 Research Article
Research Article
Non-Destructive Determination of Bulk Elemental Composition of a Japanese Bronze Coin using Negative Muon
Kazuhiko Ninomiya1,2*, Akira Sato2, Dai Tomono3, Yoshitaka Kawashima3, Akihiro Nambu2, Kentaro Terada2, Atsushi Shinohara2,4 and Tsutomu Saito5
*1Institute for Radiation Sciences, Osaka University, Japan
2Graduate School of Science, Osaka University, Japan
3Research Center for Nuclear Physics, Osaka University, Japan
4Osaka Aoyama University, Japan
5National Museum of Japanese History, Japan
Kazuhiko Ninomiya, Institute for Radiation Sciences, Osaka University, 1-1, Machikaneyama, Toyonaka, Osaka, Japan
Received Date:March 14, 2023; Published Date:December 11, 2023
Abstract
Non-destructive methods are essential for the elemental analysis of archaeological artifacts. However, analyzing metals may be difficult due to their oxidized surface. A novel elemental analysis method using a negative muon beam was developed. Despite the limitations of the experimental facility that can use muon beams, this method has excellent properties; non-destructive, multi-elemental, and position-selective analysis is possible. The elemental analysis of a Japanese bronze coin was performed at the muon facility, MuSIC, Osaka University, which was developed using a highintensity muon beam in a low-intensity accelerator. The analysis accuracy and detection limit of the method were evaluated showing an accuracy of 0.5% and a detection limit of 0.4 wt%, in roughly a half-day measurement.
Keywords:Non-destructive elemental analysis; Bulk elemental analysis; Muon elemental analysis; Bronze analysis
Introduction
Determining the elemental composition of an object is fundamental in many research fields and provides various types of knowledge. In archeology, the production period and origin of an archaeological artifact can be investigated, enabling us to understand the cultural background in which archaeological artifacts are produced. However, non-destructively determining the elemental composition of metal archaeological artifacts is difficult. Particularly, in excavated materials, the bulk elemental composition of the artifact significantly differs from that of an oxidized surface. For example, iron is known to transform into various iron hydroxide and oxide compounds [1], and bronze products form multiple layers with different chemical compositions [2] with progressing oxidation.
Non-destructive analysis methods are preferred in archaeological science, and X-ray fluorescence analysis (XRF) is the most commonly used and powerful tool. However, the applicability of XRF is limited: quantifying elements lighter than Na (Z=11) is difficult, and X-rays are predominantly emitted from the sample surface, making it challenging to detect the sample’s bulk elemental composition. The former limitation is due to the low energy of the X-rays used in XRF. Given that characteristic X-ray energies from elements lighter than Na are less than 1 keV, these X-rays are easily absorbed by minute quantities of substances such as air and detector vacuum windows.
Furthermore, using X-ray irradiation to selectively excite deep within the material is impossible, regardless of whether highenergy X-rays are used, given that the X-ray absorption efficiency is always maximized at the sample surface. Thus, obtaining only the elemental composition deep within the material by XRF is difficult because the signals from the surface layer, that is, the oxidized layer, especially for metal objects, always affects the obtained results. To remove the influence of the surface layer effect, physically scraping the surface or performing a chemical etching treatment is necessary, which severely restricts application to valuable archaeological artifacts.
Analysis methods using gamma rays, which have a higher energy than X-rays, have also been applied to archaeological artifacts. Analyses such as neutron activation [3], prompt gamma ray [4], and photon activation [5] are examples used to identify elements using gamma-ray measurements. However, the activation of these materials is problematic. In addition, the reaction probability of the nuclear process that generates gamma rays usually differs by several orders of magnitude by element (more precisely, by isotopes). The sensitivities significantly vary for different elements. This property allows the identification of trace amount elements. However, these methods have limitations on the number of applicable elements.
Here, we introduce another elemental analysis method using a negatively charged muon beam obtained from an accelerator [6].
