 Research Article
Research Article
Identification of the Organic Binding Medium and Fungi on Wall Paintings from the Victory Monument of Augustus in Nicopolis, Using HPLC-FD, Culture Media Methods, Optical Microscopy and SEM
Tziamourani E1*, Facorellis Y1, Karabotsos A1 and Zachos, K.L2
1Laboratory for the Study and Conservation of Ancient and Contemporary Cultural Property, Faculty of Applied Arts and Culture, Department of Antiquities and Works of Art Conservation, University of West Attica, Greece
2Emeritus Curator of Antiquities, Hellenic Ministry of Culture, Greece
Eleni Tziamourani, Laboratory for the Study and Conservation of Ancient and Contemporary Cultural Property, Faculty of Applied Arts and Culture, Department of Antiquities and Works of Art Conservation, University of West Attica, Greece
Received Date:May 01, 2023; Published Date:October 13, 2023
Abstract
The monument to the Actium victory raised by Octavian Augustus on the northern outskirts of Nicopolis, in the area where the Actian games were taking place, consists of an enormous podium built in ashlar masonry on the slopes of a hill sacred to Apollo. On the upper part of the terrace created by the podium, a porticus triplex was surrounding a courtyard. The area of the courtyard was occupied by a monumental altar and by two pedestals carrying bronze statues of unknown figures. The walls of the porticus and of an annex on its north wing were decorated with frescos fragments which were preserved in situ, while several other fragments were found scattered throughout the monument. In this article, we present the results of the identification of the organic binding medium, as well as the isolation of microorganisms from the wall painting fragments using HPLC and classical microbiological isolation techniques OM and SEM, respectively. Ten samples were selected for HPLC-FD analysis and compared with two reference mixtures of egg yolk with gelatin 3:1 w/w and 5:1 w/w. Six of the ten samples gave proteinaceous profile results, yielding an amino acid profile like the mixture of egg yolk/gelatin 3:1 w/w, which is relatively poor in gelatin content. The fungal species isolated were Aspergillus sp., Penicillium sp., unidentified strains of mold and fungi and Bacillus sp. This study aims to assess the present-day condition of the wall paintings for future investigation. It is well known that the metabolism of organic bindings in the substrate, like animal glue loses their binding effect when they are contaminated or when they have been in use for an extended time and consolidation is required. During a microbial infestation, these organic bindings decompose thus losing their consolidation effect. The metabolism of organic bindings is restricted to natural organic bindings and affects synthetic polymers applied for consolidation purposes.
Keywords:Nicopolis; HPLC-FD; Aspergillus sp; OM; SEM; Media cultures; Bacteria; Fungi; Amino acids
Introduction1
The monument to the Actium victory raised by Octavian Augustus on the northern outskirts of Nicopolis in the area where the Actian games were taking place, consists of an enormous podium built in ashlar masonry on the slopes of a hill sacred to Apollo. 36 bronze rams from the captivated ships of his defeated rival Antony were displayed on the southern wall of the podium, which was the monument’s façade, and according to the Latin inscription that adorned the upper part of the wall, these were dedicated to Mars and Nep tune. On the upper part of the terrace created by the podium, a porticus triplex was surrounding a courtyard. A monumental altar and two pedestals carrying bronze statues of unknown figures occupied the area of the courtyard. The walls and the sandstone pillars of the porticus and of an annex on its north wing were covered with plaster and decorated with wall paintings of which a few fragments were preserved in situ, while a few other such fragments were found scattered throughout the monument. The preserved 2.037 fragments cover a surface of roughly 3.2 m2. According to their scattering, it is estimated that all the walls and the pillars of the Stoa were decorated, thus the preserved ones represent only a minimal, representative fraction of the total.
The surface of most fragments has been maintained in good condition, while in a few others, the paint is completely obscured. As shown in Figure 1, they have a variety of colors (white, various shades of red, brown, black, yellow, brown, green, and cyan in various, intense, shades), however, except for a few samples, they do not show distinguishable drawings.
There is a plethora of painting techniques applied by various artists during the Roman period [1]. The most common painting types are the secco, where the pigments mixed with an organic binding medium, e.g., egg, are applied on a dry mortar surface, and the fresco one, consisting of a painting with pigments, dispersed in a water medium on a wet or fresh lime mortar surface [2]. Very often a second paint layer of different color was often applied a secco on top of the fresco. A variant of the fresco technique, referred to by various scholars as mezzo fresco, requires the dispersion of the pigment in lime water or lime paste, which is then applied to a mortar that has partially dried [3-5]. Finally, stucco reliefs were used for the architectural decoration of both inside and outside walls, ceilings, and floors. Traditionally stucco was made of lime, sand, and water, while animal or plant fibers were sometimes added for additional strength. Several studies have reported that these organic substances can provide a nutrient-rich substrate for microbial growth, including fungi [6-10]. Microbial growth on stone monuments can result in the accumulation of biofilms, which can produce organic acids and other compounds that contribute to the weathering and erosion of the stone surface. Similarly, microbial growth on mural paintings can lead to staining, discoloration, and deterioration of the paint layers. Some of the most common fungi that attack monuments include: Aspergillus sp., Penicillium sp., Cladosporium sp., and Alternaria sp. These genera of fungi are known for their ability to produce enzymes that break down the cellulose and other organic components in the substrate, leading to decay and deterioration. Additionally, Cladosporium sp., can produce pigmented spores that can stain and discolor surfaces [6,7]. These fungi produce enzymes, such as proteases, that can break down proteins into their constituent amino acids. When Aspergillus sp. and Penicillium sp. colonize proteinaceous materials on the surface of a monument, they secrete these proteases to break down the proteins [11,12]. This can result in the degradation and loss of structural integrity of the proteinaceous material [13]. The amino acids of animal glue and egg yolk proteins are also susceptible to degradation by proteases produced by Aspergillus and Penicillium. Animal glue is made up of collagen proteins, which contain many different types of amino acids. Proteases produced by fungi can break down the peptide bonds between these amino acids, leading to the degradation of the glue and a loss of its adhesive properties. Similarly, egg yolk proteins contain many different types of amino acids that can be degraded by proteases, resulting in the breakdown of the protein and loss of its functional properties [6]. In addition to proteases, fungi can also produce other enzymes, such as lipases and esterase’s, that can degrade other organic compounds found in proteinaceous materials, such as lipids and esters. The degradation of these compounds can further contribute to the discoloration and degradation of the proteinaceous material on the surface of a monument.
