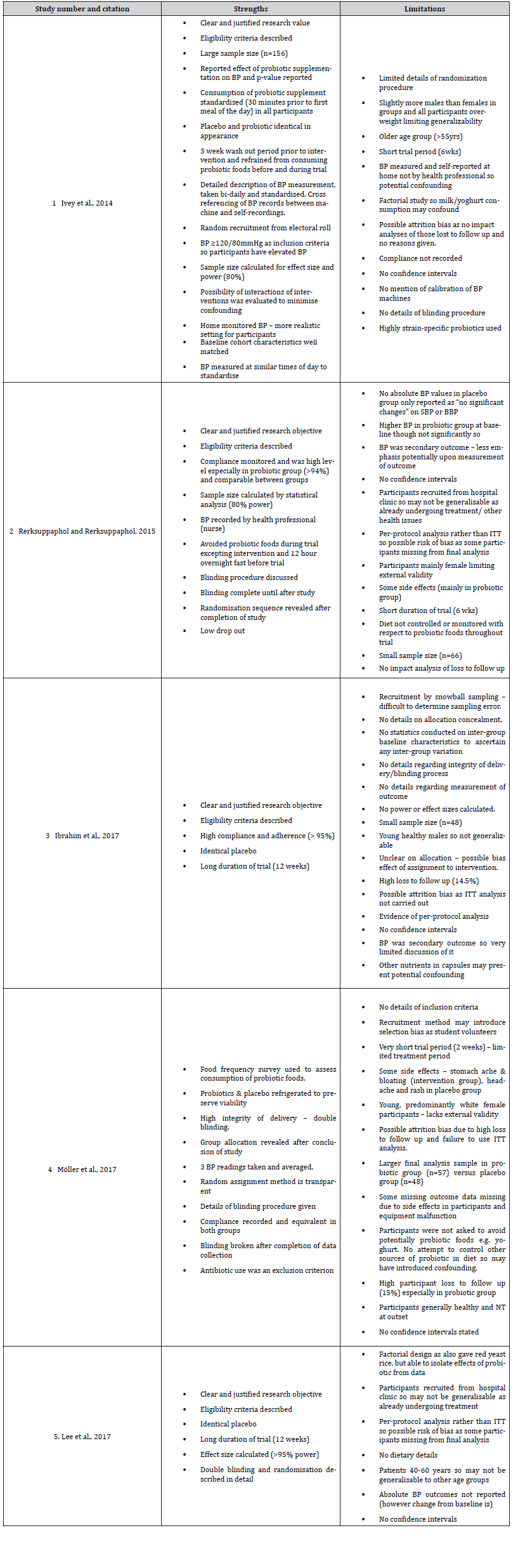
Table 6:Summary of RoB assessment of selected studies as assessed using RoB2 tool [78].
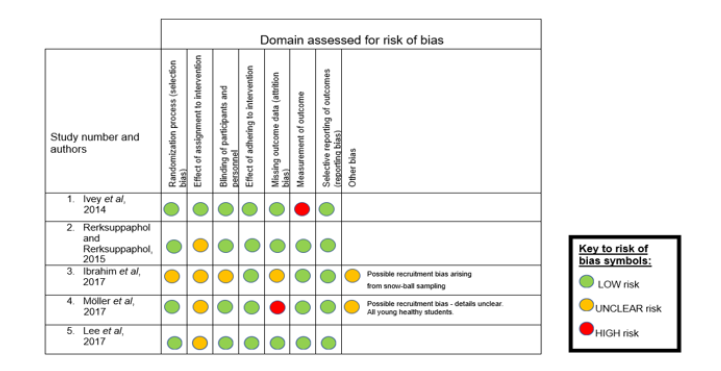
Critical analysis of included studies: Following CA and RoB assessment, it was evident that methodological quality varied between the included studies and this is detailed below.
Stronger methodological design: Three studies (1, 2 & 5) emerged as being stronger in methodological design with lower risk of bias. These were the only studies that included either pre- HT or HT participants at baseline and two of these studies (2 & 5) reported reduced BP following probiotic intervention, whilst study 1 reported no effect of the probiotic upon BP. Studies (2 & 5) supporting the intervention will be discussed first.
Study 2:Study 2 was appraised overall as high quality with low RoB, but some weaknesses were identified. Firstly, the relatively short intervention period of six weeks was regarded as a potential limitation since more effective retention of bacterial colonies have been observed with longer intervention periods [37]. Secondly there was some lack of clarity regarding assignment of intervention as some participants withdrew so it is difficult to ascertain if bias was introduced. Thirdly generalizability may be restricted as recruitment was from a clinic setting and participants had other health issues (hypercholesterolemia) [38].
Notwithstanding these potential limitations, this study was judged to be of high methodological quality for the following reasons. The study was sufficiently powered (80%) with precalculated sample size beforehand to ensure a high chance of detecting a statistically significant effect and avoiding a possible type 2 error [38]. There was high adherence to the intervention (>90% in both groups) and control of potential confounders by avoidance of probiotic food sources throughout the trial. Furthermore, measurement of outcome by a health professional was judged to possess high integrity. In summary, the overarching strengths of this study, namely high integrity and detailing in its methods, sufficiently powered calculation of effect size and minimization of confounders identified this as a robust study, which was consequently weighted highly, and would be further improved if conducted for a longer duration on a larger sample size.
