 Research Article
Research Article
Observations on Fentanyl Designer Drugs
Agnes Cua, Kevin Krock, Richard Thomas and Amadeo J Pesce*
Precision Diagnostics LLC, San Diego, CA, USA
Amadeo J Pesce, Professor, Precision Diagnostics LLC, 4215, Sorrento Valley, Boulevard, San Diego, CA 92121, United States.
Received Date: March 01, 2023; Published Date: March 23, 2023
Abstract
Designer drugs are made to serve substance abuse users and to avoid detection by the usual urine drug testing methods. Most initial screening is performed by immunoassays, but these are limited in their specificity and sensitivity. Mass spectrometry methods using LC-MS/MS methods can detect targeted designer drug derivatives of cannabinoids, benzodiazepines, and synthetic opioids such as fentanyl. However, this method of analysis is limited to a short list of designer drugs. In spite of this limitation, we added six fentanyl derivatives to our test panel in 2021. We determined the number of positive findings in more than 600,000 specimens tested in each of the years 2021 and 2022. We observed 56,569 of the 1,427,159 specimens or about 4.6% of specimens to be positive for fentanyl. However, the incidence of designer fentanyl compounds was significantly less. We considered that two of the designer fentanyl’s we observed were impurities of fentanyl synthesis.
Background
Drug testing was jump started by the need to test Vietnam war veterans for drug use. It was limited to the NIDA 5 opiate, cocaine, methamphetamine, marijuana, and phencyclidine (PCP) [1]. In the early 2000’s synthetic cannabinoids were sold to substance abusers because they gave the same “high” and were not detected by the usual drug testing techniques of immunoassay and GC mass spectrometry. The users were able to avoid detection of their drug use [2-5]. Laws were changed to include these cannabinoid drugs as prohibited. Most recently this concept of modified substance abuse compounds has been applied to those of the opiate and benzodiazepine classes [6-8]. The challenge to detect these designer drugs has fallen to laboratories that perform definitive testing using LC-MS/MS. Unfortunately, the limitation of this method is that most laboratories set their instruments to detect and quantify a limited number of compounds and thus a limited number of variants of a drug class.
Most recently fentanyl has become the drug of choice sold by the cartels, and while laboratories have altered their detection methods to detect its use, the sheer number of possible fentanyl derivatives, limits those that can be detected by this method [8-11]. In spite of this limitation, we chose to detect the use of 6 fentanyl analogs in our drug testing population. We chose to design a method based on the DEA drug seizure of fentanyl analogs based on their 2017 report [12]. These drugs and metabolites were fentanyl, acetylfentanyl, acetylnorfentanyl, acrylfentanyl, butyrylfentanyl, butyrylnorfentanyl, cis-3-methylfentanyl, cis-3-methylnorfentanyl, furanylfentanyl, and norcarfentanyl.
Methods
This study was approved by WCG IRB Puyallup, WA. From Jan 1, 2021, to November 13, 2022, we performed drug testing on 1,427,159 urine specimens received from clients, mainly pain clinics and rehabilitation centre’s [13]. The analytical method for the monitoring of drugs was that of Krock et al. [14], with the addition of the fentanyl analogs (Table 1). The precursor, quantifier, and qualifier ions are listed in Table 1 The data was downloaded into a Starlims TM database. Data tables were generated by Power BITM [15].