The purpose of this study is to identify the organic binding medium used for the wall paintings of the porticus triplex stoa and pillars, as well as that of the podium, of the Victory monument of Octavian Augustus in Nicopolis, the microorganisms that grow on their surface and the possible impact that the latter ones may have on their present-day state of preservation.
Materials and Methods
Microbiology study Sampling process
Nineteen samples (NIK 1-19) were selected for microbiological studies. The painted front side of all fragments were observed and photographed by means of an Olympus SZ61 stereomicroscope (magnification x0.67) equipped with a software system, used for PC imaging, to select the areas for samples collection. The stereomicroscope images were processed with the Infinity Analyze and Capture software for Windows (v 6.5.6) to add the scale. In Table 1, we present the stereomicroscope images of samples (painted side) selected for microbial media cultures.
Table 1:Summary of the sample’s code, location, color, and stereomicroscope images of samples selected for microbial media cultures (magnification x0.67).

Microorganisms’ isolation and characterization
Samples from the painted surface were collected under aseptic conditions from sampling areas by scraping off superficial material and plaster to a depth of 2-8 mm and then were pressed on petri plates. Culture media used for this study were Sabouraud Dextrose Agar (SDA) medium for yeasts and moulds isolation, Blood Agar/ sheep blood (BA) for culturing a wide variety of pathogenic microorganisms and Mac Conkey Agar (MAC) a medium for the growth of staphylococci and enterococci. All media were ready prepared culture media from BIOPRAPARE. The cultures were incubated at 37οC for 24-48h, and at 28οC for 5 days, to allow bacterial and fungal development, respectively. After this period, the plates stayed in incubation at the same temperature for 20 days to detect slow microbial development while the morphological characteristics of the colonies were recorded. The several colonies developed picked up to obtain pure cultures for using the slide culture technique [14]. Fungal isolates were characterized based on cultural and morphological characteristics of spore and hyphae mounted in lactophenol cotton blue stain (LPCB, Sigma-Aldrich) and identified by consulting taxonomic monographs [15-18]. Fungi that did not produce spores were categorized as sterile mycelia and if they did not show distinct morphological characters for identification were included as ‘unidentified strain’.
In addition, the fungal populations were identified and characterized by means of an optical microscope (OM) and scanning electron microscope (SEM), which are keys for their identification according to the specialized literature [15]. A Leica DM2700M optical microscope equipped with a camera system (Leica Microsystems CMS GmbH D-35578 model DFC 310 FX) was used to observe the fungal colonies. The software system used for computer imaging was Leica Application Suite (LAS).
Foot Notes
Electronic Scanning Microscope (SEM) observation
All the analyzes of the present work were carried out with the JEOL JSM 6510LV Scanning Electron Microscope (SEM) adjustable vacuum, with the possibility of magnification from 25 to 300.000 times, for morphological observation of the biological samples and obtaining high-resolution images of secondary electrons images (SE) and backscattered (BSE) images of electrons. SEM accompanied by an energy dispersive X-ray spectrometer, X-act type from Oxford Instruments (EDS, Energy Dispersive Spectrometry), for qualitative and quantitative microanalysis as well as element mapping, with INCA data analysis software.
The samples were prepared as follows:
A conductive double-sided tape was adhered to the sample holder (aluminum base). Part of the developed colony, measuring 0.5X0.5 cm, was carefully detached with a scalpel and transferred to the sample holder. This procedure was performed on all studied samples. The samples were then placed in the sublimation apparatus for 24h, in an Argon environment of 10-2 mbar and then covered with a very thin layer of gold for 2 minutes to make them conductive. The most conductive samples were transferred to the sample carrier of the SEM chamber. The SEM observation of the samples was performed at incremental magnifications from 450x to 20.000x. Microscopy was done under a vacuum of 10-9 Torr.
High-Performance Liquid Chromatography with Fluorescence Detection (HPLC-FD) Chemicals and reagents
The chemicals and reagents used for the HPLC-FD measurements are the following: Acetonitrile HPLC Grade, Methanol HPLC Grade, Ammonium mono hydrogen phosphate, anhydrous (NH4)2HPO4, Water HPLC Grade, Ammonium dihydrogen phosphate (NH4)2HPO4, 9-Fluoromethyl Chloroformate, 2-(methylthio)-ethanol, 1.0M Sodium Hydroxide (Merck Chemicals for the organic synthesis, Germany), Hydroxylamine Hydrochloride (Sigma Aldrich, USA),Boric Acid (Riedel-de Haen AG, Germany), Amino acid standard solution (Sigma Aldrich, USA), Gelatin (Fluka), Casein (FLuka).