Study 5:Some design shortcomings were identified, one being using Last Observation Carried Forward (LOCF) approach in final data analysis, which can introduce bias and distort findings [39]. Additionally, recruitment from a hospital clinic can potentially limit external validity [38]. However, despite these drawbacks, this study exhibited thorough pre-planning and transparency throughout; pre-specified highly powered sample size was calculated in advance, randomisation, allocation to intervention and probiotic therapy administration were detailed and explicit. Hence, this study was judged to provide good quality evidence and awarded higher weighting.
Study 1: Although considered overall to be a strong study, lack of clarity in the description of the methods of allocation to intervention and blinding deemed it impossible to judge whether there was an element of selection bias. Furthermore, it could be argued that in a home setting a high risk of bias may have been introduced in the measurement [40] owing to confounding by other factors in the home, for example alcohol consumption [41]. Notwithstanding these potential shortcomings, study 1 benefits from random recruitment from the electoral roll increasing external validity and minimizing recruitment bias [38]. Its methods were detailed and transparent with a pre-specified large (n=156) sample size to ensure sufficient power. Additionally, a three-week pre-intervention washout period and avoidance of probiotic foods throughout the trial sought to minimize confounding. Finally, this was the only study to study BP as a primary outcome so was highly relevant to the research aim. Overall, it was well conducted and consequently, weighted highly as evidence.
Weaker methodological design:The remaining included studies (3 & 4) were judged to be of lower quality evidence for reasons discussed below and, hence, awarded less weighting.
Study 3: A main limitation of study 3 was an absence of statistical analysis such as calculation of effect size and sample size was small (n=48) with a high loss to follow-up (>14%). Taken together it was impossible to know if this study was sufficiently powered [38]. Recruitment bias was also a potential concern as participants were recruited from a university setting by snowball sampling [38] making it difficult to determine the presence of sampling error or make inferences about populations. Furthermore, the study comprised of healthy young males (100%), restricting generalizability. Assessment of bias was hindered by a general lack of transparency in allocation to treatment and blinding procedure [38]. Despite the aforementioned limitations, study 3 does possess some merits namely a long trial period (12 weeks) allowing time for bacterial colonization to establish [42] and high adherence to the intervention (>95%). Overall, these weaknesses in design suggest that the findings should be interpreted with caution, hence this study was given less weighting.
Study 4:Many procedures in this study such as randomisation, blinding and administration of intervention exhibited high integrity and statistical analysis was conducted prior to the intervention. However, the recruitment setting diminished the external validity of results [38]. Furthermore, success of colonization and retention of the probiotic is questionable owing to the short timescale of two weeks [38,43]. Despite a large sample size (n=123), effect size was not pre-calculated and large loss to follow-up (15%) was reported, especially in the intervention group, which may skew the results [38,44,45]. Finally, this was the only study not to avoid extraneous probiotic foods during the trial potentially introducing confounders. In view of these limitations, this study was considered to provide low evidential value and less weighting was given to it.
A criticism of all the studies lies in the final analysis of results. The Consolidated Standards of Reporting Trials (CONSORT) [46] statement relating to RCTs recommends results are analysed based upon Intention to Treat (ITT) strategy meaning when conducting a comparison trial (probiotic vs placebo), participants who withdraw during the trial should be included in the final analysis [47] to reduce bias, which is often in favor of the intervention, especially when sample size is small [48]. Studies 1, 3, 4 and 5 carried out a per-protocol analysis, which omitted results from those lost to follow up in the final analysis, whilst study 2 did not report the analysis method. Disregarding “non- adhering” participants in a RCT undermines the principle of randomisation and makes it hard to draw valid comparisons between trial arms [49]. ITT analysis in RCTs is, therefore, regarded as an indicator of good practice [50]. Furthermore, lack of statistical analysis of results was evident in all five studies compromising methodological quality and potentially diminishing the statistical power of the results. Nevertheless, these studies did fulfil the stringent screening and selection process of this LR and the quality of their evidence will be discussed in the next section.
Overall quality of evidence assessment
Following CA, a number of criteria that determine methodological quality and risk of bias have been graded from very low to high (grades 1-4) across the studies (see Appendix 3), to generate an evidence profile (EP). RCT study designs are regarded as high-quality evidence [44,45] so were graded 4 for study design. Gradings were converted to a graphical representation (stellar chart) of the EP where the increasing length of each “spoke” of the “star” corresponds to increasing quality of evidence as below (Figure 2)
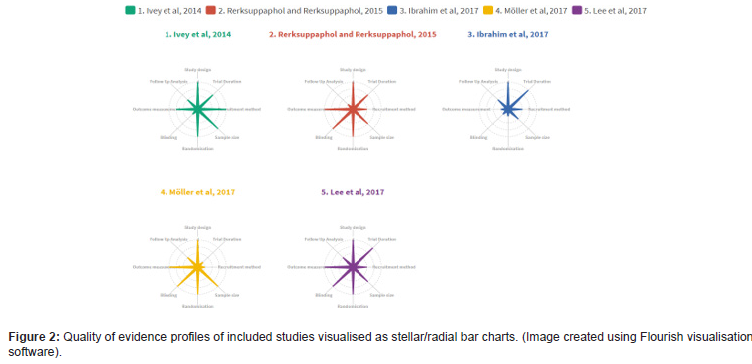
The stellar charts clearly show studies 1, 2 and 5 as high quality in most of the domains assessed, thereby increasing confidence in recommendations based on their findings. Contrastingly studies 3 and 4 displayed shortcomings in at least three domains so their findings were given less weighting.
