 Research Article
Research Article
Impact of Florida Prescription Drug Monitoring Program/Pill Mill Legislation and OxyContin® Reformulation on Tablets Dispensed in Florida and California
Paul M Coplan1,2*, Venkatesh Harikrishnan1 and Dart Richard2
1Medical Affairs Strategic Research, Purdue Pharma LP, USA
2Department of Clinical Biostatistics and Epidemiology, University of Pennsylvania Perelman School of Medicine, USA
Paul Coplan, Department of Clinical Biostatistics and Epidemiology, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104, USA
Received Date: February 2, 2023; Published Date: March 23, 2023
Abstract
Purpose: To assess whether 2 events separated by time and region had distinct effects on opioid prescriptions: 1) OxyContin® reformulation with abuse-deterrent properties nationally starting August 2010 and 2) Florida prescription drug monitoring program (PDMP)/pill mill legislation in Florida starting July 2011.
Methods: Prescriptions for extended-release (ER) and immediate-release (IR) oxycodone single-entity (SE) by tablet strength and 2 states were assessed (IQVIA Xponent™ data). The number of tablets dispensed were compared for the 1month period before reformulation to a period of 4 months after reformulation of OxyContin reformulation with abuse deterrent formulation. Similar comparisons were made before and after implementation of Florida PDMP/pill mill. The state of California was used as a control.
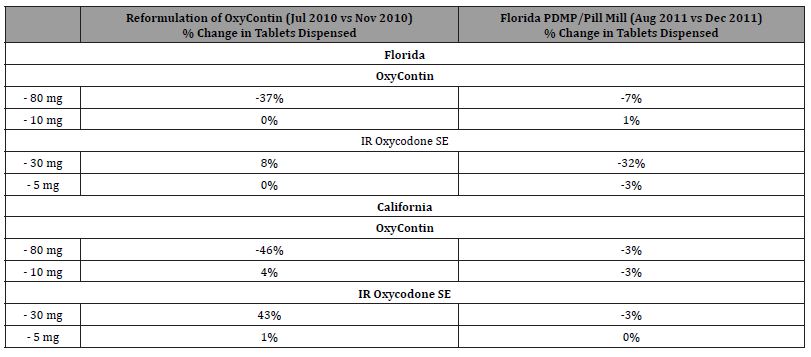
Results: The number of 80mg OxyContin tablets dispensed after its reformulation decreased in Florida (-37%) and California (-46%) with little change for 10mg OxyContin tablets (0% and +4%). In contrast, IR oxycodone SE 30mg tablets increased (+8% in Florida and +43% in California, respectively) and IR oxycodone SE 5 mg tablets did not change (0% and 1%). After implementation of Florida PDMP/pill mill legislation, IR oxycodone SE 30mg tablets decreased in Florida (-32%) but not California (-3%) with little change in IR oxycodone SE 5mg tablets (-3% and 0%), and OxyContin 80mg tablets (-7% and -3%) or 10mg tablets (+1% and -3%).
Conclusion: An analysis of change in prescription numbers of high dose and low dose tablet strengths for ER and IR oxycodone indicated a distinct effect of the reformulation of OxyContin from the effect of the Florida PDMP/pill mill legislation.
Keywords:Abuse deterrent formulation opioids; Oxycodone; Prescription drug diversion legislation Opioid abuse epidemiology; Intervention; Prevention of opioid abuse
Key Points
1. This is the first study that used real world data to measure changes in oxycodone abuse related outcomes after OxyContin reformulation and whether 2 events separated by time and region had distinct effects on opioid prescriptions: 1) OxyContin® reformulation with abuse-deterrent properties and 2) Florida prescription drug monitoring program (PDMP)/ pill mill legislation.
2. California was used as a comparator state for Florida as it was geographically separated. This allowed for minimizing effects of a legislation in Florida. California and Florida are the first and third most populous states in the US.
3. Four months after reformulation, dispensed OxyContin 80mg tablets declined in Florida ( 37%) with an analogous decline in California (-46%). Dispensed IR oxycodone SE 30mg tablets increased in both Florida (+8%) and California (+43%) during the same timeframe. IR oxycodone SE 5 mg tablets did not change (0% and 1%) with little change for 10mg OxyContin tablets (0% and +4%).
