 Letter to Editor
Letter to Editor
Update: Observations on Fentanyl Designer Drugs
Joshua Castleberry1*, Michael Leeman2, Justin Watts3 and Haley Moon4
1Department of Counselor Education and Supervision, Kent State University, USA
2Department of Communication Sciences and Disorders, Southeastern Lousiana University, USA
3Department of Rehabilitation Health Services, University of North Texas, USA
4Counseling and Psychological Services, Georgia State University, USA
Joshua Castleberry, Department of Counselor Education and Supervision, Kent State University, USA.
Received Date: August 04, 2023; Published Date: August 24, 2023
Abstract
We have previously reported the close association of designer fentanyl drugs with the presence of fentanyl. We continued this study by examining the presence of two other fentanyl structural analogs with fentanyl in urine drug tests. Based on drug testing on 1650 patient samples, we observed 119 positives for fentanyl (7.20% positivity), 23 positives for para-fluorofentanyl and 16 positives for despropionyl-p-fluorofentanyl. Both these analogs had a 100% correlation with fentanyl. In addition, we observed 8 urines positive for both xylazine and fentanyl. We do not know the source of the designer fentanyl analogs, but it appears that testing for fentanyl alone is adequate to detect fentanyl abuse.
Keywords:Fentanyl; Designer drugs; Drug testing; Fentanyl analogs
Background
One of the purposes of illicit designer drugs is to avoid detection by the usual urine drug testing methods. As a consequence, laboratories set up methods to detect these compounds. Analytical methods using LC-MS/MS methods can detect targeted designer drug derivatives of cannabinoids, benzodiazepines, and synthetic opioids such as fentanyl. There has been much interest in fentanyl analogs being present in substance abuse users [1-5]. Because of the specificity termed targeted testing, the laboratory is limited to a short list of designer drugs so it must be selective to detect the most prevalent ones [6]. The state of Massachusetts MADDS program identified two fentanyl analogs prevalent in its substance abuse users: para-fluorofentanyl and despropionyl-p-fluorofentanyl [7]. In addition, the CDC [8], and the State of Michigan [9], observed many overdose deaths associated with the p-fluoro analog. We examined a number of specimens from rehabilitation programs to determine the association of these two analogs with fentanyl.
Methods
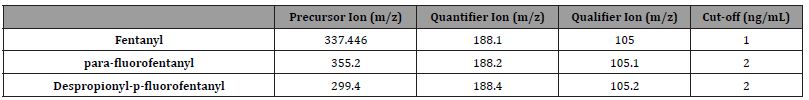
This study was approved by WCG IRB Puyallup, WA. Convenience specimens from rehabilitation facilities were tested using a modification of the method of Krock et al. [10,11]. The standards were obtained from Cerilliant and Cayman Chemical. The Sciex instruments were set at 355.2 m/z for the parent parafluorofentanyl, and the qualifier ion set at 105.1 m/z and the quantifier at 188.2 m/z. For the despropionyl-p-fluorofentanyl, the instruments were set at 299.4 m/z for the parent despropionylp- fluorofentanyl, and the qualifier ion set at 105.2 m/z and the quantifier at 188.4 m/z. The precursor, quantifier, and qualifier ions are listed in Table 1. The data was assembled using previously reported method [12].
Table 1:MRM transitions and cutoffs for fentanyl and its analogs.
Results
Based on drug testing on 1650 patient samples, we observed 119 positives for fentanyl (7.20% positivity), 23 positives for parafluorofentanyl and 16 positives for despropionyl-p-fluorofentanyl, both these analogs had a 100% correlation with fentanyl. In addition, we observed 8 urines positive for both xylazine and fentanyl.
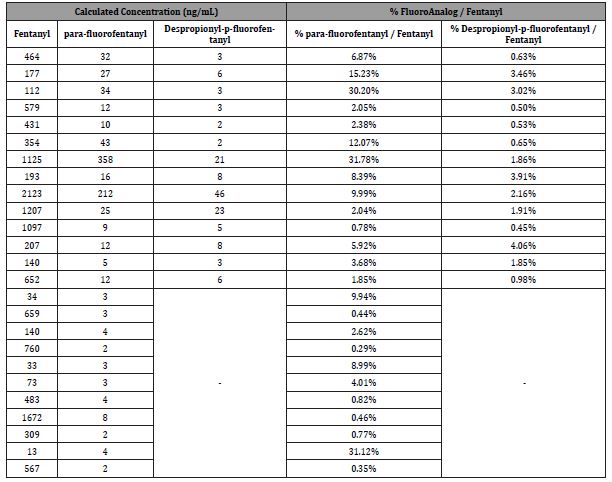
We observed a large difference in the mean fentanyl concentrations between the ones positive for the p-fluoro and those not positive. Those positive for p-fluoro have much greater fentanyl concentrations 544 ng/mL and about 198 ng/mL for the negative ones. The incidence of designer fentanyl compounds was observed only about 10% of the time.