A negatively charged muon is an elementary particle with a mass that is 207 times that of an electron. When the muon is introduced into a material, it forms an exotic atomic system known as a muonic atom, that is, in which “muon” atomic orbits are formed around a nucleus. Muonic X-rays are X-rays emitted from muonic atoms as a result of the interorbital transition of muons. Considering that the mass of muons is 207 times that of electrons, the energies of muonic X-rays are 207 times higher than those of electrons. Therefore, the problem of low X-ray energies in XRF can be solved by using muonic X-rays in elemental analysis. In addition, by adjusting the muon incident energy in the material, the muon can control the stop position (depth), enabling position-selective analysis. In addition, the muonic X-ray emission probability does not change significantly depending on the element.
This feature enables simultaneous multi-elemental analyses. Muonic X-ray measurement using muon irradiation allows for multi-element, position-selective, and non-destructive elemental analysis. Elemental analysis methods using muons (muon-induced X-ray emission [MIXE]) have rapidly developed over the last decade. This method has already been applied to archaeological metal artifacts, such as Chinese bronze mirrors [7,8], Chinese bronze coins [9], Japanese coins [8,10], and Roman coins [11-13]. The method has also been applied to the content analysis of medicine bottles that cannot be opened [14]. Muon elemental analysis requires an accelerator to generate a muon beam, which is a limit to its application. The requirement of a proton accelerator with a highenergy and high-current proton beam increases the cost of analysis. Recently, a method to generate an intense muon beam despite low accelerator power was developed [15]. This paper introduces the results of muon elemental analysis of archaeological artifacts using a new type of muon generator.
Materials and Methods
Samples
A Japanese bronze coin, the Tempo-tsuho sample, minted in the Edo period (19th century) and held by the National Museum of Japanese History, was selected as a muon analysis sample. The elemental composition of Tempo-tsuho differs depending on the site location. The Tempo-tsuho is oval in shape, with a length of 49 mm and a width of 32 mm. The coin has a thickness of approximately 2 mm, with a 6 × 6 mm square hole in the center for the coin. A photograph of the sample is shown in Figure 1(a). From the typology the Tempo-tsuho was considered authentic as it was produced by the central government of Edo Shogunate.

Muon facility
Muon elemental analysis experiments were conducted at the MuSIC-M1 beamline of the Research Center for Nuclear Physics (RCNP), Osaka University. The maximum energy and current of the proton beam of the RCNP accelerator were 392 MeV and 1.1 μA, respectively. This is much lower than other muon facilities; for example, the other muon facility, J-PARC (Japan Proton Accelerator Research Complex, Ibaraki, Japan), has 8 times higher proton energy and 1000 times higher proton current [16]. Despite the low accelerator power in MuSIC, it achieves a muon beam intensity comparable to that of a highly efficient muon collection system around the muon production target. In addition, the continuous beam structure, which is different from the pulsed beam structure in J-PARC, solves the problem of detector saturation and allows for measurements under high-efficiency conditions. The detailed method of muon production and the properties of MuSIC are provided elsewhere [15].
Muon elemental analysis
An experimental muon setup was constructed at the exit of the MuSIC-M1 beamline. The muon beam generated by the accelerator was extracted into air through a polyimide vacuum window. The muon beam was then irradiated on the sample after passing through two plastic scintillation counters with thicknesses of 0.5 and 2 mm, respectively, to determine the muon arrival time on the sample and a 2.2 mm aluminum plate for optimizing the muon energy. The generated muonic X-rays were detected using a highpurity germanium semiconductor detector (Mirion Technology, BE3830) placed 5 cm away from the sample, which is suitable for detecting high-energy X-rays. Figure 1(b) shows the experimental setup. The duration of muon irradiation for the bronze coin was approximately 60 min. The energy of the muon beam was tuned to 15.9 MeV that corresponded to a muon stopping depth of 1.47 mm. The size of the muon beam was 3×3 cm. The detailed experimental procedures are described in a previous study [17].