Evidence statement
This research study presents moderate evidence based upon National Institute for Health and Care Excellence (NICE) guidelines [51] (see Appendix 4), from two robust studies (2 & 5) suggesting a net benefit of probiotic supplements in reducing BP. If the methodologically weaker studies (3 & 4) are excluded, the balance of evidence strengthens further towards probiotic supplementation being beneficial for HTN reduction. However, further research with larger scale trials using target groups such as pre-HTs/HTs for longer duration is recommended.
Discussion
Statement of findings
Two of the studies (2 & 5) observed a significant decrease in BP in the probiotic groups. Importantly, the probiotic cohorts of these studies had a higher mean baseline BP compared to the control group and classified as pre-HT and HT respectively [52]. The third study (1) composed of participants with mid pre-HT SBP and normotensive DBP at baseline and reported no effect of probiotic supplements on BP. This systematic literature review, therefore, adds to the growing body of research [22,53,42,54] indicating that baseline BP status prior to intervention may be influential upon the efficacy of the probiotic supplement.
Although included studies of this LR did not conduct GM analysis, and so it is impossible to determine whether dysbiosis was an underlying condition, wider research suggests its role in pathogenesis and maintenance of HTN [19,20,55-57].
Dysbiosis association with HTN
Existing evidence appears to support an association between gut dysbiosis and elevated blood pressure. The precise aetiology of HTN remains elusive and risk factors are multifaceted [58], however, as aforementioned, a disrupted gut microbial profile has been identified as a possible driver [20,25,56,59]. Whilst individual GM are dynamic and variable, HTs have been shown to exhibit an inverse relationship between microbial diversity and HTN [19]. A seminal study by Durgan DJ, et al. [60], in animal models demonstrated that dysbiosis causes HTN. A causal link between the GM and HTN is further supported by faecal microbiota transplantation (FMT) studies in animal and human models. Microbiota from HT donor rats was able to induce HTN in NT recipients [55,60,62]. Transferability of HBP through FMT from HT humans’ microbiota to germ free (GF) mice has also been demonstrated [59]. Taken together these results suggest the GM is susceptible to manipulation and may offer a potential therapeutic focus for HBP management. A further important finding is that dysbiosis appears to precede the development of HTN. Prehypertension (pre-HTN) is defined as a BP of 120–139 mmHg and/or a diastolic blood pressure of 80– 89 mmHg [61]. GM analysis shows minimal variation between pre- HT and HT microbial profiles in both animal and human models, but were distinctly altered from a NT GM [56,59] (suggesting that in pre-HTN dysbiosis has already occurred. Collectively, these findings suggest that ideally interventions to restore GM balance should commence at an early stage to prevent HTN. Although further research is required to clarify mechanisms through which the GM influences BP, the following section outlines hypotheses.
How dysbiosis may induce HTN: Bacterial metabolic byproducts may be important signalling molecules for BP. Short chain fatty acids (SCFAs), predominantly acetate, propionate and butyrate are produced during bacterial fermentation of dietary fibre in the colon [62,65] mainly by Firmicutes and Bacteroidetes phyla [66]. Whilst functional pathways are yet to be elucidated and specific bacterial genera of bacteria are not yet confirmed, SCFAs are thought to act as important signalling metabolites between the GM and BP [65-68]. Murine studies suggest SCFAs may signal via specialized chemoreceptors found in locations associated with BP regulation, including the walls of blood vessels and kidney tissue [65] known as G protein-coupled receptors (GPCRs) [65,67]. Moreover, depletion of some SCFA-producing bacteria, as observed in dysbiosis, are associated with development of HTN [67,69-72]. Induction of HTN in rat models correlated with reduced butyrateproducing bacteria and downregulation of butyrate metabolisms [60], whilst acetate supplementation correlated with a reduction in dysbiosis, observed as a reduced F/B ratio and reduced BP in HT animals [69,71]. Notwithstanding the limited human research, from these findings it could be hypothesized that a gut microbial shift may alter metabolite production, such as SCFAs, which may impact BP regulation as summarised in Figure 3.
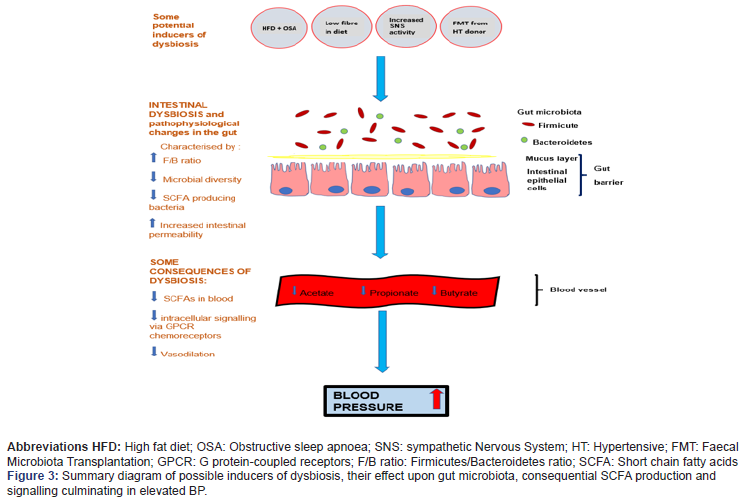
Dysbiosis facilitates inflammation: Other studies suggest the presence of gut microbiota appears necessary for HTN to develop possibly by facilitating inflammation [73], which underlies HTN [56,57,69]. HT human participants in a small Brazilian study were found to have dysbiosis and an inflamed immune profile compared to their NT counterparts [58]. Additionally, several SCFA (mainly butyrate) producers were diminished in the HTs [57]. Juanola O, et al. [72], also report diminished levels of SCFAs associated with an inflammatory profile and HTN. Although based on relatively limited samples, these findings suggest interplay between the GM, HTN and the immune system. Consequently, interventions targeting the GM, such as probiotics, may offer anti-HT potential. A number of physiological mechanisms by which probiotics may influence blood pressure are explored below.