4. Four months after the introduction of the Florida PDMP/ pill mill legislation, dispensed IR oxycodone SE 30mg tablets declined considerably in Florida (-32%) but not in California (-3%) with little change in IR oxycodone SE 5mg tablets (-3% and 0%), and little change in OxyContin 80mg tablets (-7% and -3%) or 10mg tablets (+1% and -3%) respectively in the 2 states.
5. OxyContin reformulation and Florida PDMP/pill mill legislation had a separate and independent impact on OxyContin and oxycodone abuse.
Previous Presentations
1. Coplan P, DeVeaugh-Geiss A, Gyarmathy A, Harikrishnan V. Impact of Florida PDMP introduction, pill mill legislation and OxyContin reformulation on tablets dispensed in Florida and California [poster 575]. Poster presented at: 33rd International Conference on Pharmacoepidemiology & Therapeutic Risk Management (ICPE); August 26-30, 2017; Montreal, Canada.
2. Coplan P, DeVeaugh-Geiss A, Gyarmathy A, Harikrishnan V. Impact of Florida PDMP introduction, pill mill legislation and OxyContin reformulation on tablets dispensed in Florida and California [poster 92]. Poster presented at: College on Problems of Drug Dependence 79th Annual Scientific Meeting; June 17-22, 2017; Montreal, Canada.
Introduction
The abuse and misuse of opioid analgesics have contributed to a serious and growing public health problem [1]. A range of national recommendations and local interventions have been used to help curb the epidemic. Teasing out the effectiveness of different interventions and their impact on abuse, addiction, overdose and death is needed to provide evidence-based solutions to the opioid epidemic.
There is substantial debate about the value of opioids with abuse-deterrent properties (ADPs) in reducing opioid abuse and its consequences. Several studies have shown a positive effect of decreased abuse of specific reformulated opioids with ADPs, but not on overall opioid abuse. In August 2010, reformulated OxyContin was introduced nationwide to deter abuse by physicochemical barriers to crushing, snorting and injection. Several studies have reported meaningful reductions in OxyContin abuse rates after ADP were added compared to other opioids in the same period [2- 6], though there are some exceptions [7,8]. Reductions in abuse of OxyContin range from 12% to 75% and non-oral routes showed a greater effect than for oral abuse [1].
While various authorities have endorsed the concept of opioids with abuse deterrent properties, others have questioned their usefulness. For example, the US Food and Drug Administration (FDA) has questioned “whether the observed decline in OxyContin abuse is due to the ADF and not due to other concurrent interventions” [8]. The Institute for Clinical and Economic Review (ICER) concluded that OxyContin possessed ADP, but was not cost effective at its current price.(ref) Others have questioned that “real-world evidence has been limited to simple time series that have examined abuse rates before and after the reformulation of OxyContin …”[9]. The main limitation of time series analyses is that the effect of other concurrent interventions may confound the ability to separate the role of an abuse deterrent formulation from other opioid-related policies, although a control group can measure the secular changes due to other opioid-related policies. Retrospective study designs that can isolate the effects of reformulation with ADP from other interventions have not been conducted and was the topic of a FDA public workshop to obtain expert advice [10].
The introduction of legislation in Florida to shut down “pill mills” and a state prescription drug monitoring program (PDMP) are competing explanations for an observed decrease in OxyContin abuse [8,11,12]. Pill mills are pain clinics that prescribe and dispense large amounts of prescription opioids without clear medical necessity for the purposes of diversion, while prescription drug monitoring program (PDMP) facilitate tracking and enforcing the state legislation on pill mills. The Florida legislation was approved April 12, 2011 [13], became active between July and September 2011 and was associated with a reduction in oxycodone related deaths in Florida [14]. Like most state medical examiner systems, the state medical examiner database in Florida cannot differentiate between death caused by different formulations of oxycodone such as IR or ER oxycodone.
One method that has been used to study the effect of different opioid interventions is surveillance of trends in prescribing of opioid analgesics. Hwang et al reported a change in the annual growth rate of OxyContin use in the US from 4.9% prior to the reformulation to -23.8% during the year after the reformulation; during the same period, there were no statistically significant changes associated with the sales of alternative ER or immediate release (IR) opioids. However, they did not assess changes in OxyContin prescriptions by tablet strength or by state. An Australian study contrasted person level changes in high versus low dose OxyContin tablets after the introduction of reformulated OxyContin and reported much larger reductions in prescription for high tablet strengths than low tablet strengths, particularly among younger people [15].