Results
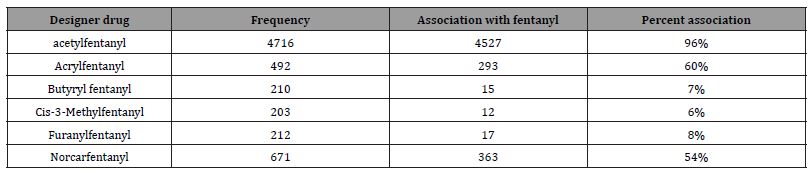
The number of positive fentanyl urine specimens for 2021 and 2022 was 25,544 and 31,025 respectively. In our system we noted more norfentanyl than fentanyl positive urines. We attribute this to the difference in cut offs and that it appears that the metabolite is in greater quantity than the parent drug. With the median value of urinary fentanyl at 47ng/mL while that of the norfentanyl was 206ng/mL. The distribution curves are presented in Figure 2 & 3. Table 2 shows the frequency of the designer fentanyls and their norfentanyl metabolites. Both the parent drug and its metabolite always occurred together. From this we determined It appears that like fentanyl the nor metabolites are a major metabolic pathway. Compared to fentanyl, the acetyl fentanyl and acetylnorfentanyl had lower median concentrations of 5.7ng/mL and 18.6ng/mL respectively. Their distribution curves are presented