Authentic samples
Ten samples (NIK-1, 7, 10, 11,13, 13, 13, 13, 14, 15, 16, 18, 19) collected for HPLC-FD analysis by scraping the surface of fragments with a scalpel blade. The sampling of powder samples was carried out with the highest possible selectivity as to the layers and samples weighed approximately 5-10 mg.
Reference samples
Pure proteinaceous materials such as casein and gelatin were
used. Also, artificial specimens of two different media were prepared,
generally following traditional instructions to artists in their
preparation. They were:
i. gelatin in water solution 6% w/w and
ii. egg yolk diluted with an equal volume of water. The two
media were painted on a plaster layer of lime and sand. After drying
at room temperature for five days, was dried in the oven for 2h at
60οC. The surface of these paint layers was scrapped off, and 1-5
mg powder of samples was taken for analysis of the proteinaceous
binders with HPLC-FD. Two reference samples were also prepared
by mixing powders from egg yolk/white lead 2FbCO3 Pd (OH)2 2%
and gelatin layers in different proportions (5:1 w/w and 3:1 w/w).
Apparatus and chromatographic conditions
A YL9100 HPLC system equipped with an YL9110 quaternary pump and GBC LC 1250 fluorescence detector was used for the separation and quantification of the fluorescent amino acid derivatives in reference and authentic samples. Amino acids were derivatized with 9-fluorenymethyl chloroformate (FMOC-Cl) using a method developed by Haynes et al., [16]. FMOC-Cl was dissolved in acetonitrile as a 4.16 mg/mL solution (16 mM). Borate buffer was prepared from 200 mM boric acid solution adjusted to pH 8.5 with 5 M sodium hydroxide solution. An alkaline cleavage reagent was prepared daily by mixing 170μL of 850 mM sodium hydroxide solution with 75μL of 500 mM hydroxylamine hydrochloride solution and 5μL of 2-(methylthio)-ethanol. The quenching reagent was acetonitrile- glacial acetic acid (8:2).
Separation of the FMOC-AA derivatives was carried out using a
ternary gradient elution. Three different eluents were used:
i) eluent A consisting of 20 mM ammonium dihydrogen phosphate
(pΗ 6.5) in 15% methanol 85% water,
ii) eluent B consisting of 15% methanol in water and
iii) eluent C consisting of 10% water in acetonitrile. An Hypersil
C18 (ODS) column with dimensions 250x4.6 mm, 3μm was
used in a 54-minute gradient program. The column temperature
was set at 38°C and the solvent flow rate at 0.8mL/min.
The excitation wavelength of the fluorescence detector was at
270 nm and the emission at 316 nm.
Analytical procedure
The analytical procedure for the determination of proteinaceous binders in wall painting samples was applied to all the samples. The optimization of the derivatization and chromatographic conditions were performed using an amino acid standard solution (AA) and amino acid standard solutions of gelatin and casein hydrolysate (from Fluka). In AA standard solution the amino acids concentration compounds are in 0.1N HCl at the indicated concentrations ± 4% as follows: 2.50 μMoles/mL of alanine (ala) and glycine (gly), valine (val), leucine (leu), isoleucine (ile), serine (ser), methionine (met), phenylalanine (phe), aspartic acid (asp), glutamic acid (glu), lysine (lys) tyrosine (tyr), proline (pro), threonine (thr), arginine (arg), histidine (his), and 1.25 of μMoles/mL of cysteine (cys). Recognition of the nature of the protein binding medium was affected by a quantitative determination of amino acids and their characteristic ratios. Chromatographic data were also subjected to statistical treatments. In particular, correlation coefficients are quite successful and provide an easy means for comparing large data sets. The amino acid similarity pattern [S (A, B)] between two kinds of proteins is calculated with equation (1), where ai (i=1-n) and bi (i=1-n) are the amino acid contents of two corresponding kinds of proteins [17].
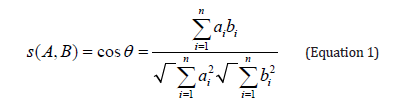
This method has been applied successfully to proteinaceous binding media and adhesives by Sinkai and Sugisita [18]. The analytical procedure can be summarized as follows: Aliquots of the amino acid standard solution (2.5 mM) were added to the borate buffer (pH 8.5) in different proportions. A volume of 100μL of amino acid buffer solutions were derivatized by adding successively 100μL of FMOC-Cl solution and 60μL volume of cleavage reagent and 140μL of quenching reagent. The reaction was then stopped by the addition of quenching reagent. Aliquots of 5μL, from the prepared reaction solutions, were injected into the HPLC system. Reference mural painting samples (5-10 mg) were subjected to acid hydrolysis under flow of nitrogen (1mL HCl 6N, 110°C for 24h). At the end of hydrolysis, the samples were let to be cold and then placed in an oven at 40°C till they became completely dry. In the remaining dry material, 1 mL H2O was added, to prepare a stock solution and stored at 4°C. Aliquots (10μL) of the hydrolysate were added to 490μL of borate buffer, pH 8.5. From this solution 100μL were taken up for derivatization following the routine procedure and the mixture was used for HPLC-FD analysis. Duplicate hydrolysis and derivatizations were performed. One HPLC injection (20μl) was performed for each reaction mixture. This procedure allows the determination of the following amino acids: aspartic acid (Asp), glutamic acid (Glu), hydroxyproline (Hyp), serine (Ser), glycine (Gly), threonine (Thr), alanine (Ala), proline (Pro), tyrosine (Tyr), arginine (Arg), valine (Val), isoleucine (Iso), leucine (Leu) and phenylananine (Phe).