Modulation of dysbiosis: A causal link has been demonstrated between dysbiosis and HTN [60]. Probiotics in HT rat models have been seen to ameliorate dysbiosis by reducing an elevated F/B ratio and increasing SCFA producers along with an accompanying decrease in BP [75], however they appeared ineffective on NT animals [71,75], which may imply an absence of dysbiosis. Although not human studies, these results appear to support the findings of this LR, which suggests that probiotic supplements are only effective when BP is elevated. A possible reason for this is that the HBP was driven by dysbiosis so that restoration of microbial balance by the probiotics ameliorated the HTN. Interestingly, study 1 appeared unresponsive to probiotics, which could be related to baseline BP status. This study (1) cohort was marginally pre-HT for SBP, but NT for DBP from which it could be inferred that dysbiosis was either absent or not sufficiently advanced to cause HTN. Further studies utilizing GM analysis would be useful to establish whether HTs have dysbiosis prior to probiotic intervention and the impact on the GM and BP post-intervention.
Anti-inflammatory effect and upregulation of NO improve Endothelial Function: Probiotics may reduce pro-inflammatory status and related endothelial dysfunction. Inflammation has been shown to be strongly associated with HT [57] and associated impairment of endothelial function possibly by reduction of Nitric Oxide (NO), a vasodilator [76]. Lactobacillus strains administered to SHR appear to reduce inflammatory status and upregulate bioavailability of NO, thereby improving endothelial function [76]. It is of interest that the studies used in this LR all included lactobacillus strains, but not all reduced BP suggesting other conditions may need to be present, such as dysbiosis. In conclusion, this LR provides moderate evidence for efficacy of probiotic supplements in reducing HTN. In arriving at this judgement, the presence of elevated BP at baseline is an important factor along with the relative quality of evidence from five included studies.
Evaluation of included studies
This literature review found moderate evidence for the efficacy of probiotic supplements in reducing HTN. In arriving at this judgement, the presence of elevated BP at baseline is an important factor along with the relative quality of evidence from the five included studies. Only three studies (1, 2 & 5) of the five included participants with either HTN or pre HTN prior to intervention. Two of the studies (2 & 5) are particularly relevant to the research aim as their participants had baseline BP ≥130/85mmHg, which has been found to produce a more significant reduction with probiotics than baseline BP below these values [22] and are more generalisable to clients likely to be encountered in a clinic setting. Study 2 on pre-HT participants (mean baseline BP 139.5/87.7mmHg) demonstrated improved SBP following probiotic supplementation. Despite some limitations following CA, as discussed previously, this study was considered to be of particular value to the research as it used participants with elevated BP at the outset. Study 5 also reported a significant reduction in DBP in the HT cohort (mean baseline BP was 141/85mmHg). Notwithstanding the limitations discussed previously, this highly powered study was of good methodological quality, so its findings are highly weighted. If, as research suggests, dysbiosis is causal in HTN [19,57,60], then one possible explanation for the findings in studies 2 and 5 may be that participants had imbalanced gut microbiota, which was restored by administration of the probiotic and so reduced the BP, however without GM analysis, this remains hypothetical. Contrastingly, Study 1 reported opposing findings as despite the participants having only elevated SBP (131± 13mmHg), probiotics did not lower BP. Although critically appraised to be a highquality study, there are a number of factors that may account for non-effect of the probiotic. Firstly, other literature has suggested consumption of probiotics may have a greater effect when baseline BP is borderline HT [22,42,54] with Khalesi S, et al. [23], noting a significant reduction if BP ≥ 130/85mmHg. As the SBP in study 1 was only marginally raised, the effect of the probiotic, if any, may have been insignificant. A second important factor to consider is that strain specificity of probiotics may affect efficacy. Study 1 used a two-strain preparation containing Lactobacillus acidophilus La5 and Bifidobacterium animalis subsp. lactis Bb12, which have been reported as less effective in other trials [76]. Furthermore, anti-HT effect appears more significant when daily dose is >5 x109 CFU/ day with an intervention period ≥8 weeks [42]. Study 1 used 3 x109 CFU/day for 6 weeks. Collectively these factors could mean both strains, dosage and duration were sub-optimal in study 1 to see any effect of the probiotic. Thirdly the participants in study 1 were overweight so it is possible that this was a factor in their elevated SBP rather than dysbiosis in which case probiotic consumption may have had little benefit. Several issues, therefore, emerge from study 1 that remain unclear. Without GM analysis, it is impossible to ascertain whether dysbiosis was present. Furthermore, as the DBP was not elevated and SBP was marginally pre-HT, if present, dysbiosis may not have been sufficiently advanced to have yet impacted upon BP, hence the failure to observe a response to the probiotic. Therefore, on the strength of the most robust evidence of studies 1, 2 and 5, probiotic supplements do appear to reduce BP with the caveat that the participants are HT at baseline. The other two included studies (3 & 4) were judged to be of lower quality and, importantly, were conducted upon normotensives. When these weaker studies are disregarded, the findings of this LR lend further support for the use of probiotic supplements to reduce BP in HTs. However, they indicate a need for further research using longer duration studies on HTs with specific strains of probiotics administered in capsule form. A minimum dosage of 5x109 CFU/ day would be recommended. GM analysis at baseline and postintervention would also be advantageous to establish whether dysbiosis is initially present and, if so, the effects of the probiotic upon the GM. Other SRs are consistent with the findings of this LR; [54], examined probiotic supplementation and found a short-term reduction in HBP. Whilst a large number (n=23) of RCT studies were included, methodological quality was variable, however an advantage was that nine studies were in HTs and, in seven of those, no HTN medication was being taken so the effects of the probiotic could be examined in isolation. Chi C, et al. [53], reported a significant reduction in SBP and DBP in HT human participants, particularly if Diabetes mellitus (DM) was present, although the anti-HT effect appeared age specific (≤ 60 years). Collectively these studies corroborate the findings from this literature review that probiotics can be of benefit in reduction of HTN and suggest further study focus should be upon dosage and strains in larger scale, longer duration trials on HT humans. The findings of LR are significant as, although modest BP reductions were observed in this study and the wider literature, it is suggested that even a 3.3/1.4mmHg reduction may reduce risk of a serious cardiovascular event by 22% [78].