Results and Discussion
Figure 2 shows the spectrum obtained through muon irradiation of the sample. Muonic X-ray peaks with energies of 115 keV (Cu, 4f- 3d), 140 keV (Pb, 7i-6h), 158 keV (Sn, 5 g–4f), 233 keV (Pb, 6h–5 g), 330 keV, 336 keV (Cu, 3d-2p), 345 keV, 350 keV (Sn, 4f-3d), 431 keV, 438 keV (Pb, 5 g–4f)), 445 keV, 451 keV (Cu, 4d-2p), 938 keV, 972 keV (Pb, 4f-3d), 976 keV, 982 keV (Sn, 3d-2p), 1507 keV, and 1513 keV(Cu, 2p-1s) were identified. All of these peaks originate from muonic X-rays from Cu, Sn, and Pb [18]. Given that Sn plates were placed around the detector as a radiation shield, the background signals of muonic Sn X-rays originated from the muon stop in the radiation shield. The contribution of the background signal was estimated from the muon irradiation results for the Fe metal plate sample obtained using the same experimental setup. In addition, small muonic X-ray peaks corresponding to N, O, and Al were observed. These three elements originated from the background signal of the muon stop in the air around the sample and sample holder.

The intensity of each muonic X-ray emitted from the sample was determined by considering the detection efficiency of a high-purity germanium semiconductor detector. The energy dependence of the detection efficiency was determined from a simulation experiment using the Monte Carlo code, EGS5 (Electron Gamma Shower 5) [19]. The emission intensity of the muonic X-rays almost corresponds to the elemental composition. This demonstrates that the sample was mainly comprised of Cu, Sn, and Pb and was made of bronze. The experimental muonic X-ray intensity ratios of Sn to Cu and Pb to Cu were determined as I(Sn(4f-3d)/Cu(3d-2p)) = 0.178 ± 0.030 and I(Pb(5g-4f)/Cu(3d-2p)) = 0.165 ± 0.017. In the analysis, the 350 keV peak of the muonic Sn 4f-3d transition was not considered because this peak contained a small contribution from muon capture in Pb.
To determine the elemental composition quantitatively, a calibration curve between the elemental composition and muonic X-ray intensity determined from qualified standard samples reported previously was applied [10]. The elemental mass ratios were determined to be Sn/Cu = 0.252 ± 0.043stat ± 0.015syst and Pb/Cu = 0.196 ± 0.020stat ± 0.018syst. A statistical error arises from the measurement in this study, and the systematic error originates from the calibration curve reported in the literature [10]. Therefore, more precise quantitative values can be obtained by increasing the accuracy of the calibration curve. If the sample comprised only these three elements, the analytical value was Cu:69.1 ± 2.2stat ± 1.1syst, Sn:17.4 ± 3.0stat ± 1.1syst and Pb:13.5 ± 1.4stat ± 1.2syst, as shown in Table 1.
Table 1:Elemental compositions of the Tempo-tsuho analyzed in this work together with these by XRF and muon analysis at J-PARC [8].
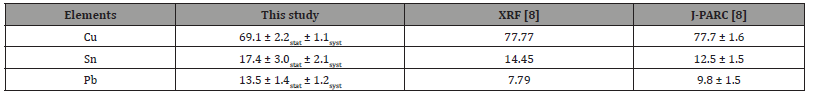
The analytical values obtained in this study were compared to those obtained by XRF. Table 1 depicts a comparison of the results of the muon elemental analysis and XRF analysis [8]. The results measured at another muon facility, J-PARC, are also shown [8]. The muon analysis values of the sample were in good agreement. In addition, the XRF result was consistent with the analysis result of this study; however, the Pb content from the muon elemental analysis was slightly higher than that from XRF. This difference may be due to the fact that the muon elemental analysis examined the interior of the material, whereas the XRF analysis included a significant contribution from the sample’s surface. This research yielded an analytical precision of a few percentage points, which is comparable to previous reports of muon elemental analysis [8]. As aforementioned, MuSIC is a relatively inexpensive muon generator, and its high muon collection efficiency and high-efficiency measurement system provide the same level of analytical precision as analysis using another large accelerator facility.