in Figures 4 & 5. These lower concentrations are consistent with the possibility that these were process impurities. Using a data set that encompassed the years 2016 to 2022, in order to establish this possibility, we compared the frequency of the designer fentanyls with fentanyl (Table 3). We examined the frequency at which we observed both fentanyl and acetylfentanyl together and this was about 96%%. Both these observations of low urinary concentrations and correlation with fentanyl lead us to believe that the observed acetylfentanyl is a by-product of the fentanyl synthesis production process. Acrylfentanyl was observed 492 times at the at low concentration of 4ng/mL and about 60% of the time associated with fentanyl making it not possible to definitively establish it as a synthetic impurity. The remaining designer fentanyl compounds were correlated with fentanyl in very low frequency (Tables 2 & 3). These derivatives did not have a close relationship with fentanyl and were considered as true designer fentanyl compounds.
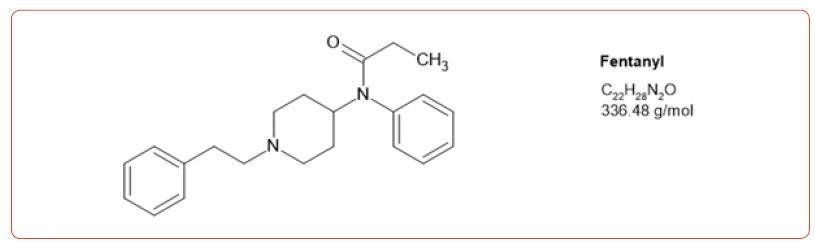
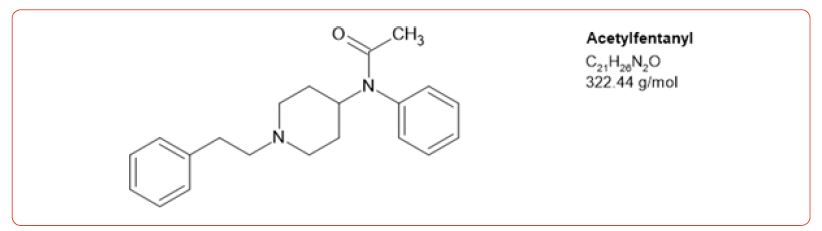
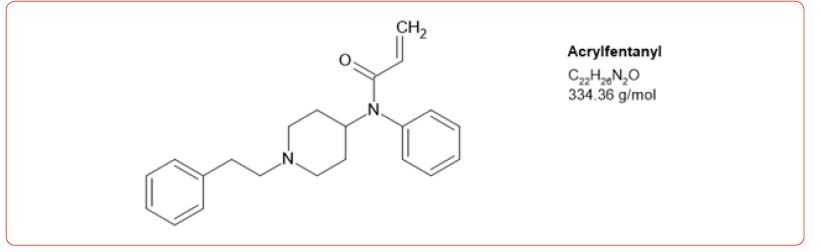
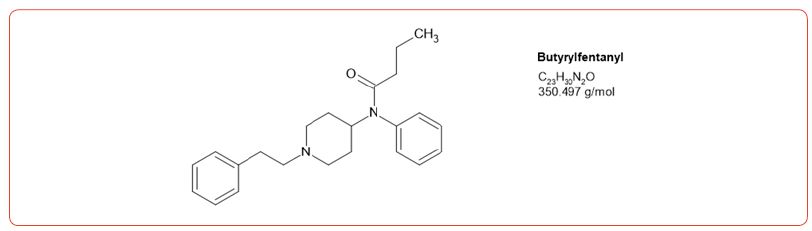
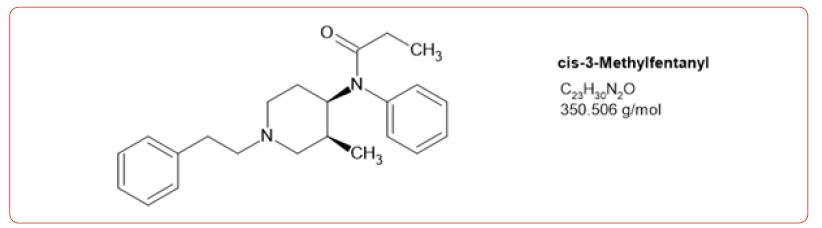
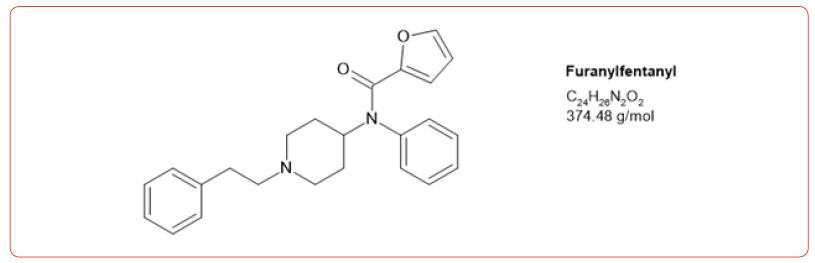
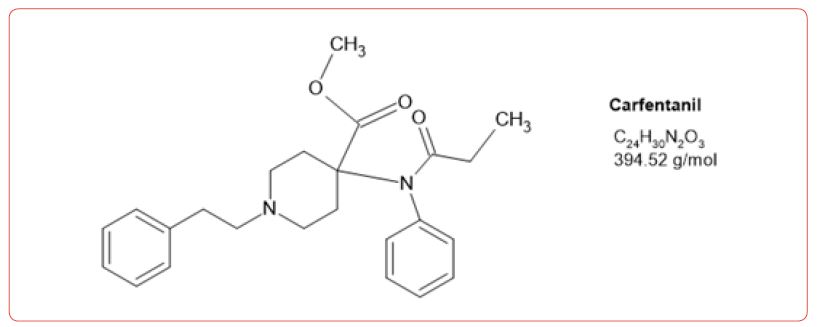
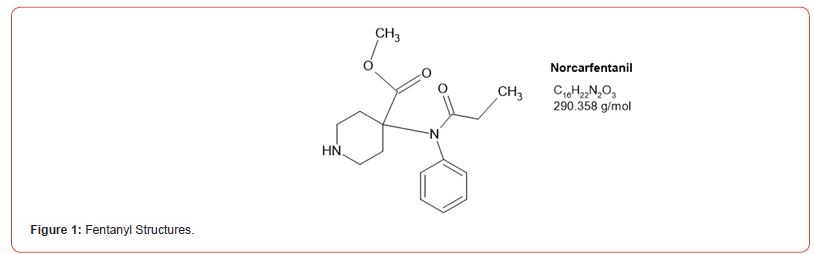
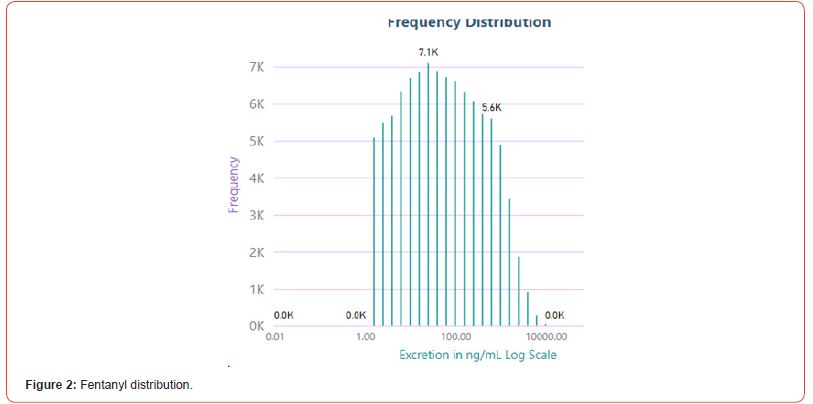
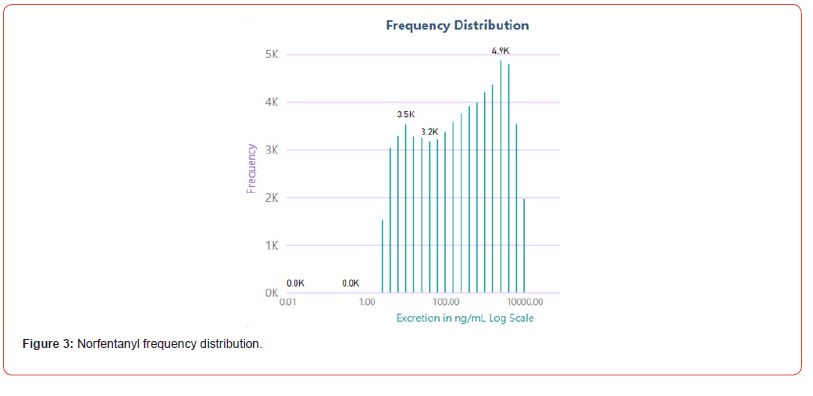
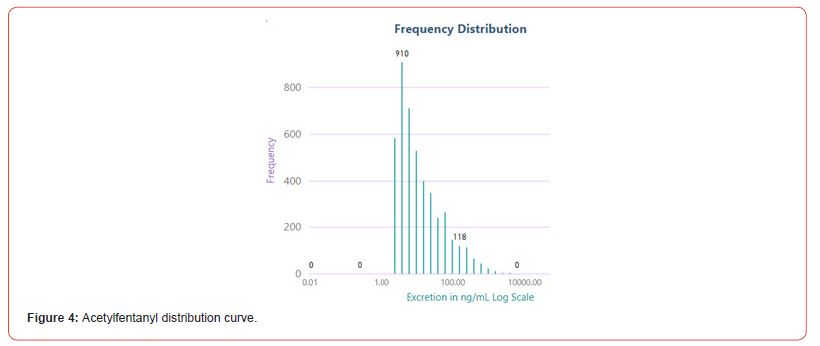
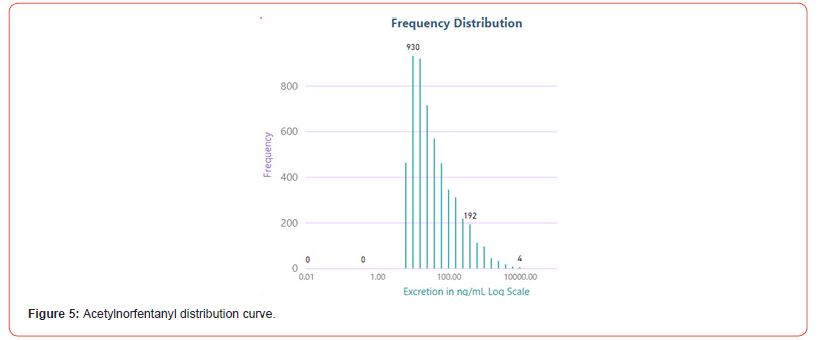
Table 1:MRM transitions and cutoffs for fentanyl and its analogs.
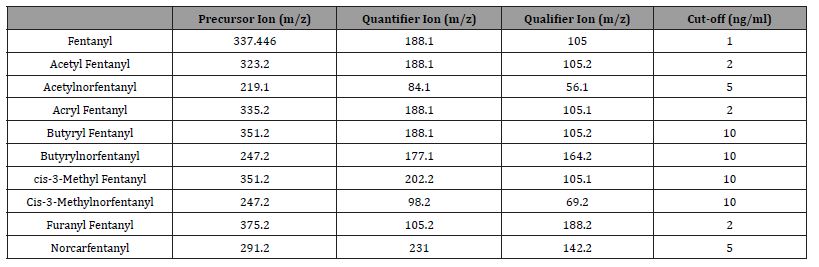
Table 2:Frequency of detection of fentanyl and fentanyl analogs from 2021 to 2022.
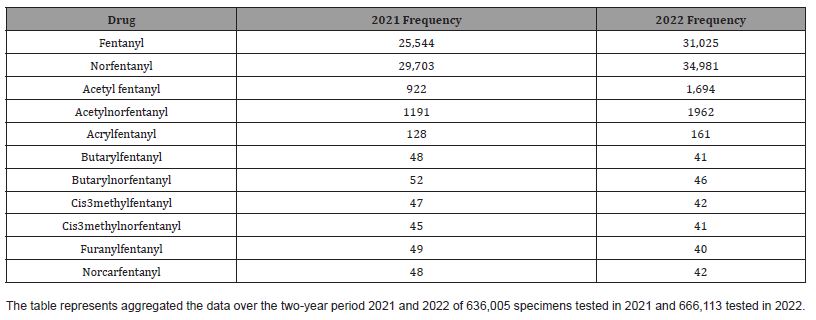
Table 3:Frequency of association of possible designer fentanyls with fentanyl based on observations from 2016 to 2022.