Results and Discussion
Microbiology Study
For this study, we decided to proceed with a classical microbiological isolation technique, and we didn’t use a molecular biology research or metagenomic approach, since our aim was to record the present-day condition of the wall paintings.
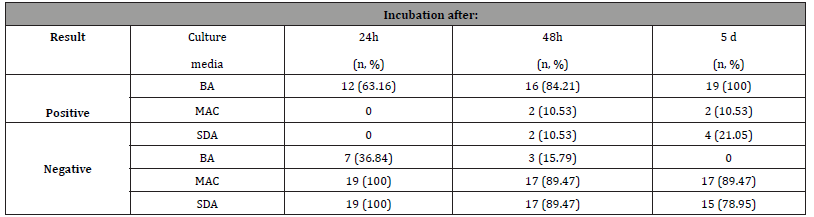
A total of 57 cultures (19 samples for each medium) were examined during the study period. Evaluation of the cultures at 24th hour yielded 12 (63.16%) positive results for BA. Out of 7 cultures (36.84%), that were found to be negative in the first 24th hour’s evaluation, 16 cultures (84.21%), were isolated positive for BA, 2 cultures (10.53%) for McA and SDA at 48th hour. During the 5th day evaluation of the cultures yielded 19 (100%) positive results for BA 2 (10.53%) for MA and 4 (21.05%) for SDA (Table 2). In BA agar, several unknown colonies were developed in each sample as BA is a non-selective medium, good for culturing many bacterial species including Gram- negative and Gram-positive species. Due to similar morphological characteristics with other bacteria mold and fungi, they are presented as unidentified strains. SDA is a selective medium primarily used for the isolation of dermatophytes, other fungi and yeasts but can also grow filamentous bacteria. The acidic pH of this medium (pH ~ 5.0) inhibits the growth of bacteria but permits the growth of yeasts and most filamentous fungi. McA is an indicator, a selective and differential culture medium for bacteria designed to selectively isolate Gram-negative bacilli. The crystal violet and bile salts inhibit the growth of Gram-positive organisms which allows for the selection and isolation of gram-negative bacteria. The fungal species isolated (Figures 2, 3 and 4), were Aspergillus species (15.79%), Penicillium and Altenaria species (10.53%), unidentified strains mold and fungi (68.42%) and Bacillus species (5.26%). (Figures 2 - 4)


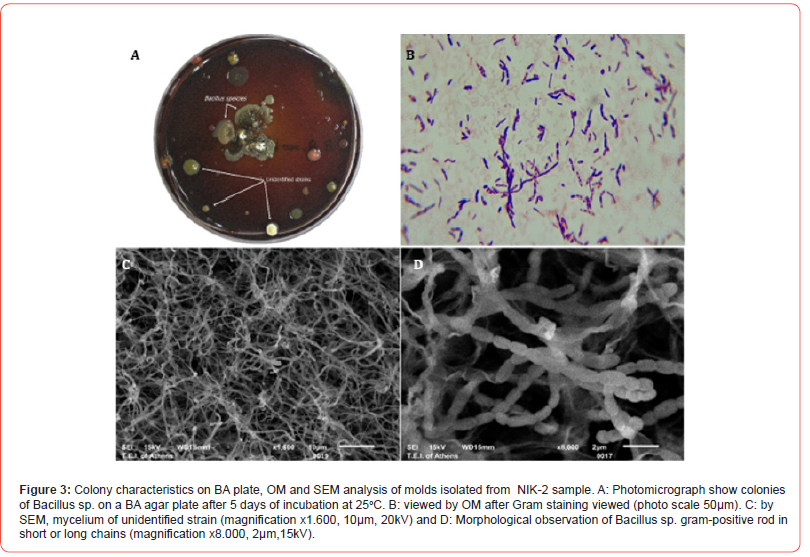
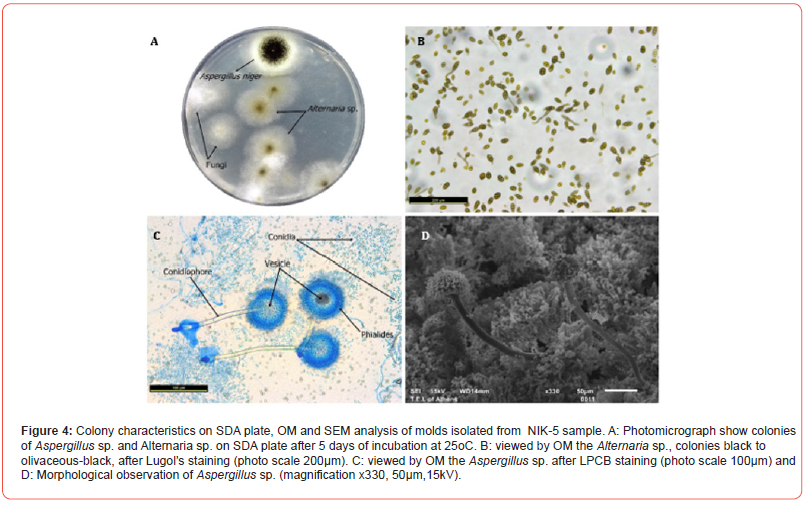
Table 2:Culture results according to incubation times.
