 Review Article
Review Article
“Urban Mine” A Modern Source of Materials: Part I Battery Recycling
Farouk Tedjar*
Energy Research Institute, Nanyang Technological University, Singapore
Farouk Tedjar, Energy Research Institute, Nanyang Technological University, 1 Cleantech Loop, 637141, Singapore.
Received Date: May 04, 2020; Published Date: May 22, 2020
Introduction
Today world population increase and related economy expands are accompanied with important growth of substantial need for natural resources. Consequently, in order to sustain same level of economic activities a great amount of energy and resource are consumed [1]. The energy preoccupation linked to a potential “peak oil” introduces a strong actions on fuel saving and utilization of clean energy. In particular emerging of climate changing problematic and global warming issues from Kyoto protocol [2,3] with impact of greenhouse gases and CO2 are the most important challenges the last 3 decades.
Within all resources and just after water and energy, in third position we have the metals. In other hand today, no doubt those industrial emerging segments such as s solar panel, wireless products, and e-mobility and wind turbines will have a great growth and demand. However almost all those equipment are using strategic and critical metals facing a security of supply.
In particular, the situation of pressure on strategic and critical materials was recently completed by the Geopolitical aspect coming out from the high level of availability (and then control) of some natural resources such as Cobalt (65% owned by Congo) and Rare Earths (85% owned by China). Today the consideration on economic importance vs. supply risk of each basic materials leads to a strong increase of interest on recycling. In front fear of scarcity of resources in recent years, several initiatives were enhancing the deep transition from a traditional linear economy to a new mode of resource flow based on circular economy [4,5].
Among the energy sector, batteries are the commercial segment facing the most important growth of last decades. In fact, the development of electric and electronic devices is giving now the model of consumption to “a wireless age”. The growth of miniaturization in one hand and energy density need lead to rapid development of new chemistry for batteries as observed during last decade particularly in secondary systems. However, dark side of the progress is always in environment impact and scarcity of resources. Bit implementation of collection and recycling processes could lead to a large decrease of environment impact and important recovery of resources. To maintain a sustainable and safe supply chain to the economic cycle of the battery segment, it is critical to operate a deep change. The Circular Economy is the most relevant to face this challenge [6].
Urban Mine and Circular Economy
This new concept of Circular Economy is based on strong modification of traditional model based on “production of goods – use said goods by the society– put through away at end of life”. One of the pillars of the circular economy is recycling in order that wastes will be converted to valuable materials substituting the natural resources. A link between recycling /recovering/reuse and circular economy is the status of the waste. For this reason, “Urban Mining”, concept developed since 2004 [7] is now well integrated in the recycling and sustainability landscape and largely used as key concept within any end-of-life sustainability approach. Scarcity or strategic metals [4,5] as well as Climate Change issues [8,9] are pillars supporting this concept We built this concept on our experience on primary recycling batteries [10], Steel Dust from electrical arc furnace [11], Fly ash from Municipal Solid Waste Incineration [12], Asbestos conversion [13] and lithium ion [14]. In particular, within a study for EU Commission during 5th Framework Program (1999-2002) we observed that lithium content of batteries is higher than the most concentration natural deposit [15]. From this study, the name was registered [7]. The concept is based on the need to shift from traditional mine with classical ores to “Urban Mine “with new “Urban ores” as illustrated in Figure 1.