Recommendations as to the use of probiotic supplements as an intervention when supporting HT clients in a Nutritional Therapy setting
The findings of this research study suggest probiotic supplements may be a beneficial intervention for pre-HT/HT clients, particularly where dysbiosis may be suspected to be present. The following factors should be considered when recommending probiotic supplements.
Strain specificity:A lack of human trials made it impossible to definitively determine efficacy of specific strains, however Lactobacillus sp. is present in both effective studies (2 & 5), which is consistent with the wider literature [42]. A number of studies suggest multi-strain probiotics have shown effectiveness [23,53]. The recommendation, therefore, would be a multi-strain preparation including Lactobacillus species.
Dosage and duration:Study 5 suggests dosage is effective at >2 x 108 CFU/day. Other studies suggest greater benefit may be found with a larger dosage [23,42,79]. However, there is disparity in the wider literature and clients would need to be made aware of potential side effects such as bloating or abdominal cramps. Although studies 2 and 5 reported an anti- HT effect after 6 and 8 weeks respectively, a duration of at least 8 weeks is recommended in the wider literature [42].
Age:Age of client is also a consideration as this research study reported beneficial results in 40–60-year-olds, however limited benefit was observed in over 60-year-olds [53], but no adverse effects were reported either. Further research would be required to determine efficacy in different age strata.
Administration:Both studies (2 & 5) administered the probiotic in capsule form, which is in line with recommendations in other studies [42]. Although study 5 administered the supplement post-prandially morning and evening, due to the small numbers of studies it was not possible to establish the best time to take a supplement and these may vary depending upon manufacturer’s guidelines.
Recommendations
In summary, it is recommended that a multi-strain probiotic including Lactobacillus species is taken for a minimum of eight weeks administered as per the manufacturer’s instructions. A minimal dosage of 5 x 109 CFU/day in capsule form appears to be of most benefit in HTs below 60 years.
Overview of Study Limitations
Although the empirical studies selected for this research were all RCTs, regarded as high-quality evidence when evaluating the efficacy of an intervention, the stringent eligibility criteria applied may have excluded some studies with HT participants. Excluding studies with medical co-morbidities such as T2DM was deemed necessary to prevent confounding from other health conditions and medications. However, this reduced the scope for inclusion of studies where participants were pre-HT/HT at baseline. Furthermore, none of the studies analysed GM composition so it was not possible to know if HT participants were dysbiotic at baseline and whether any reduction in BP was due to the probiotic effect on the GM. As probiotics are a growing area of research, in retrospect, searching grey literature, such as unpublished clinical trials, may have been useful to ensure that the most recent evidence was appraised [80].
Conclusion
This study found moderate evidence that probiotic supplements can reduce HTN where baseline BP is borderline HT and supports the growing body of evidence suggesting a potential role for probiotics in reduction of HTN. The research indicates dysbiosis in the GM can be a causal factor in HTN. Probiotics may restore GM balance and increase metabolites involved in BP modulation to reduce HTN. The findings of this literature review provide moderate evidence that probiotic supplements lower blood pressure where baseline BP is borderline HT and supports the growing body of evidence suggesting a potential role for probiotics in reduction of HTN. It is, therefore, recommended that probiotics are used as a complementary therapy to other HTN interventions such as dietary and lifestyle modifications to provide gut and cardiovascular support, particularly where dysbiosis is suspected. Since HTN can be linked to dietary and lifestyle factors, a Nutritional Therapist is well qualified to recommend probiotic supplements as an adjunct to additional personalized dietary/lifestyle interventions to support the cardiovascular system, for example the DASH diet [81], and advise upon incorporation of probiotic/prebiotic food sources to support general gut health. It is anticipated that probiotic supplements may form a short/medium term convenient intervention, whilst a client transitions to an improved diet. Any recommendations would need to be considered in conjunction with a client’s medical advice as any consequent BP reduction may require a BP medication review.