The most important of the deterioration problems in wall painting induced or influenced by microorganisms, can be summarized as deterioration due to biofilms, metabolism of organic binding as substrates, alterations of mineral pigments, alteration of mineral pigments, excretion of mineral or organic acids, and interaction of microbes and faunal elements [19,20]. For that reason, the characterization of the microbial flora present on frescoes or easel paintings has been limited to selected groups of microorganisms rather than to all types of microorganisms that might be present on a given substrate. On the other hand, given the wide range of organic and inorganic molecules that are present in wall paintings, many different types of microorganisms may grow on such substrates provided that favorable environmental conditions (humidity, temperature, light, and, to a lesser extent, pH) are met [20]. Mycological research on biodeterioration in monuments (frescoes) is mainly based on classical cultivation methods using a variety of standardized media as Malt Extract Agar (MEA), Dichlorane Rose Dengal Chloramphenicol Agar (DRBC), Potato Dextrose Agar (PDA), Czapek–Dox Agar (CDA), Tryptic Soy Agar (TSA), Sabouraud Dextrose agar (SDA) [18-25]. Nevertheless, the use of modern molecular techniques for the detection of fungi on and in materials of cultural heritage will provide a deeper insight and understanding of fungal community structures and their consequences for the material [19]. Despite the fact that in this study we used conventional nutrient materials, that agrees with the assertion of several researchers who reported that the genus Aspergillus and genus Penicillium are the main fungi isolated from wall paintings [18-25].
HPLC-FD Calibration of amino acid analysis
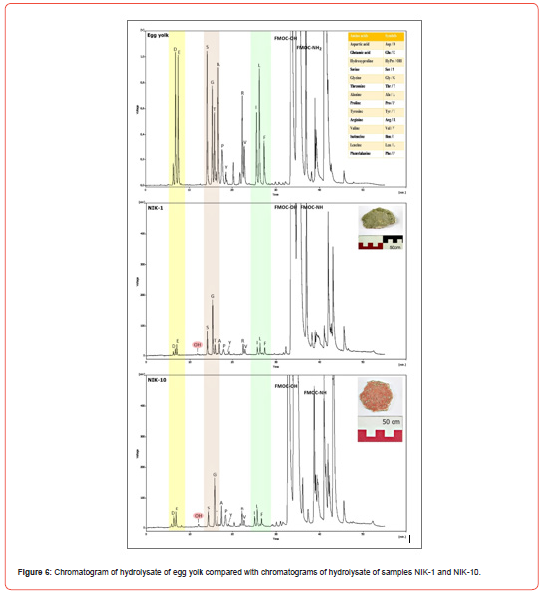
Calibration of amino acid analysis typically involves analyzing the amino acid standard at a number of concentrations. Figure 5 presents a representative chromatogram illustrating the elution pattern of amino acids expected in an acid hydrolysate of gelatin and egg yolk. Essentially baseline resolution is achieved for all derivatives.
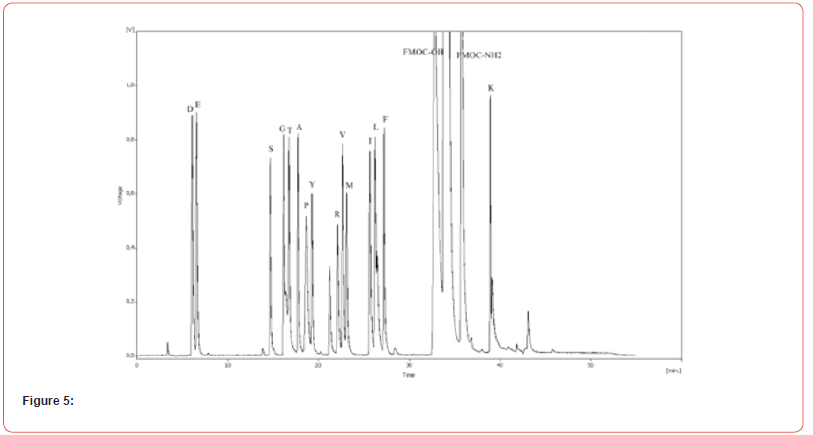
Five amino acid standard levels were analyzed to determine the range of analysis for each amino acid. The linearity of fluorescence was investigated over a concentration range of 62.5 to 250 pmoles per amino acid. Peak areas obtained for each amino acid were plotted versus the known concentration for each of the amino acids in the standard dilution. Response linearity is obtained in the range of 5 to 62.5 pmoles with correlation coefficients exceeding 0.97. Detection limit is considered to be 5 pmoles for the amino acid derivatives.
Reference samples
The analytical results of the basic reference binding media are presented in Table 3, in the form of relative amino acid content (percentage areas). The table also includes the amino acid composition data for two reference mixtures of egg yolk with gelatin 3:1 w/w and egg yolk with gelatin 5:1 w/w. Table 4 shows the values of the concentration ratios between different amino acids for each tempera. Each binder was characterized by a specific set of amino acid percentages and ratio values, which can be used as a ‘fingerprint’ [5].