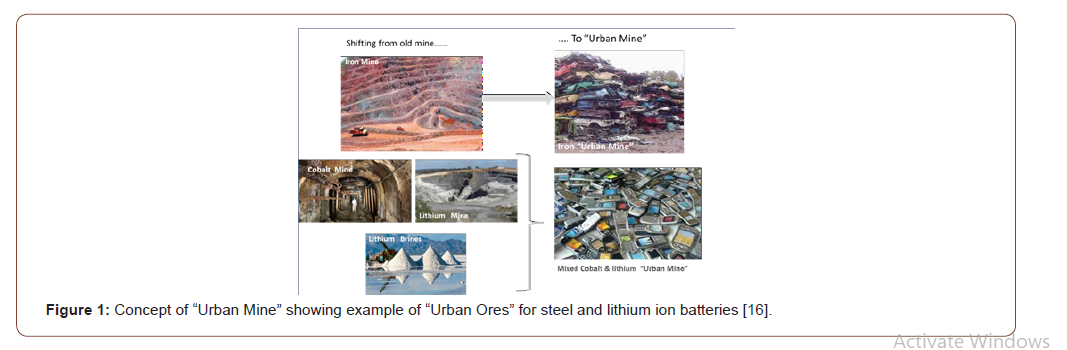
Urban Mine and Sustainable City
Circular Economy and “Urban Mine” are two key for the achievement of the Sustainable City. Every family as large producer of plastic, paper, organic wastes, aluminum, batteries, lamp and electronic used goods. They are the “daily “feeder” of the “Urban Mine”, while the organic domestic wastes are important source of energy (incineration, methane conversion) or materials for agriculture (composting). In particular, for countries likes Europe, USA, Japan Korea, Singapore, today the resources of critical and strategic metals are not in far countries owner of the natural resources but within the cities.
Clearly today, the Cities face economic growth from waste resource use while decreasing the environmental impact, which introduce a beneficial balance on economic, environmental and social sides going forward to the Sustainable City. [17,18]. Exploitation of the “Mine in the City” can contribute largely to achievement of Sustainable City.
Battery Segment
The development of electric and electronic devices is giving now the model of consumption to “a wireless age”. The growth of miniaturization in one hand and energy density need lead to rapid development of new chemistry for batteries as observed during last decade particularly in secondary systems. The growth of miniaturization in one hand and energy density need lead to rapid development of new chemistry for batteries as observed during last decade particularly in secondary systems [19] (Figure 2).
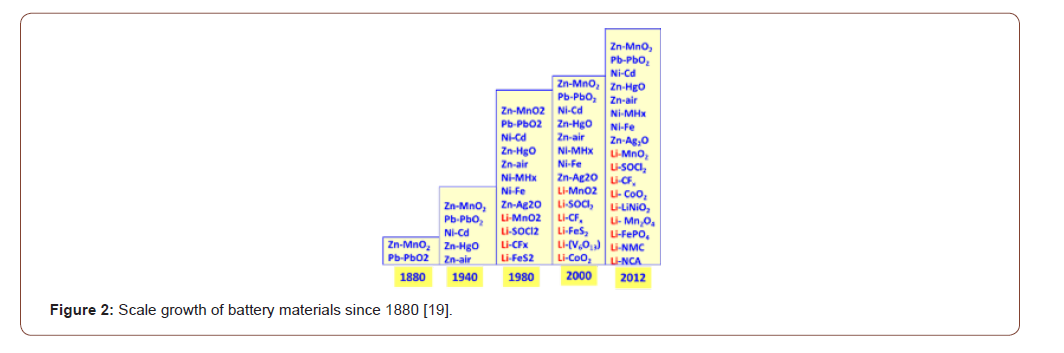
This market is constantly in search of higher electrochemical performance (mainly energy density) in front of increasing miniaturization. The use of primary and rechargeable batteries is growing considerably and among the problematic waste, batteries have a predominant place due to the heavy metals and electrolyte/ solvents. Dark side of the progress is always in environment impact and scarcity of resources. After use of batteries, when discharged, they become an important source of pollution as they contain substantial amounts of toxic elements such as lead, cadmium, nickel or mercury. Though manufacturers have decreased the mercury content, batteries and used batteries remain an important source of heavy metals (lead, cadmium, indium, nickel, zinc and manganese). This aspect bound to the environment and connected to the economy of raw materials imposes recycling measures. The collection and the treatment of batteries and used accumulators should help economize on raw materials and thus contribute to the protection of the environment. The composition of those batteries imposes a particular consideration of their end of life management due to the environment impact and valuable resource conservation for materials.
Consequently, any treatment and recovery process of used batteries must allow high recovery rate of strategic and critical materials with low environment impact. Among the impact, CO2 emission is the most important as metals where always processed using pyro metallurgy way. Emission of CO2 is accompanied (fowling the kind of batteries and the composition) by emission of harmful gases (Cadmium and zinc vapor, chlorine oxide of sulfur and nitrogen oxides as well as dioxins). Beside gas emission all metallurgical process are accompanied with by-products as solid waste, which concentrate pollutants (fly ash and slag).