Further recommendations
It is evident from this review that further large-scale, longer duration empirical studies using probiotic supplements are required on HT participants to identify most effective strains, dosage, administration as well as duration of any effect. GM analysis at baseline and following intervention would also be useful to establish whether dysbiosis is concurrent with the HTN and the effect of the probiotics on both GM and HTN. This may provide evidence to develop personalized probiotic protocols in the future.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare no competing interests.
- Aagaard T, Lund H, Juhl C (2016) Optimizing literature search in systematic reviews – are MEDLINE, EMBASE and CENTRAL enough for identifying effect studies within the area of musculoskeletal disorders? BMC Med Res Methodol 16(1): 161.
- Stanaway JD (2018) Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Stu. Lancet 392(10159): 1923-1994.
- National Institute for Health and Care Excellence (2019) Hypertension in adults: diagnosis and management.
- World Health Organization (2020) Improving hypertension control in 3 million people: country experiences of programme development and implementation.
- Public Health England (2017) Health matters: combating high blood pressure.
- World Health Organisation (WHO) (2019) Hypertension.
- Allen NB, Siddique J, Wilkins JT, Shay C, Lewis CE, et al. (2014) Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age. JAMA 311(5): 490-497.
- Kearney PM, Megan Whelton, Kristi Reynolds, Paul Muntner, Paul K Whelton, et al. (2005) Global burden of hypertension: analysis of worldwide data. Lancet 365(9455): 217-223.
- Rahman M, M Mostafa Zaman, Jessica Yasmine I, Jalil Chowdhury, Ham Nazmul Ahsan, et al. (2018) Prevalence, treatment patterns, and risk factors of hypertension and pre-hypertension among Bangladeshi adults. J Hum Hypertens 32(5): 334-348.
- Zhou B (2017) Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet 389(10064): 37-55.
- National Health Service (2019) High blood pressure (hypertension).
- Moore TJ, PR Conlin, J Ard, LP Svetkey (2001) DASH (Dietary Approaches to Stop Hypertension) Diet Is Effective Treatment for Stage 1 Isolated Systolic Hypertension. Hypertension 38(2): 155-158.
- Marques FZ, Mackay CR, Kaye DM (2018) Beyond gut feelings: how the gut microbiota regulates blood pressure. Nat Rev Cardiol 15(1): 20-32.
- Tang WHW, Kitai T, Hazen SL (2017) Gut microbiota in cardiovascular health and disease. Circ Res 120(7): 1183-1196.
- Yang T, Zubcevic J (2017) Gut-brain axis in regulation of blood pressure. Front Physiol 8: 845.
- Al Khodor S, Reichert B, Shatat I F (2017) The microbiome and blood pressure: Can microbes regulate our blood pressure?’. Front Pediatr pp: 138-138.
- Qin J, Ruiqiang Li, Jeroen Raes, Manimozhiyan A, Kristoffer Solvsten B, et al. (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464(7285): 59-65.
- Kelly TN, Lydia A Bazzano, Nadim J Ajami, Hua He, Jinying Zhao, et al. (2016) Gut Microbiome Associates with Lifetime Cardiovascular Disease Risk Profile among Bogalusa Heart Study Participants. Circulation Research 119(8): 956-964.
- Sun S, Anju Lulla, Michael Sioda, Kathryn Winglee, Michael C Wu, et al. (2019) Gut microbiota composition and blood pressure: The CARDIA study. Hypertension 73(5): 998-1006.
- Yang T, Monica M Santisteban, Vermali Rodriguez, Eric Li, Niousha Ahmari, et al. (2015) Gut Dysbiosis is Linked to Hypertension. Hypertension 65(6): 1331-1340.
- Food and Agriculture Organization of the United Nations/World Health Organization (2001) Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria.
- Chen Y, Liu W, Xue J, Yang J, Chen X, et al. (2014) Angiotensin-converting enzyme inhibitory activity of lactobacillus helveticus strains from traditional fermented dairy foods and antihypertensive effect of fermented milk of strain H9. J Dairy Sci 97(11): 6680-6692.
- Khalesi S, Jing Sun, Nicholas Buys, Rohan Jayasinghe (2014) Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension 64(4): 897-903.
- Upadrasta A, Madempudi R S (2016) Probiotics and blood pressure: Current insights. Integr Blood Press Control pp: 33-42.
- Daliri EBM, Lee BH, Oh DH (2017) Current Perspectives on Antihypertensive Probiotics. Probiotics and Antimicrob Proteins 9(2): 91-101.
- Robles Vera I, Marta Toral, Miguel Romero, Rosario Jiménez, Manuel Sánchez, et al. (2017) Antihypertensive Effects of Probiotics. Curr Hypertens Rep 19(4): 1-8.
- Moher D, Alessandro Liberati, Jennifer Tetzlaff, Douglas G Altman, PRISMA Group (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097.
- The Cochrane Collaboration (2008) Standards for the REPORTING of new Cochrane Intervention Reviews.
- The Cochrane Collaboration (2008) Chapter 8: Assessing risk of bias in a randomized trial.
- Liberati A (2009) Guidelines and Guidance The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS medicine 6(7): 1-28.
- Liberati A (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology pp: e1-e34.
- Bolderston A (2008) Writing an Effective Literature Review. J Medic Imaging Radiation Sciences 39(2): 86-92.
- The Cochrane Collaboration (2008) Data extraction forms.
- The Cochrane Collaboration (2008) MECIR Manual.