We assessed changes in prescribing associated with the introduction of OxyContin with ADP and the Florida legislation. We hypothesized that changes caused by reformulation of OxyContin should affect all states. In contrast, changes specific to a single state like the Florida pill mill and PDMP implementation should not induce changes in another state.
Methods
Introduction of oxycodone ER with ADP occurred simultaneously throughout the United States. In contrast, the pill mill legislation affected only Florida and occurred well after the reformulation was introduced. We used the method by Hwang et al, modified by adding the use of 1) different tablet strengths, 2) state specific analyses and 3) cash payment.
We compared the number of units dispensed for oxycodone IR and ER formulations in Florida and California. California was selected a priori as a comparator before study data was available because it is also a highly populated state, but geographically distant from Florida and therefore interventions enforced in Florida illicit market would not be expected to affect prescribing in California.
The study period to evaluate the effect of reformulation was the one-month period before reformulation compared to the 4-month period after reformulation of OxyContin (July 2010 versus November 2010). The four-month period was selected because it represents the effects of reformulation without the effect of legislation, which did not start until July 2011.
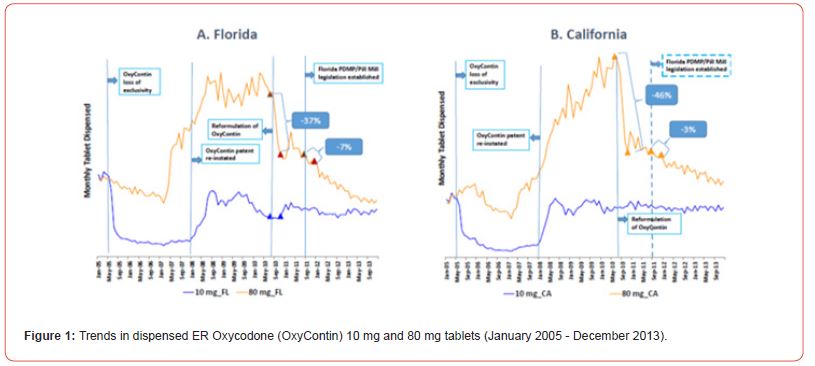
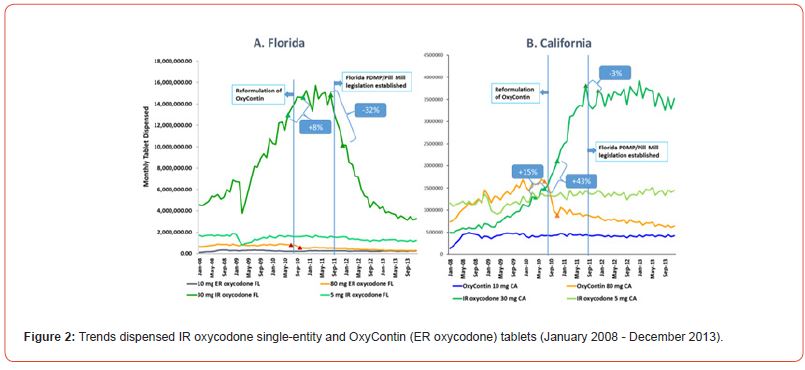
We analyzed different tablet strengths because higher tablet strengths have a greater probability of being abused and higher illicit resale value [10]. This analysis included IR oxycodone singleentity 30 mg and OxyContin (ER oxycodone) 80 mg tablets as they were the most illegally sold table by dealers in Florida [10]. The prescription volume of lower tablet strengths reflects trends in the medical use of a type of opioid analgesic and so can capture trends due to changing numbers of patients prescribed a type of opioid due to popularity of an opioid type or market access trends due to insurance coverage of an opioid product. The diverging trend for the higher versus lower tablet strengths may provide tool for differentiating trends driven by abuse changes versus trends due to medical use changes such as insurance changes or prescribing preferences. Dispensing data for IR oxycodone single entity products and for OxyContin were obtained from a common commercial source, a national prescription database (IQVIA Xponent™). Cash payments were also assessed because prescriptions for diversion purposes are often paid by cash to avoid discovery by insurers and law enforcement agencies [Figures 1&2].