For the environment and recycling rate issues, battery recycling was subject of intense research for non-thermal processes [10, 20- 24].
Recycling of alkaline and zinc carbon batteries
Zinc carbon batteries (known as Leclanche cell) and its alkaline version are the most popular primary batteries. Generic composition is reported in Figure 3.
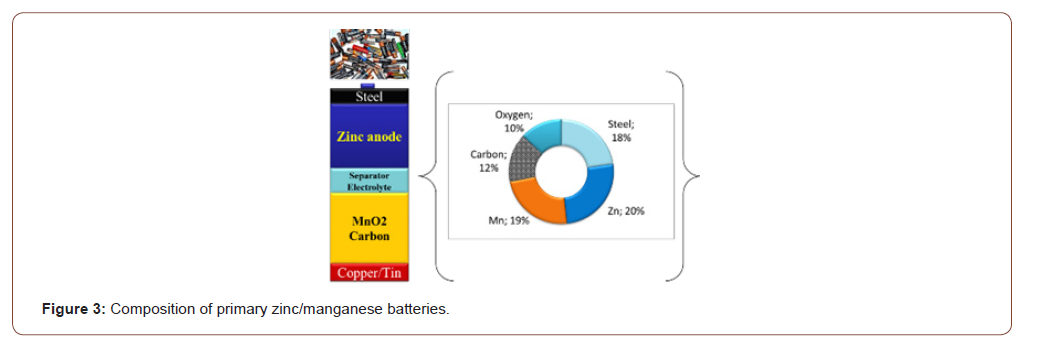
From the average composition, we can see that this kind of batteries could be a substantial source of raw materials such as steel, zinc and manganese as well as carbon (graphite). A quick analysis of the physical and chemical properties as well as behavior of those materials indicate that metallurgical route is not efficient and sustainable way for clean recycling and high recycling rate. Indeed if the process allows recovering steel and zinc, unfortunately manganese is lots in slag while carbon graphite is lost as CO2 during thermal process. A mechanochemistry route was investigated and patent in 1994 [10] leading to the recovery of
1) Steel after shredding and magnetic separation
2) Graphite carbon in first acid leaching step
3) Zinc metal and manganese dioxide by double electro wining after purification leaching solution (Figure 4)
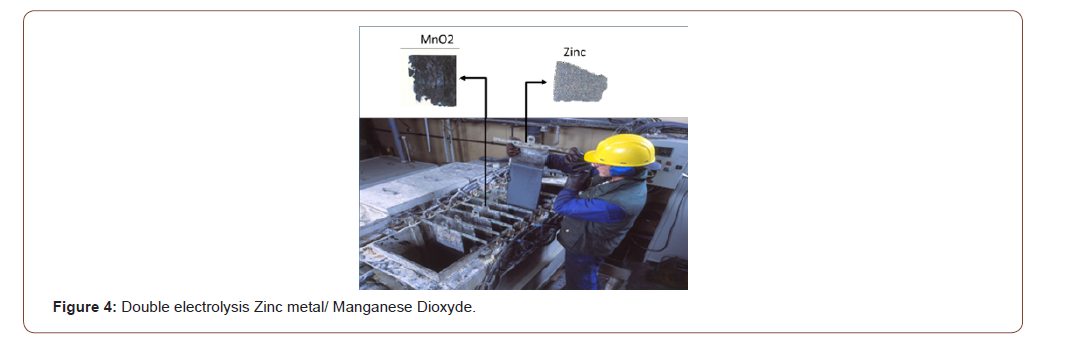
(The picture shows zinc anode plate extracted manually from electrolysis pilot plant)
The analysis of zinc metal shows a purity of 98.8% while the X-Ray diffraction analysis shows that the recovered product has a same fingerprint than a market production EMD (Electrolytic Manganese Dioxide).