- Schuneman H, et al. (2013) GRADE handbook.
- National Health Service (2019) What is blood pressure?.
- Yue Y, Xiaoxi Xu, Baoyu Yang, Jing Lu, Shuwen Zhang, et al. (2020) Stable Colonization of Orally Administered Lactobacillus casei SY13 Alters the Gut Microbiota. Biomed Res Int.
- Greenhalgh, T (1997) Assessing the Methodological Quality of Published papers. BMJ 315(7103): 305-308.
- Lachin JM (2016) Fallacies of last observation carried forward analyses. Clin Trials 13(2): 161-168.
- Gonçalves VSS, Keitty RC Andrade, Kenia MB Carvalho, Marcus T Silva, Mauricio G Pereira, et al. (2018) Accuracy of self-reported hypertension: A systematic review and meta-analysis. J Hypertens 36(5): 970-978.
- Obara T, Takayoshi Ohkubo, Michihiro Satoh, Nariyasu Mano, Yutaka Imai (2010) Home and Office Blood Pressure Control among Treated Hypertensive Patients in Japan: Findings from the Japan Home versus Office Blood Pressure Measurement Evaluation (J-HOME) Study. Pharmaceuticals 3(2): 419-432.
- Liu J, Dan Zhang, Yingze Guo, Hongwei Cai, Keyuan Liu, et al. (2020) The Effect of Lactobacillus Consumption on Human Blood Pressure: a Systematic Review and Meta-Analysis of Randomized Controlled Trials. Complement Ther Med 54: 1-7.
- Unno T, Jung Hye Choi, Hor Gil Hur, Michael J Sadowsky, Young Tae Ahn, et al. (2015) Changes in human gut microbiota influenced by probiotic fermented milk ingestion. J Dairy Sci 98(6): 3568-3576.
- Guyatt, G, Andrew DO, Elie A Akl, Regina Kunz, Gunn Vist, et al. (2011) GRADE guidelines: 1. Introduction - GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64(4): 383-394.
- Guyatt GH, Andrew D Oxman, Gunn Vist, Regina Kunz, Jan Brozek, et al. (2011) GRADE guidelines: 4. Rating the quality of evidence - Study limitations (risk of bias). J Clin Epidemiol 64(4): 407-415.
- CONSORT (2010) Transparent reporting of trials.
- Del Re AC, Natalya CM, Janet CB, John WF (2013) Intention-to-treat analyses and missing data approaches in pharmacotherapy trials for alcohol use disorders. BMJ Open 3(11): 1-6.
- Bondemark L, Abdulraheem S (2018) Intention to treat (ITT) analysis as reported in orthodontic randomized controlled trials—evaluations of methodology and recommendations for the accurate use of ITT analysis and handling dropouts. Eur J Orthod 40(4): 409-413.
- Rangathan P, Pramesh C, Aggarwal R (2016) Common pitfalls in statistical analysis: Intention-to-treat versus per-protocol analysis. Perspect Clin Res 7(3): 144-146.
- McCoy C (2017) Understanding the Intention-to-treat Principle in randomized controlled Trials. West J Emerg Med 18(6): 1075-1078.
- National Institute for Health and Care Excellence (2020) Developing NICE guidelines: the manual.
- Svetkey LP (2005) Management of Prehypertension. Hypertension pp: 1056-1061.
- Chi C, Li C, Wu D, Buys N, Wang W, et al. (2020) Effects of Probiotics on Patients with Hypertension: a Systematic Review and Meta-Analysis. Curr Hypertens Rep 22(5): 34.
- Qi D, Nie XL, Zhang JJ (2020) The effect of probiotics supplementation on blood pressure: a systemic review and meta-analysis. Lipids Health Dis19(1): 79.
- Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, et al. (2017) Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics 49(2): 96-104.
- Santisteban MM, Yanfei Qi, Jasenka Zubcevic, Seungbum Kim, Tao Yang, et al. (2017) Hypertension-Linked Pathophysiological Alterations in the Gut. Circ Res 120(2): 312-323.
- Silveira Nunes G, Danielle Fernandes D, Luiz Roberto ADO, Eloisa Helena Medeiros C, Tatiani Uceli M, et al. (2020) Hypertension Is Associated with Intestinal Microbiota Dysbiosis and Inflammation in a Brazilian Population. Front Pharmacol 11: 1-14.
- Pazoki R, Abbas Dehghan, Evangelos Evangelou, Helen Warren, He Gao, et al. (2018) Genetic predisposition to high blood pressure and lifestyle factors: Associations with midlife blood pressure levels and cardiovascular events. Circulation 137(7): 653-661.
- Li J, Fangqing Zhao, Yidan Wang, Junru Chen, Jie Tao, et al. (2017) Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 5(14): 1-19.
- Durgan DJ, Bhanu P Ganesh, Julia L Cope, Nadim J Ajami, Sharon C Phillips, et al. (2016) Role of the Gut Microbiome in Obstructive Sleep Apnea-Induced Hypertension. Hypertension 67(2): 469-474.
- Mell B, Jala Venkatakrishna R, Anna V Mathew, Jaeman Byun, Harshal Waghulde, et al. (2015) Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics 47(6): 187-197.
- Toral M, Iñaki Robles Vera, Néstor de la Visitación, Miguel Romero, Tao Yang, et al. (2019) Critical Role of the Interaction Gut Microbiota-Sympathetic Nervous System in the Regulation of Blood Pressure. Front Physiol 10: 1-14.