Results
Dispensing after OxyContin Reformulation
In Florida, XX tablets of OxyContin 80 mg were dispensed in the pre-period, which decreased to XX tablets in the post period, a relative decrease of 37% (Table 1). Dispensing of OxyContin 10 mg was unchanged compared to the pre-period. In contrast, oxycodone SE 30 mg, XX tablets were dispensed in the post-period, a relative increase of 8%. Dispensing of oxycodone 5 mg was unchanged in the post-period.
In California, XX tablets of OxyContin 80 mg were dispensed in the pre-period, which decreased to XX tablets in the post period, a relative decrease of 46% (Table 1). Dispensing of OxyContin 10 mg was increased 4%. In contrast, oxycodone SE 30 mg, XX tablets were dispensed in the post-period, a relative increase of 43%. Dispensing of oxycodone 5 mg was unchanged in the post-period.
Dispensing after Florida PDMP/Pill Mill Implementation
In Florida, XX tablets of OxyContin 80 mg were dispensed in the pre-period, which decreased to XX tablets in the post period, a relative decrease of 7% (Table 1). Dispensing of OxyContin 10 mg was unchanged compared to the pre-period. In contrast, oxycodone SE 30 mg, XX tablets were dispensed in the post-period, a relative decrease of 32%. Dispensing of oxycodone 5 mg decreased 3%.
In California, XX tablets of OxyContin 80 mg were dispensed in the pre-period, which decreased to XX tablets in the post period, a relative decrease of 3% (Table 1). Dispensing of OxyContin 10 mg also decreased 3%. Oxycodone SE 30 mg, XX tablets were dispensed in the post-period, a relative decreased of 3%. Dispensing of oxycodone 5 mg was unchanged in the post-period.
Table 1:Changes in tablets dispensed for OxyContin and IR oxycodone single entity by tablet strength and time period surrounding two interventions designed to reduce opioid abuse.
Florida trends in the ratio of IR oxycodone and OxyContin higher-strength tablets
The ratio of dispensed IR oxycodone 30 mg tablets versus 80 mg OxyContin tablets changed from 16.7 before to 28.7 four months after OxyContin reformulation. The ratio of dispensed IR oxycodone 30 mg tablets versus 80 mg OxyContin changed from 29.0 before to 21.3 four months after establishment of Florida PDMP/pill mill legislation
California trends in the ratio of IR oxycodone and OxyContin higher-strength tablets
The ratio of dispensed IR oxycodone 30 mg tablets versus 80 mg OxyContin changed from 0.9 before to 2.4 four months after OxyContin reformulation. The ratio of dispensed IR oxycodone 30 mg tablets versus 80 mg OxyContin did not change, from 4.3 before to 4.3 four months after, establishment of Florida PDMP/pill mill legislation.
Cash payment prescriptions in Florida
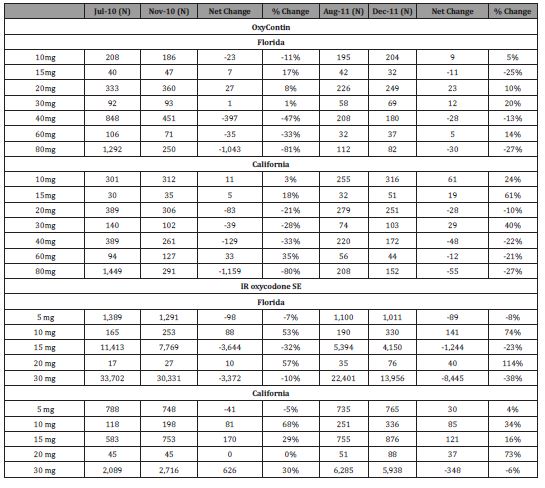
In 4 months after OxyContin reformulation (Table 2), the number of cash-paid prescriptions decreased 81% for 80 mg OxyContin tablets compared to 11% decrease for 10 mg OxyContin tablets and 10% and 7% decrease for IR oxycodone SE tablets (30 mg and 5mg tablets), respectively).
In 4 months after the Florida legislation (Table 2), the number of cash-paid prescriptions decreased more for 30 mg IR oxycodone SE tablets (-38%) compared with 5 mg IR oxycodone SE tablets (-8%). OxyContin 80 mg tablets decreased by -27% after the Florida legislation; however, the absolute number of prescriptions for OxyContin 80 mg tablets decreased by -1,043 and -30 after the OxyContin reformulation and the Florida PDMP/ Pill mill legislation respectively. The impact of OxyContin reformulation on cash paid 80 mg tablets was more than Florida PDMP/ Pill mill legislation [Figure 2].