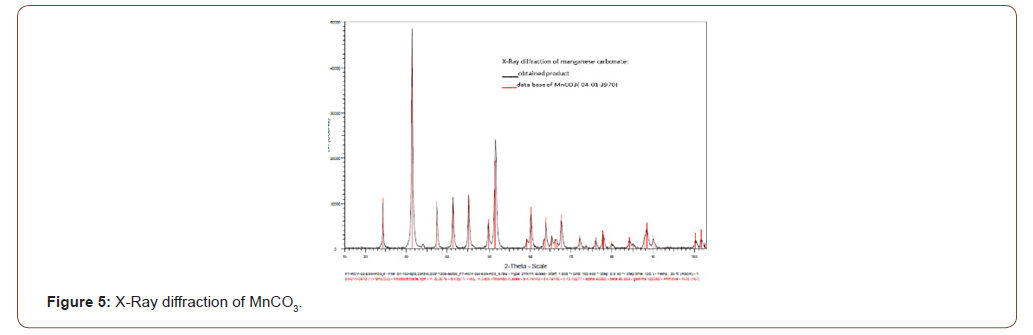
In second project, the process was improved within a second patent [20] avoiding electrolysis (heavy investment part) and leading to chemical product based on zinc and manganese salts. The manganese has been obtained as manganese carbonate (MnCO3) and X-Ray diffraction shows that all the peaks are fitting with the Rhodochrosite mineral product as reported in Figure 5.
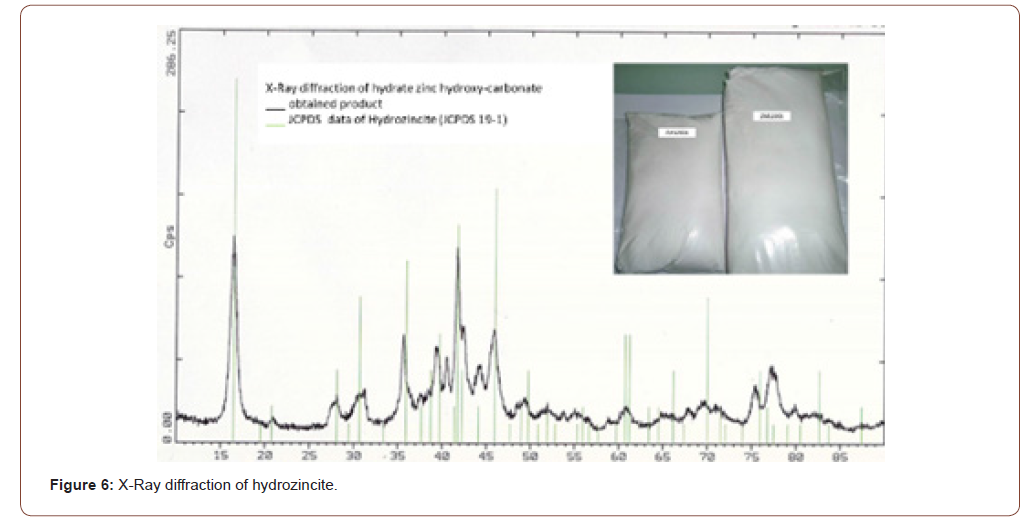
In second step, the zinc was precipitated as Hydro incite which is and hydroxyl-carbonate with a formula of Zn5(OH)6(CO3)2. X-Ray diffraction patterns of the product are reported in Figure 6.
Beside the recovery of steel, zinc and manganese, this process allows to separate a clean phase of carbon-graphite, which leads to substantial increase of recycling rate according to EU Directive 06-66 [25].
Recycling of lithium ion batteries
Within the Figure 2 we can observe that the lithium segment is facing a jump from electrochemical series (all non-rechargeable systems) before 1990 to more than 19 systems including new rechargeable systems has been observe during last decade. New electrolytes, conductive salts and new oxide composition have recently enriched the Lithium battery chemistry.
Lithium ion battery (LIB) becomes today the most largely adopted for portable electronic devices. Recently new chemistries of both cathodes and anodes allow the LIB system to be the best system candidate for in EV segment. However, dark side of the progress is always in environment impact and scarcity of resources. Therefore, particular composition of those batteries imposes a specific consideration of the two aspects of environment impact / Resources conservation. In particular, we estimate today the world consumption of mobile phones at up to 7.5 billion (means more than the world population). Beside this segment, e-mobility is now the most challenging sector for climate change and decrease of fossil energy consumption. The forecasted quantity of EV should be around 3 million in 2020 and up to 20 million within 10 years (2030) (Figure 7).