Table 2 shows the frequency of the designer fentanyls and fentanyl. Both the parent drug and its analog always occurred together. The analogs were always present at lower concentrations. These lower concentrations are consistent with the possibility that these were added separately.
Table 2:Summary data for Fentanyl, para-fluorofentanyl, Despropionyl-p-fluorofentanyl.
Discussion
The observations of the presence of these compounds from sources such as the Massachusetts MADDS program, the CDC Morbidity and Mortality letter, and the Michigan death study would indicate this compound is a major problem [7-9]. These studies would indicate that laboratories should be set up to monitor these designer analogs. The data presented here indicates that the presence of fentanyl itself is a good indicator of illicit drug use.
We do not know the source of these analogs. Because of their low concentrations compared to fentanyl, one possibility is that they are made from poorly sourced fentanyl precursors. A second possibility is that the addition of these compounds enhances the opiate high, although the pharmacogenomics in humans is not well known [13]. The Massachusetts Drug Supply Data Stream (MADDS) report states that the 4 fluorofentanyl has a higher toxicity risk than fentanyl [7]. Because our data is consistent with the p-fluoro analog being added to the fentanyl supply as an additive we favor this explanation.
Acknowledgement
None.
Conflict of Interest
Author declared no conflict of interest.
References
- The NIH Almanac. National Institute on Drug Abuse (NIDA). National Institutes of Health (NIH).
- Armenian P, Kathy T Vo, Jill Barr-Walker, Kara L Lynch (2018) Fentanyl, fentanyl Analogs and novel synthetic opioids: A comprehensive review. Neuropharmacology 134(Pt A): 121-132.
- Mirjam de Bruin-Hoeg´ee, Djarah Kleiweg, Daan Noort, Arian C. van Asten (2021) Chemical attribution of fentanyl: The effect of human metabolism. Forensic Chemistry 24: 100330.
- (2016) EMCDDA–Europol Joint Report on acetylfentanyl. Joint Reports. European Monitoring Centre for Drugs and Drug Addiction.
- (2017) Report on the risk assessment of N-(1‑phenethylpiperidin-4-yl)-N-phenylacrylamide (acryloylfentanyl) in the framework of the Council Decision on new psychoactive substances. Risk Assessments. European Monitoring Centre for Drugs and Drug
- Agnes Cua, Kevin Krock, Richard Thomas, Amadeo J Pesce (2023) Observations on Fentanyl Designer Drugs. Open Access J Addict & Psychol 6(5): 1-7.
- Massachusetts Drug Supply Data Stream (MADDS) Community Drug Supply Alert.
- Biting J, O’Donnell J, Mattson CL (2022) Notes from the Field: Morbidity and Mortality Weekly report Overdose deaths involving para fluorofentanyl in the United States, July 2020–June 2021. Centers for Disease Control and Prevention Morbidity and Mortality Weekly Report 71(39): 1239-1240.
- Emerging Trend: Para-fluorofentanyl (pFF) Increase in Michigan 2018-2022 Year-to-Date.
- Amadeo Pesce, Raymond Sundyhanata, Dennis Ritz, Richard Thomas, Gregory Ackerman, et al (2021) Effects of a Pandemic and Isolation on Alcohol and Psychoactive Medication Use in a Population of Rehabilitation and Pain Patients. Ann Clin Lab Sci 51(5): 694-697.
- Krock K, Pesce A, Ritz D, Thomas R, Cua A, et al. (2017) Lower Cutoff for LC-MS/MS Urine Drug Testing Indicates Better Patient Compliance. Pain Physician 20(7): E1107-E1113.
- Pesce AJ, Chandler N, Ackerman G (2021) Information Technology Structure for Urine Drug Testing Reports, 21st Century Pathol 1 (1): 103.
- Hassanien SH, Bassman JR, Naccarato CMP, Twarozynski JJ, Traynor JR, et al. (2020) In vitro pharmacology of fentanyl analogs at the human mu opioid receptor and their spectroscopic analysis. Drug Test Anal 12(8): 1212–1221.
-
Agnes Cua, Kevin Krock, Richard Thomas and Amadeo J Pesce*. Update: Observations on Fentanyl Designer Drugs. Open Access J Addict & Psychol 7(3): 2023. OAJAP.MS.ID.000665.
Fentanyl; Designer drugs; Drug testing; Fentanyl analogs; Cannabinoids; Benzodiazepines; Synthetic opioids; Drug derivatives; Substance abuse users; Rehabilitation; Drug use
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