- Zhang W, Li N (2011) Prevalence, Risk Factors, and Management of Prehypertension. Int J Hypertens.
- Miyamoto J, Mayu Kasubuchi, Akira Nakajima, Junichiro Irie, Hiroshi Itoh, et al. (2016) The role of short-chain fatty acid on blood pressure regulation. Curr Opin Nephrol Hypertens 25(5): 379-383.
- Natarajan N, Daijiro Hori, Sheila Flavahan, Jochen Steppan, Nicholas A Flavahan, et al. (2016) Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol Genomics 48(11): 826-834.
- Chung WSF, Alan W Walker, Petra Louis, Julian Parkhill, Joan Vermeiren, et al. (2016) Modulation of the human gut microbiota by dietary fibres occurs at the species level. BMC Biol 14(3): 1-13.
- Pluznick JL, Ryan J Protzko, Haykanush Gevorgyan, Zita Peterlin, Arnold Sipos, et al. (2013) Olfactory receptor responding to gut microbiotaderived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A 110(11): 4410-4415.
- de la Cuesta Zuluaga J, Noel T Mueller, Rafael Álvarez Quintero, Eliana P Velásquez Mejía, et al. (2018) Higher Fecal Short-Chain Fatty Acid Levels Are Associated with Gut Microbiome Dysbiosis, Obesity, Hypertension and Cardiometabolic Disease Risk Factors. Nutrients 11(51): 1-16.
- Marques FZ, Erin Nelson, Po-Yin Chu, Duncan Horlock, April Fiedler, et al. (2017) High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation 135(10): 964-977.
- Yan Q, Yifang Gu, Xiangchun Li, Wei Yang, Liqiu Jia, et al. (2017) Alterations of the gut microbiome in hypertension. Front Cell Infect Microbiol pp: 1-9.
- Ganesh Bhanu P, James W Nelson, Joshua R Eskew, Arunkumar Ganesan, Nadim J Ajami, et al. (2018) Prebiotics, probiotics, and acetate supplementation prevent hypertension in a model of obstructive sleep apnea. Hypertension 72(5): 1141-1150.
- Yang F, Hengwen Chen, Yonghong Gao, Na An, Xinye Li, et al. (2020) Gut microbiota-derived short-chain fatty acids and hypertension: Mechanism and treatment. Biomed Pharmacother 102(7): 1-15.
- Karbach SH, Tanja Schönfelder, Ines Brandão, Eivor Wilms, Nives Hörmann, et al. (2016) Gut Microbiota Promote Angiotensin II-Induced Arterial Hypertension and Vascular Dysfunction. J Am Heart Assoc 5(9): 1-29.
- Juanola O, José Ferrusquía A, Rocío García V, Pedro Z, Marta Magaz, et al. (2019) Circulating levels of butyrate are inversely related to portal hypertension, endotoxemia, and systemic inflammation in patients with cirrhosis. FASEB J 33(10): 11595-11605.
- Robles Vera I, Marta Toral, Néstor de la Visitación, Manuel Sánchez, Manuel Gómez-Guzmán, et al. (2020) Probiotics Prevent Dysbiosis and the Rise in Blood Pressure in Genetic Hypertension: Role of Short-Chain Fatty Acids. Mol Nutr Food Res 64(6): 1-13.
- Gómez Guzmán M, Marta Toral, Miguel Romero, Rosario Jiménez, Pilar Galindo, et al. (2015) Antihypertensive effects of probiotics Lactobacillus strains in spontaneously hypertensive rats. Mol Nutr Food Res 59(11): 2326-2336.
- Asemi Z, Samimi M, Tabassi Z, Rad MN, Foroushani AR, et al. (2013) Effect of daily consumption of probiotic yoghurt on insulin resistance in pregnant women: A randomized controlled trial. Eur J Clin Nutr 67(1): 71-74.
- Sleight P, S Yusuf, J Pogue, R Tsuyuki, R Diaz, et al. (2001) Blood-pressure reduction and cardiovascular risk in HOPE study. Lancet 358(9299): 2130-2131.
- Szulińska M, Igor Łoniewski, Katarzyna Skrypnik, Magdalena Sobieska, Katarzyna Korybalska, et al. (2018) Multispecies probiotic supplementation favorably affects vascular function and reduces arterial stiffness in obese postmenopausal women—A 12-week placebo-controlled and randomized clinical study. Nutrients 10(11): 1672.
- Conlin PR, D Chow, ER Miller, L P Svetkey, PH Lin, et al. (2000) The effect of dietary patterns on blood pressure control in hypertensive patients: Results from the dietary approaches to stop hypertension (DASH) trial. Am J Hypertens 13(9): 949-955.
- The Cochrane Collaboration (2011) RoB2.0: A revised Cochrane risk-of-bias tool for randomized trials.
-
Helen J Broomfield, Miranda D Harris, Joanna L R Goldie. Could Probiotic Supplements Be an Effective Intervention to Reduce Hypertension? A Systematic Literature Review. On J Complement & Alt Med. 7(3): 2022. OJCAM.MS.ID.000661.
-
Probiotic Supplement; Hypertension; Dysbiosis; Blood Pressure; Gut microbiome; Probiotic Supplements; Effective Intervention; Reduce Hypertension; Probiotic supplementation; Medical interventions; World Health Organization; Sympathetic nervous system; Randomised controlled trial
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