In particular, the following remarks can be made as follow:
Gelatin binder: Presence of hyproxyproline, the highest percentage of glycine, the highest values of gly/ileu, ala/phe, pro/leu and hyp/leu ratios and the lowest ones of ser/gly leu/ala and val/ ala. Egg binder: the highest percentages of aspartic acid, glutamic acid, serine and leucine. Compared to gelatin, egg yolk has the lowest percentages of glycine and proline. The highest values of ala/phe, asp/pro ratios and the lowest one of glu/asp. Five ratio values (ser/ileu, ala/gly, pro/leu, ser/gly and ala/pro) close to 1.5 and three ratio values (gly/ileu, leu/ala, and val/ala) close to 1. Casein: the highest percentages of glutamic acid, proline and leucine. The highest values of leu/ala, ser/gly and pro/leu and the lowest ones of ala/pro, asp/pro, and gly/ileu. As expected, the mixtures of egg yolk with gelatin have higher quantity of glycine and proline, compared to egg yolk and the protein component of mixtures of egg yolk and gelatin is characterized by ratios of amino acids in between the egg yolk’s ratios and the gelatin’s ratios.
Authentic samples
Six of the ten samples which were collected showed proteinaceous profile results (Table 5), giving an amino acid profile like egg yolk. The presence of small amounts of hyproxyproline detected in all samples, as well as a relatively high content in glycine was an indication of the presence of animal glue at low levels. The fact that the hyproxyproline was observed in very low amounts suggests that animal glue was not the main binder used in the paint. A possible source of animal glue in the paint layer is contamination from plaster. Further knowledge as to the extent of the contamination of gelatin in the egg tempera layers of the Nicopolis samples was gained from the comparison of the characteristic amino acid ratios in the authentic samples NIK-1, NIK-7, NIK-10, NIK-16, NIK-18, and NIK-19 with those of the two reference samples of egg yolk containing different amounts of gelatin Tables 4 and 6.
Table 3:Relative percentage content of amino acids in reference samples.

Table 4:Amino acid concentration ratios of reference samples.
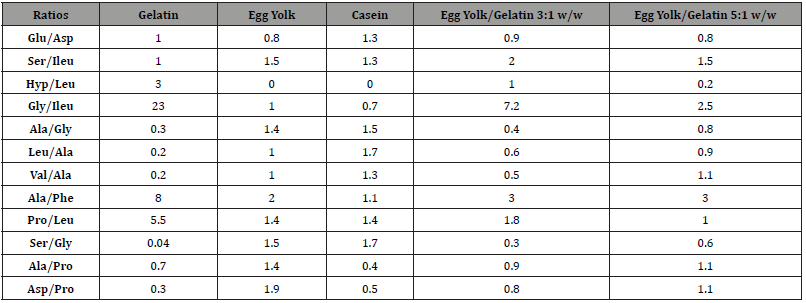
Table 5:Relative percentage content of amino acids in authentic samples.
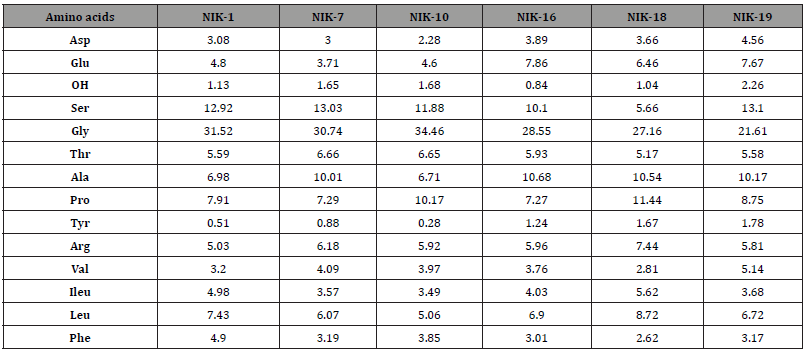
Table 6:Amino acid concentration ratios of authentic samples.
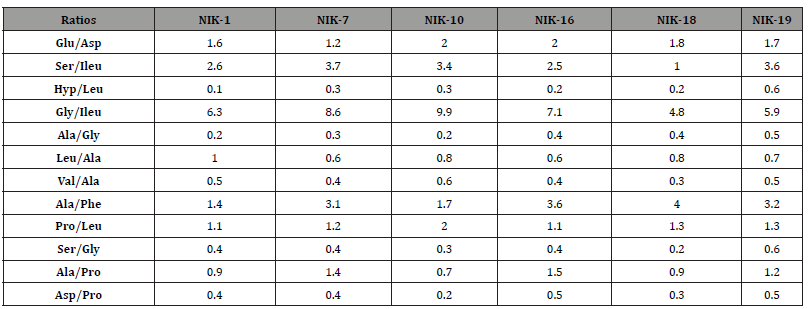
It becomes obvious that the ratios of the authentic samples have mean values that are closer to the ones of the mixture egg yolk/gelatin 3:1 w/w which is relevantly poor in gelatin content Figure 6. It should be stressed that the amino acid concentration ratios are much more helpful for binder identification than amino acid percentages, though the latter are indispensable whenever binder degradation processes are suspected [28]. Those findings agree with previous relevant studies [28-30].