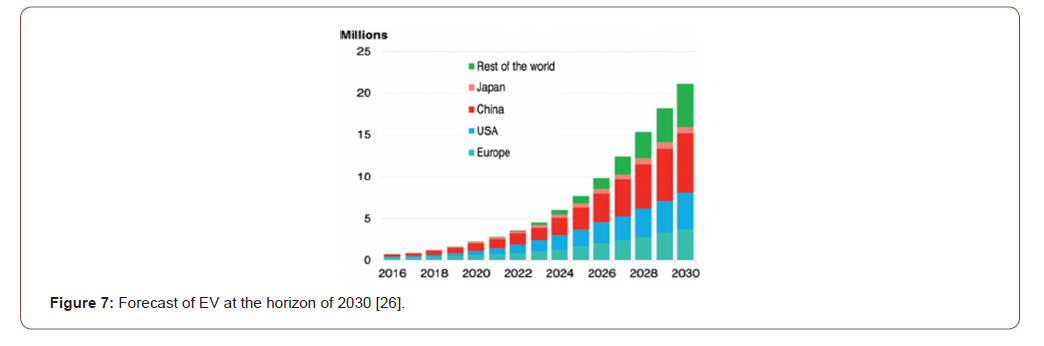
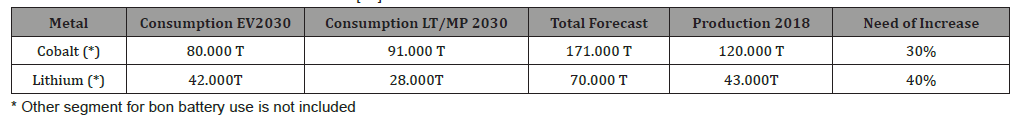
Translating those numbers of EV in battery materials, we jump from a demand of 500.000 T in 2020 to 5 million of tons in 2030. The amount of 5 million of battery materials means an average quantity of cathode materials (all chemistries included) of 1.200.000 tons of cathode materials. If we consider only cobalt (in NMC and NCA cathodes) and Lithium in all chemistries, the expected amounts within 10 years and the need of production increase are reported in Table 1.
Table 1:Characteristic of the patients with AED withdrawal (n=162).
One of the solutions for Sustainability of this segment is to convert the used batteries as “Urban Mine” of lithium cobalt and graphite beside manganese and nickel (for NMC) and iron/ phosphate for LFP.
As seen for recycling of alkaline batteries pyro metallurgy leads to
1) The loss of manganese and lithium trapped in the slag fraction
2) The conversion of carbon/graphite to CO2.
3) The emission of fluorine coming from all fluorinated materials (lithium salts, Polyvinyfluoriden and some fluorinated solvent such as Perfluoropolyethers).
Around the early days of lithium ion battery, we built and introduced in 1999, a project within n the 5th FP GROWTH program European call. The VALIBAT project (G1RD-CT-2000-00232) coordinated by RECUPYL was dedicated to recycling all lithium based batteries [15]. This preliminary project was the Proof of Concept. This first project was with a pilot demonstration as Proof of Value [14]. The work led to an internationally granted patent [15]. The process based on same approach as recycling alkaline batteries process but operating in inert atmosphere due to high reactivity of lithium based batteries. After separation of metals and plastic using various physical unit operations, specific chemical process is carried out. When we apply the process to laptop and mobile phone batteries based on lithium cobaltite cathode and LiC6 anode, the main materials recovered are:
1) Graphite/carbon
2) Cobalt carbonate
3) Lithium carbonate.
Graphite leached in acid media has substantial purity as reported in Figure 8 and Table 2.
The X-Ray diffraction patterns show a very good fitting with graphite indexed material, while SEM picture shows the two species of carbon (flakes of graphite from anode insertion material and nanoparticles of carbon black from cathode as conductive materials). Table 2 reports the purity of the obtained material after leaching, washing and drying steps.
Table 2:Chemical composition of carbon/graphite.