Table 2:Changes in cash prescription for OxyContin and IR oxycodone single entity by tablet strength and time period surrounding two interventions designed to reduce opioid abuse.
Discussion
Our results indicate that nationwide introduction of OxyContin with ADP was followed by similar dispensing changes in Florida and California. An abrupt drop in dispensed 80 mg tablets of OxyContin states was observed, but little change of 10 mg OxyContin or IR oxycodone 5 mg. During the same time, there were modest increases in dispensed IR oxycodone 30 mg tablets, which appear to be part of an increasing trend that preceded the reformulation of OxyContin.
In contrast, the Florida PDMP/pill mill legislation intervention a year later (without analogous actions in California ) led to large and rapid decreases in dispensed IR oxycodone 30 mg tablets in Florida and no change in California, but only a small decrease in IR oxycodone 5 mg tablets in Florida, in the second half of 2011. The Florida PDMP/pill mill legislation intervention had a relatively small effect on dispensed OxyContin – approximately 4% decrease on OxyContin 80 mg tablets. The two interventions can therefore be differentiated by changes in oxycodone tablets dispensed.
Dart et al have shown a parallel relationship between the availability of prescription opioid analgesics through legitimate pharmacy channels and the diversion and abuse of these drugs and associated adverse outcomes including overdose mortality [2].
Demonstrating the effect of interventions to prevent opioid abuse and overdose that uses population levels of abuse over time is challenging due to confounding in secular trends of opioid abuse, i.e. trends over time unrelated to the intervention of interest. Use of a comparator population, such as a geographically distant state, is helpful but different regional secular trends can still confound the interpretation. Using short periods of observation tends to limit the impact of confounding by secular trends. Different tablet strengths can differentiate secular trends due to market access, price and availability from trends due to less desirability for purposes of abuse, since higher tablet strengths were generally preferred by abusers. In this study, we used prescription data as a proxy measure of abuse and diversion, with the measurement of abrupt changes in prescription and volume, contrasted across states, tablet strengths and formulations of oxycodone as an approach to tease out the effect of intervention from secular trends. Through these 4 contrasts (region, tablet strength, time, formulation), the ecological study design is strengthened. To date, no alternative to the quasiexperimental comparator study design has been used to evaluate the effect of abuse deterrent opioids on reducing its abuse in the real world.
Some authors have suggested that an undesirable consequence of reductions in OxyContin abuse after its reformulation was an in increase in abuse of other opioids; as a result, the net effect of OxyContin with ADP on opioid overdose was neutral with the decrease in OxyContin-associated overdose being offset by increases in overdoses from other opioids [1]. In this study, there were small changes in IR oxycodone use with the introduction of OxyContin with ADP in Florida and a large increase in California. However, IR oxycodone was increasing rapidly in California preceding the introduction of reformulated OxyContin. For example, prescriptions for 30 mg IR oxycodone tablets increased 43% in the 4 months after OxyContin reformulation but increased 15% in the 4 months before OxyContin reformulation. In addition, OxyContin represented about 10% of oxycodone prescriptions and about 2.9% of the total opioid prescription [3], as well as 5% of overall opioid deaths [16], and therefore the ability of an intervention focused on abuse of OxyContin tablet could not be expected to impact the other 95% of the opioid overdoses (i.e., the etiologic fraction is small).
Limitations
This analysis assumes that higher doses are generally preferred for abuse over lower doses. This may not be true but had been reported in studies among drug dealers in Florida [9]. There may have been other interventions in California and Florida that occurred simultaneously with the introduction of reformulated OxyContin in August 2010. However, the co-occurrence of the decreases in dispensed 80 mg tablets of OxyContin in both states in the same 4-month period, the specificity of the effect for 80 mg OxyContin tablets, and the abruptness of the decreases in the 4 months after both interventions suggests this effect is likely to be small relative to that of the 2 interventions studied. There may have been market access issues that influenced these findings, such as products being added or removed from insurance formularies. However, such access issues affect all tablet strengths of products similarly, so the specificity of the effects for higher doses suggest this may be unlikely. The analysis did not evaluate the reasons and frequency for prescriptions and uses only prescription volume data captured in a commercial database.