Correlation coefficients
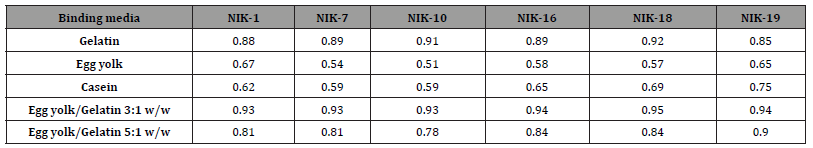
Amino acid similarity pattern between the authentic samples and the reference proteinaceous binders was calculated with equation 1. Correlation coefficients of samples are listed in Table 7. results reported in Table 7 it becomes obvious that the binding media of authentic samples are similar to gelatin, presenting correlation coefficients having mean values that approach 0.90. However, the amino acid similarity pattern values between the aforementioned samples and the reference sample of egg yolk/gelatin mixture 3:1 w/w even higher, exceeding 0.90. The results show that the egg yolk has been the main medium for paint layers throughout the detected samples, while animal glue is basically present in plaster, possibly applied directly on the preparation ground surface just before painting. The binding media used in samples NIK-11, NIK-13, NIK-14 and NIK-15 could not be identified. The results of a quantitative amino acid analysis for protein media and the identification are not always unmistakable. Differences between fresh and aged samples could be attributable to losses of amino acids caused by the process of ageing. During acid hydrolysis, some amino acid derivatives resulting from ageing will be hydrolyzed by severe acid treatment. Others, however, with changed or blocked side groups, will no longer be detectable in their common amino acid form [31]. One the other hand, the fungal growth in depth can cause degradation of proteins. The fungal growth in depth degrading some of its components (especially glues and binders), while hyphae penetrated the painted layer, which resulted in a decrease in the cohesion of the painted layers, thus giving rise to exfoliations, cracking, and loss of the paint. To these damages one should add those inflicted by metabolites, often acidic in nature, and by extracellular enzymes excreted by microorganisms. These compounds may modify the colors as well as the stability of the painted layer and of the substrate [32].
Table 7:Correlation coefficients of authentic samples.
Conclusion
In conclusion, the Victory Monument of Octavian Augustus in Nicopolis stands as a remarkable testament to the craftsmanship and artistry of ancient Rome. Its grandeur is evident in the vast platform adorned with bronze rams captured from Antony’s defeated ships and the intricate decorations that once embellished the walls and pillars of the triplex portico. The surviving fragments of these frescoes, although representing only a fraction of the original, reveal a rich palette of colors and various painting techniques employed by Roman artists.
However, as it is common with many historical artifacts, the passage of time has left its mark, and the surfaces of these paintings have not been immune to the presence of microbial growth. Fungi such as Aspergillus, Penicillium, Cladosporium, and Alternaria have manifested, leading to staining, discoloration, and the deterioration of both painted surfaces and the organic binders mentioned. These microorganisms, with their enzymatic activities, have played a significant role in the breakdown of proteins, lipids, and esters within the panels, contributing to their current state of preservation.
The in-depth analysis of authentic samples using chromatographic methods has provided profound insights into the composition of binders employed in historical artworks. Notably, the mean values of amino acid concentration ratios in these samples closely mirror those found in the 3:1 w/w egg yolk/gelatin mixture, a composition characterized by low gelatin content. This underscores the significance of considering amino acid concentration ratios when identifying binders, as they yield more informative data than mere amino acid percentages.
What is particularly noteworthy is that the amino acid similarity pattern values between these samples and the reference sample of the 3:1 w/w egg yolk/gelatin mixture exceed 0.90. This strongly suggests that egg yolk served as the primary medium for creating the paint layers in the examined samples, while animal glue appears to have been predominantly employed in plaster, likely applied to the ground preparation surface immediately before painting.
In summary, this research documents the current state of the Monument’s wall painting fragments and underscores the intricate interplay between artistic materials, aging processes, and microbial activity in the preservation and degradation of cultural heritage objects. Understanding these multifaceted factors is imperative for developing effective conservation strategies and ensuring the longterm preservation of these invaluable works of art for future generations to appreciate and study.
Acknowledgement
None.
Disclosure of interest
No Conflict of interest.
References
- Cuní J (2016) What do we know of Roman wall painting technique? Potential confounding factors in ancient paint media analysis. Herit Sci 4: 44.
- Apostolaki Ch, Perdikatsis V, Repuskou E, Brekoulaki H, Lepinski S (2006) Analysis of Roman Wall Paintings from Ancient Corinth/Greece. 2nd International Conference on Advances in Mineral Resources Managements and Environmental Geotechnology, Greece.
- Coccato A, Caggiani MC, Occhipinti R, Mazzoleni P, Dalessio A (2021) The Irreplaceable Contribution of Cross Sections Investigation. Painted Plasters from the Sphinx Room (Domus Aurea, Rome). Minerals 11(1): 4.
- Mazzocchin GA, Vianello A, Minghelli S, and Rudello D (2010) Analysis of Roman wall paintings from the thermae of ‘Iulia Concordia’. Archaeometry 52: 644-655.
- Paladini A, Toschi F, Colosi F, et al., (2015) Stratigraphic investigation of wall painting fragments from Roman villas of the Sabina area. Appl Phys A 118: 131-138.
- Tyagi P, Verma RK, Jain N (2021) Fungal degradation of cultural heritage monuments and management options. Current Science 121 (12): 1553-1560.
- Zucconi L, Canini F, Isola D, Caneva G (2022) Fungi Affecting Wall Paintings of Historical Value. A Worldwide Meta-Analysis of Their Detected Diversity. Appl Sci 12(6): 2988.
- Caselli E, Pancaldi S, Baldisserotto C, Petrucci F, Impallaria A (2018) Characterization of biodegradation in a 17th century easel painting and potential for a biological approach. PLoS ONE 13(12): e0207630.
- Suphaphimol N, Suwannarach N, Purahong W, Jaikang C, Pengpat K (2022) Identification of Microorganisms Dwelling on the 19th Century Lanna Mural Paintings from Northern Thailand Using Culture-Dependent and-Independent Approaches. Biology 11(2): 228.