Discussion
We chose to monitor 6 fentanyl analogs out of a possible hundred or more. Our selection was based on the 2017 DEA report on synthetic fentanyl compounds [12]. The highest positivity rate was for acetyl fentanyl, but this was about 3% of the incidence of the fentanyl positivity. To our surprise, it appears that the acetyl fentanyl and the acrylfentanyl may be impurities made during the fentanyl synthesis process. We did not observe a high frequency of the other designer fentanyl compounds. This contrasts with what we expected from the DEA report. We may have missed some of the other designer compounds which is illustrative of the problem of identifying these compounds in a high throughput laboratory using a targeted drug system.
The observations of Maximo J Marin, and Xander MR van Wijk for designer benzodiazepines apply to designer fentanyls. “Based on the history of cannabinoids and benzodiazepines, we expect that we shall observe more of these derivatives as substance abusers attempt to avoid detection.” [12].
Conclusion
We also observed that the nor metabolites appeared to be a major metabolic pathway for these compounds and often appeared to be present in higher concentrations than the parent drug.
Acknowledgement
None.
Conflict of Interest
Author declared no conflict of interest.
References
- The NIH Almanac. National Institute on Drug Abuse (NIDA), National Institutes of Health (NIH).
- Synthetic cannabinoids.
- Synthetic cannabinoids drug profile. European monitoring centre for drugs and drug addiction.
- Liana F, Walter F (2011) Beyond THC: The New Generation of Cannabinoid Designer Drugs. Front Behav Neurosci 5: 60.
- Vera LA, João LG, Joselin A, Helena MT, José SC (2020) The synthetic cannabinoids phenomenon: from structure to toxicological properties. A review. Crit Rev Toxicol 50(5): 359-382.
- Pietro B, Raffaele G, Adriano T, Marilyn AH, Francesco PB (2021) Designer Benzodiazepines: A Review of Toxicology and Public Health Risks. Pharmaceuticals (Basel) 14(6): 560.
- Maximo JM, Xander MR van Wijk (2019) The Evolution of Designer Benzodiazepines Challenges for detection and monitoring. Clinical Laboratory News.
- Armenian P, Kathy TV, Jill BW, Kara LL (2017) Fentanyl, fentanyl Analogs and novel synthetic opioids: A comprehensive review. Neuropharmacology 134(Pt A): 121-132.
- Mirjam de Bruin-Hoeg´ee, Djarah Kleiweg, Daan Noort, Arian C van Asten (2021) Chemical attribution of fentanyl: The effect of human metabolism. Forensic Chemistry 24: 100330.
- (2016) EMCDDA–Europol Joint Report on a new psychoactive substance: N-phenyl-N-[1-(2-phenylethyl) piperidin-4-yl] acetamide (acetylfentanyl). EMCDDA–Europol joint publication EMCDDA–Europol Joint Report on acetylfentanyl.
- (2017) EMCDDA–Europol Joint Report on a new psychoactive substance Report on the risk assessment of N-(1-phenethylpiperidin-4-yl)-N-phenylacrylamide (acryloylfentanyl) Joint Reports.
- (2017) 2017 National Drug Threat Assessment. US Department of Justice Drug Enforcement Administration.
- Amadeo P, Raymond S, Dennis R, Richard T, Gregory A, et al. (2021) Effects of a Pandemic and Isolation on Alcohol and Psychoactive Medication Use in a Population of Rehabilitation and Pain Patients. Ann Clin Lab Sci 51(5): 694-697.
- Krock K. Pesce A, Ritz D, Thomas R, Cua A, et al. (2017) Lower Cutoff for LC-MS/MS Urine Drug Testing Indicates Better Patient Compliance. Pain Physician 20(7): E1107-E1113.
- Pesce AJ, Chandler N, Ackerman G (2021) Information Technology Structure for Urine Drug Testing Reports, 21st Century Pathol 1 (1): 103.