Conclusion
Interventions to decrease opioid abuse have a dynamic and distinct impact on the volume of opioid prescriptions. The effect of the Florida PDMP/pill mill legislation was distinct in timing and type of oxycodone tablets dispensed from the OxyContin reformulation effect. When assessing the effects of the reformulation of OxyContin on abuse-related outcomes, the Florida PDMP/pill mill legislation is unlikely to be a big confounder as indicated by the small effect that the Florida PDMP/pill mill legislation had on dispensed OxyContin tablets, particularly the prescriptions for higher tablet strengths of OxyContin. The use of 4 contrasts (region, tablet strength, time, formulation) in temporal trends in prescription opioid data provides an innovative approach to assess the impact of different interventions to curb prescription opioid abuse.
Acknowledgement
None.
Conflict of interest
None of the authors have a conflict of interest.
References
- Curfman GD, Beletsky L, Sarpatwari A (2018) Benefits, Limitations, and Value of Abuse-Deterrent Opioids. JAMA Intern Med 178(1): 131-132.
- Dart RC, Surratt HL, Cicero TJ, Parrino MW, Severtson SG, et al. (2015) Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med 372(3): 241-248.
- Coplan PM, Chilcoat HD, Butler SF, Sellers EM, Kadakia A, et al. (2016) The effect of an abuse-deterrent opioid formulation (OxyContin) on opioid abuse-related outcomes in the post marketing setting. Clin Pharmacol Ther 100(3): 275-286.
- Severtson SG, Ellis MS, Kurtz SP, Rosenblum A, Cicero TJ, et al. (2016) Sustained reduction of diversion and abuse after introduction of an abuse deterrent formulation of extended release oxycodone. Drug Alcohol Depend 168: 219-229.
- Sessler NE, Downing JM, Kale H, Chilcoat HD, Baumgartner TF, et al. (2014) Reductions in reported deaths following the introduction of extended-release oxycodone (OxyContin) with an abuse-deterrent formulation. Pharmacoepidemiol Drug Saf 23(12): 1238-1246.
- Chilcoat HD, Coplan PM, Harikrishnan V, Alexander L (2016) Decreased diversion by doctor-shopping for a reformulated extended release oxycodone product (OxyContin). Drug Alcohol Depend 165: 221-228.
- Cicero TJ, Ellis MS (2015) Abuse-deterrent formulations and the prescription opioid abuse epidemic in the united states: lessons learned from Oxycontin. JAMA Psychiatry 72(5): 424-430.
- Jones CM, Muhuri PK, Lurie PG (2017) Trends in the nonmedical use of OxyContin®, United States, 2006 to 2013. Clin J Pain 33(5): 452-461.
- Good BC, Manolis C, Shramk W (2018) There’s little evidence abuse-deterrent opioids work. Why should we use them?
- (2018) Data and Methods for Evaluating the Impact of Opioid Formulations with Properties Designed to Deter Abuse in the Post market Setting: A Scientific Discussion of Present and Future Capabilities.
- Kennedy-Hendricks A, Richey M, McGinty EE, Stuart EA, Barry CL, et al. (2016) Opioid Overdose Deaths and Florida's Crackdown on Pill Mills. Am J Public Health 106(2): 291-297.
- Rutkow L, Chang HY, Daubresse M, Webster DW, Stuart EA, et al. (2015) Effect of Florida's Prescription Drug Monitoring Program and Pill Mill Laws on Opioid Prescribing and Use. JAMA Intern Med 175(10): 1642-1649.
- (2018) Attorney General Bondi's Statement on the Passage of Pill Mill Legislation by the House Appropriations Committee.
- Delcher C, Wagenaar AC, Goldberger BA, Cook RL, Maldonado-Molina MM (2015) Abrupt decline in oxycodone-caused mortality after implementation of Florida's Prescription Drug Monitoring Program. Drug Alcohol Depend 150: 63-68.
- Schaffer AL, Buckley NA, Degenhardt L, Larance B, Cairns R, et al. (2018) Person-level changes in oxycodone use after the introduction of a tamper-resistant formulation in Australia. CMAJ 190(12): E355-E362.
- Hirsch A, Proescholdbell SK, Bronson W, Dasgupta N (2014) Prescription histories and dose strengths associated with overdose deaths. Pain Med 15(7): 1187-1195.