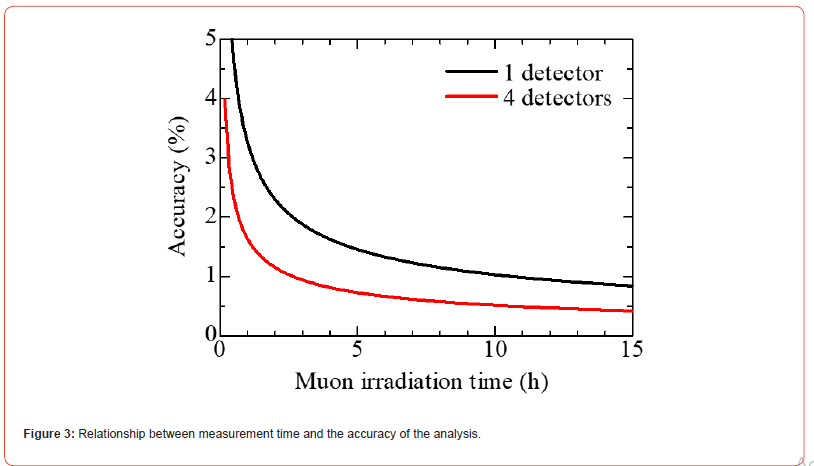
The performance of the muon elemental analysis method at MuSIC was estimated on the basis of the results of this study. The accuracy of Cu for Sample 1 was 3.8%, excluding the systematic error derived from the calibration data. This analysis was performed through muon irradiation for 60 min, and a longer analysis time will enable highly accurate analysis. In addition, only one detector was used for analysis, but there was sufficient space around the sample to accommodate additional detectors, as shown in Figure 1(b). A more accurate analysis was possible by including three detectors (four detectors in total). Figure 3 shows the relationship between the measurement duration and the accuracy of the analysis results.
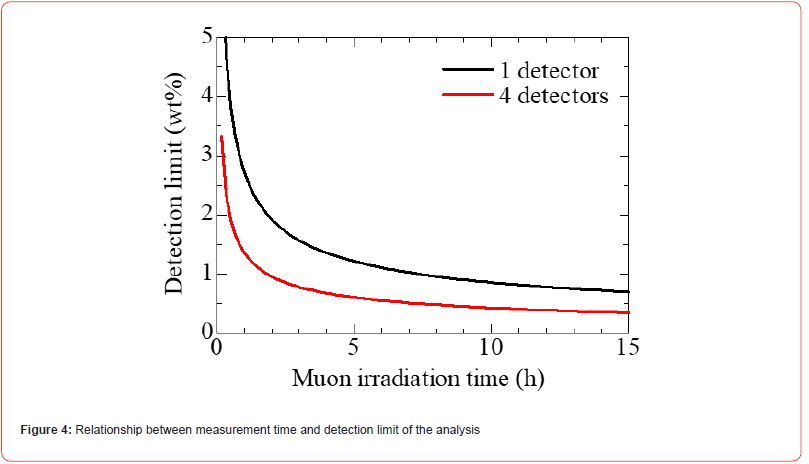
For example, an analysis accuracy of 0.5% or less can be achieved by performing measurements using four detectors for 11 h. We also estimated the detection limit of the experimental setup. The count of Pb(5 g–4f) was 490 counts, whereas the error in the background in this energy region was 30. Assuming 3.3 times of the quantification limit [20], the detection limit of this method was estimated as 2.1wt%. The detection limit is also expected to improve by extending the measurement time and increasing the number of detectors. Figure 4 shows the dependence of the muon irradiation duration on the detection limit. From Figure 4, detection of 0.4wt% component will be achieved through a 12-hour measurement with a 4-detector experimental setup.