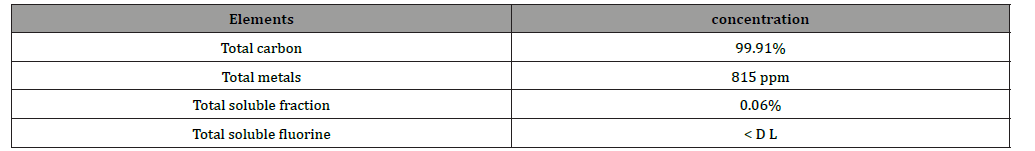
The specification of this carbon/graphite allows the use as battery materials as reported in Figure 8 and 9.
The electrochemical performances were reproduced within six campaigns of initial fine fraction (black mass) coming out from mechanical processing / refining of lithium ion batteries. Figure 10 is the plot of specific capacity mAh/g against cycle number.
The testing of materials obtained within six different campaigns shows a very good reproducibility [28]. The recovery of the carbon/ graphite within this process allows:
1) Closing loop with reuse in batteries, leading Resources conservation
2) Economy of 590 kg of CO2/T of batteries
3) Direct impact on EU Recycling Rate as gravimetric value (up to 70 %)
Cobalt carbonate is precipitated after carbon/graphite recovery as shown in Figure 11.
Finally, the solution free from heavy metals was subject to carbonation for recovery of lithium as lithium carbonate. The characteristic of this lithium carbonate are reported in Figure 12.
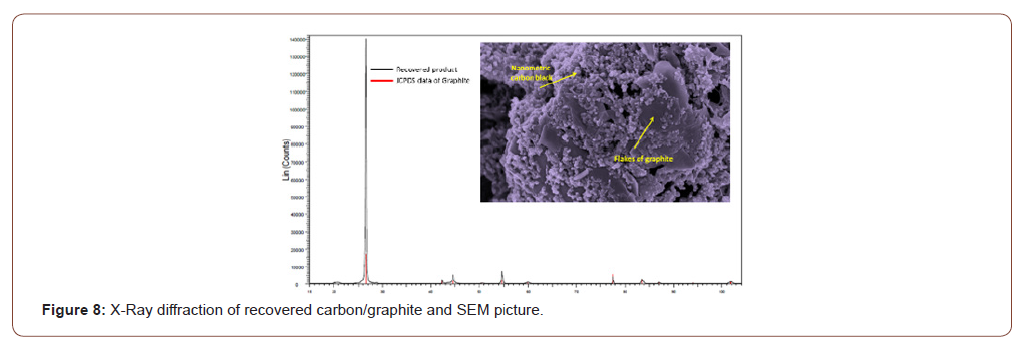
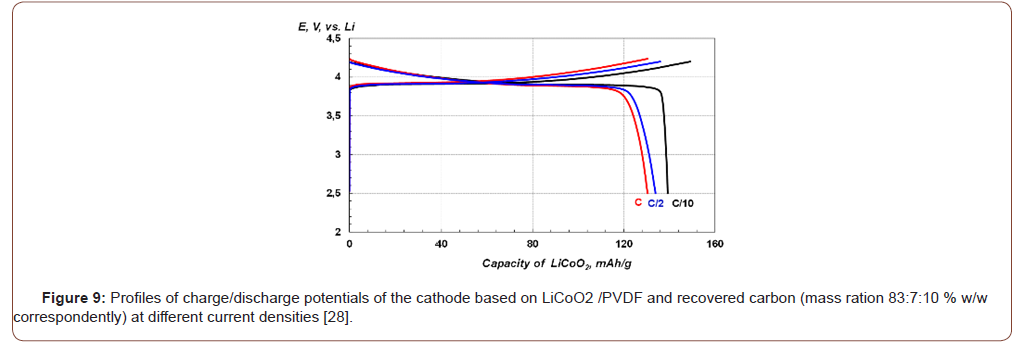

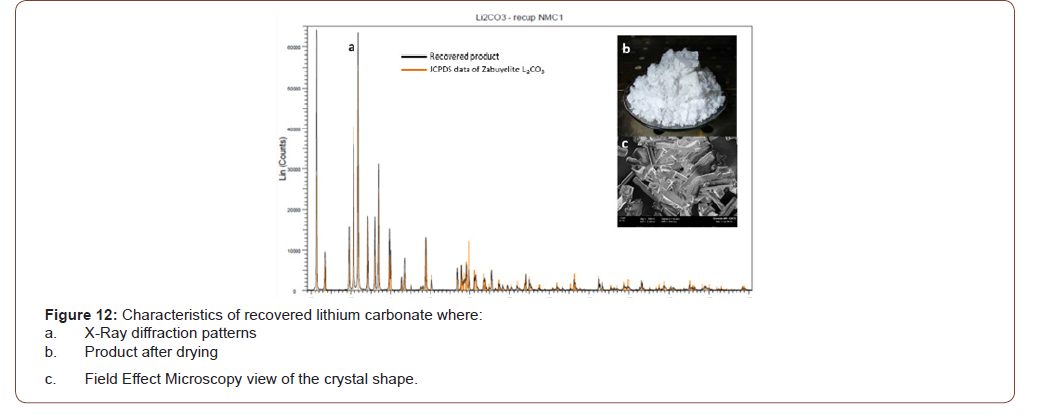
Table 3:Of battery grade lithium carbonate.