- Zucconi L, Gagliardi M, Isola D, Onofri S, Andaloro M (2012) Biodeterioration agents dwelling in or on the wall paintings of the Holy Saviour’s cave (Vallerano, Italy). International Biodeterioration and Biodegradation 70: 40-46.
- Sterflinger K, Piñar G (2013) Microbial deterioration of cultural heritage and works of art-tilting at windmills? Applied Microbiology and Biotechnology 97(22): 9637-9646.
- Garg KL, Kamal KJ, Mishra AK (1995) Role of fungi in the deterioration of wall paintings. The Science of the Total Environment 167(1-3): 255-271.
- Colombini MP, Fuoco R, Giacomelli A, Muscatello B (1998) Characterization of Proteinaceous Binders in Wall Painting Samples by Microwave-Assisted Acid Hydrolysis and GC-MS Determination of Amino Acids. Studies in Conservation 43(1): 33-41.
- Dhingra OD, Sinclair JB (1995) Basic Plant Pathology Methods Chapter 9, Bright-Field Microscopy Techniques, Slides Culture Techniques and Preparation of Permanent Mounts. (2nd edn), pp. 819-338.
- Milanesi C, Baldi F, Vignari R, Ciampolini F, Faleri C (2006) Fungal deterioration of medieval wall fresco determined by analysing small fragments containing copper. International Biodeterioration and Biodegradation 57(1): 7-13.
- Haynes PA, Sheumack D, Kibby J, and Redmond JW (1991) Amino acid analysis using derivatisation with 9-fluorenylmethyl chloroformate and reversed-phase high performance liquid chromatography. Journal of Chromatography 540: 177-185.
- Tamura S, and Osawa F (1969) Amino acid pattern similarity between foods. Journal of Japan Society of Nutrition and Food Sciences 2: 494-496.
- Sinkai T, and Sugisita R (1990) Identification of protein-containing binding media and adhesives in works of art by amino acid analysis. Journal of Archaeological Science of Japan 35: 1-12.
- Petersen K (2006) Conservation Science: Heritage Materials. Royal Society of Chemistry. 1st edition, Chapter 10, Wall Paintings: Aspects of deterioration and restoration Vol. 241-265.
- Sterflinger K (2010) Fungi: Their role in deterioration of cultural heritage. Fungal Biology Reviews 24(1-2): 47-55.
- Caselli E, Pancaldi S, Baldisserotto C, Petrucci F, Impallaria A (2018) Characterization of biodegradation in a 17th century easel painting and potential for a biological approach. PLOS ONE 13(12): e0207630.
- Guglielminetti M, De Giuli Morghen C, Radaelli A, Bistoni F, Carruba G (1994) Mycological and ultrastructural studies to evaluate biodeterioration of mural paintings. Detection of fungi and mites in Frescos of the monastery of St Damian in Assisi. International Biodeterioration & Biodegradation 33(3): 269-283.
- Unković N, Dimkić I, Stupar M, Stanković S, Vukojević J, et al. (2018) Biodegradative potential of fungal isolates from sacral ambient. In vitro study as risk assessment implication for the conservation of wall paintings. Plos One 13(1): e0190922.
- Rosado T, Silva M, Dias L, Candeias A, Gil M, et al. (2017) Microorganisms and the integrated conservation-intervention process of the renaissance mural paintings from Casas Pintadas in Évora-Know to act, act to preserve. Journal of King Saud University-Science 29(4): 478-486.
- Moza MI, Mironescu M, Georgescu C, Florea A, Bucşa L (2012) Isolation and characterization of moulds degrading mural paintings. Annals of RSCB Vol. XVII: 1.
- Tamura S, and Osawa F (1969) Amino acid pattern similarity between foods. Journal of Japan Society of Nutrition and Food Sciences 22: 494-496.
- Sinkai T, and Sugisita R (1990) Identification of protein-containing binding media and adhesives in works of art by amino acid analysis. Journal of Archaeological Science of Japan 35: 1-12.
- Colombini MP, Fuoco R, Giacomelli A and Muscatello B (1998) Characterization of proteinaceous binders in wall painting samples by microwave-assisted acid hydrolysis and GC-MS determination of amino acids. Studies in Conservation 43(1): 33-41.
- Wei S, Schreiner M, Rosenberg E, Guo H, Qinglin MA (2011) The identification of the binding media in the tang dynasty chinese wall paintings by using py-gc/ms and gc/ms techniques. Int J Conserv Sci 2(2): 77-88.
- Schilling MR and Khanjian HP (1996) Gas Chromatographic Analysis of Amino Acids as Ethyl Chloroformate Derivatives. Part 2, Effects of Pigments and Accelerated Aging on the Identification of Proteinaceous Binding Media. Journal of the American Institute for Conservation 35(2): 123.
- Karpowicz A (1981) Ageing and Deterioration of Proteinaceous Media. Studies in Conservation 26(4): 153.
- Cifferri O (1999) Microbial Degradation of Paintings. Applied And Environmental Microbiology 65(3): 879-885.
-
Tziamourani E*, Facorellis Y, Karabotsos A and Zachos, K.L. Identification of the Organic Binding Medium and Fungi on Wall Paintings from the Victory Monument of Augustus in Nicopolis, Using HPLC-FD, Culture Media Methods, Optical Microscopy and SEM. Open Access J Arch & Anthropol. 5(1): 2023. OAJAA.MS.ID.000604.
-
Organic Binding Medium, Nutrient-rich, Microorganisms’ isolation, Sabouraud Dextrose Agar
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