Muonic elemental analysis can be used to investigate the elemental composition of bulk samples non-destructively and independent of the surface. Even if the surface of a sample is highly oxidized, the internal composition can be determined without any surface treatments. Furthermore, if the object is surface treated or painted, the interior can be analyzed while retaining the surface layer. The muon elemental analysis method has similar sensitivity for all elements. Therefore, this method is not suitable for identifying trace amounts of elements, but it is a powerful tool for analyzing major elements in valuable archaeological artifacts. Furthermore, isotope compositions and chemical states can be obtained through muonic X-ray measurements.
The energies of muonic X-rays differ slightly depending on the nucleus size, that is, isotopes [18]. Therefore, detailed spectroscopy of muonic X-rays enables non-destructive isotope analysis. Nondestructive isotope analysis of lead, which is widely used to investigate artifact origins, has been reported [21]. In addition, the emission probability of muonic X-rays changes depends on the chemical state of the element. By using such a “chemical effect,” the elemental composition can be investigated, and the chemical composition, such as oxidation state, can be identified [22]. Using this property, it can be determined whether the interior of the artifact analyzed by the muon contains a metal layer or an oxidized component.
Conclusion
A non-destructive elemental analysis method using muons was applied to analyze a Japanese Tempo-tsuho bronze coin, which was minted in the 19th century. The bronze coin is made of copper, tin, and lead, and the compositions were quantitatively determined. Muon analysis can analyze only the interior of the sample, and the influence of the surface oxidized layer can be ignored. The performance of this analysis in MuSIC was evaluated. By improving the detection system, elements with an accuracy of 0.5% and detection of 0.4 wt% component can be quantified in a half-day muon experiment. In this way, a relatively low-cost muon source, MuSIC, can achieve a highly accurate analysis. Muon elemental analysis is a powerful tool to analyze valuable archaeological artifacts; it can analyze multiple elements simultaneously, nondestructively, and regioselectively. Moreover, this method can provide information on isotope compositions and chemical states.
Acknowledgments
This experiment was performed as part of the MuSIC facility development. We wish to express our gratitude to the RCNP accelerator group and all those involved for their participation. This research was partially supported by Grant-in-Aid for Scientific Research B (22H02107) and Grant-in-Aid for Scientific Research on Innovative Areas (18H05460).
Conflict of Interest
This study declares no conflict of interests.
References
- T Misawa, K Hashimoto, S Shimodaira (1974) The mechanism of formation of iron oxide and oxyhydroxides in aqueous solutions at room. Corros. Sci 14(2): 131-149.
- LR obbiola, JM Blengino, C Fiaud (1998) Morphology and mechanisms of formation of natural patinas on archaeological Cu–Sn alloys. Corros Sci 40(12): 2083-2111.
- L Szentmiklósi, Z Kis, M Tanaka, B Maróti, M Hoshino et al. (2021) Revealing hidden features of a Japanese articulated iron lobster via non-destructive local elemental analysis and 3D imaging. J Anal At Spectrom 36(11): 2439-2443.
- JMP Cabral, MA Gouveia, AM Alarcão, J Alarcao (1983) Neutron activation analysis of fine grey pottery from Conimbriga, Santa Olaia and Tavarede, Portugal. J Archaeol Sci 10(1): 61-70.
- Chr Segebade (2013) Edward’s sword? – A non-destructive study of a medieval king’s sword. AIP Conf. Proc. 1525: 417-421.
- Ninomiya (2019) Non-destructive, position-selective, and multi-elemental analysis method involving negative muons. J. Nucl. Radiochem. Sci 19: 8-13.
- MK Kubo, H Moriyama, Y Tsuruoka, S Sakamoto, E Koseto et al. (2008) Non-destructive elemental depth-profiling with muonic X-rays. J Radioanal Nucl Chem 278(3): 777-781.
- Kazuhiko Ninomiya, Michael K Kubo, Patrick Strassesr, Takashi Nagatomo, Yoshio Kobayashi et al. (2015) Elemental Analysis of Bronze Artifacts by Muonic X-ray Spectroscopy. JPS Conf Proc 8: 033005.
- K Ninomiya, T Nagatomo, K Kubo, TU Ito, W Higemoto et al. (2012) Development of nondestructive and quantitative elemental analysis method using calibration curve between muonic X-ray intensity and elemental composition in bronze. Bull. Chem. Soc. Jpn 85: 228-230.
- Kazuhiko Ninomiya, Michael Kenya Kubo, Takashi Nagatomo, Wataru Higemoto, Takashi U, Ito et al. (2015) Nondestructive Elemental Depth-Profiling Analysis by Muonic X-ray Measurement. Anal. Chem 87(9): 4597-4600.
- Bethany V Hampshire, Kevin Butcher, Katsu Ishida, George Green, Don M Paul et al. (2019) Using Negative Muons as a Probe for Depth Profiling Silver Roman Coinage. Heritage 2(1): 400-407.
- George Alexander Green, Katsu Ishida, Bethany V Hampshire, Kevin Butcher, A Mark Pollard et al. (2021) Understanding Roman Gold Coinage Inside Out. J Archaeol Sci. 134: 105470.
- GA Green, K Ishida, K Domoney, T Agoro, AD Hillier (2022) Negative muons reveal the economic chaos of Rome’s AD 68/9 Civil Wars. Archaeol. Anthropol. Sci 14: 165.
- Kayoko Shimada-Takaura, Kazuhiko Ninomiya, Akira Sato, Naomi Ueda, Motonobu Tampo et al. (2021) A novel challenge of nondestructive analysis on OGATA Koan’s sealed medicine by muonic X-ray analysis. J Nat Med 75: 532-539.
- S Cook, R D'Arcy, A Edmonds, M Fukuda, K Hatanaka et al. (2017) Delivering the world’s most intense muon beam. Phys. Rev. Accel Beams 20: 030101.
- Wataru Higemoto, Ryosuke Kadono, Naritoshi Kawamura, Akihiro Koda, Kenji M. Kojima et al. (2017) Materials and life science experimental facility at the Japan Proton Accelerator Research Complex IV: the muon facility. Quantum Beam Sci 1(1): 11.
- Kazuhiko Ninomiya, Majino Kajino, Makoto Inagaki, Kentaro Terada, Akira. Sato et al. (2020) Per atom muon capture ratios and effects of molecular structure on muon capture by γ-Fe2O3 and Fe3O4. J. Radioanal. Nucl. Chem 324: 403-408.
- R Engfer, H Schneuwly, JL Vuilleumier, HK Walter, A Zehnder (1974) Charge-distribution parameters, isotope shifts, isomer shifts, and magnetic hyperfine constants from muonic atoms. Atom. Data Nucl. Data Tabl 14: 509-597.
- H Hirayama, Y Namito, AF Bielajew, SJ Wilderman, WR Nelson (2005) The EGS5 code system. SLAC-Report, pp. 730.
- Lloyd A Currie (1968) Limits for qualitative detection and quantitative determination. Application to radiochemistry. Anal. Chem 40: 586-593.
- Kazuhiko Ninomiya, Takuto Kudo, Patrick Strasser, Kentaro Terada, Yosuke Kawai et al. (2019) Development of non-destructive isotopic analysis methods using muon beams and their application to the analysis of lead. J Radioanal. Nucl. Chem 320: 801-805.
- Kazuhiko Ninomiya, Meito Kajino, Akihiro Nambu, Makoto Inagaki, Takuto Kudo et al. (2022) Non-destructive composition identification for mixture of iron compounds using chemical environmental effect on muon capture process. Bull Chem Soc Jpn 95(12): 1769-1774.
-
Kazuhiko Ninomiya*, Akira Sato, Dai Tomono, Yoshitaka Kawashima, Akihiro Nambu, Kentaro Terada, Atsushi Shinohara and Tsutomu Saito. Non-Destructive Determination of Bulk Elemental Composition of a Japanese Bronze Coin using Negative Muon. Open Access J Arch & Anthropol. 5(2): 2023. OAJAA.MS.ID.000608.
-
Demokritos, Archaeometry, lyophilizer, Radiocarbon, Carbohydrates, Neolithic
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