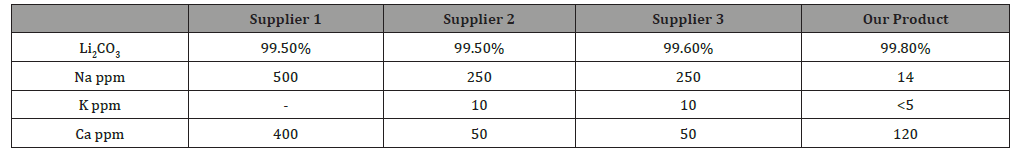
In particular, lithium carbonate is largely fitting with the specification of battery grade available currently on the market (Table 3)
From those specifications of materials as well as first testing of carbon as material electrode, it is clearly demonstrated that used batteries within portable and e-mobility could be the new source of strategic materials within” Urban Mine”.
Conclusion
Among all resources and just after water and energy, in third position we have the metals. In other hand today, no doubt those industrial emerging segments such as s solar panel, wireless products, e-mobility and wind turbines will have a great growth and demand. However almost all those equipment are using strategic and critical metals facing a security of supply. Among the emerging sectors, batteries are one of the commercial segments facing the most important growth of last decades. The sustainability of this segment imposes a strict application of an industrial/commercial model based on Circular economy. In same times, “Urban Mining”, concept developed since 2004 is now well integrated in the recycling and sustainability landscape. It is also largely used as key concept within any end-of-life sustainability approach. Within the life cycle of every good/equipment, “Urban Mine” is one the most important key in the supply chain [33]. In the 3 processes of battery recycling, it has been demonstrated that used batteries became now a real “Urban Ores” taking in the future an important place of supply chain shifting from traditional mine to the “Urban Mine”, a real mine in the city avoiding:
1) A far shipment of commodities,
2) Heavy processing of natural ores,
3) A constraint of geopolitical dependence.
Further report is in progress for another strategic segment of industry were “Urban Mine” can strongly contribute to sustainability of this sector as well has equilibrating the geopolitical supply chain.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- COP21 Paris (France) United Nations Report 12-2015
- (2012) United Nations Environment Program (UNEP). Metal stocks and recycling rates
- (2017) EU Communication on the 2017 list of Critical Raw Materials for the EU.
- (2017) EU Report on critical metals. pp. 15
- Urban Mine, INPI 093650442, WIPO 104248, Int Trade Mark 1371937
- (1997) Kyoto Treaty (UNFCCC Protocol).
- (2015) International Energy Agency. Energy and climate change, IEA Publishing.
- Tedjar, C. Poinsignon, French Patent 944000740
- Tedjar, G. Roux, F. Juif FR9509548, WO062991 (2001), US 00010156A1
- Tedjar , EP 1047478B1
- Tedjar, P. Rivat, M. Garnier FR9703151
- Tedjar, JC. Foudraz WO patent 2005101564-A2, F.Tedjar US patent 2007_0198725-A1
- VALIBAT European Commission data N° 51959, RECUPYL SAS (FR), https://cordis.europa.eu/project/rcn/51959/factsheet/en.
- Tedjar, Asian Regional Summit Hello Tomorrow , 7 November 2019
- UNEP Engine of sustainability Report, November 2017
- Tedjar, Urban Mine for Sustainable City, Int. Congress on Sustainable City, Sétif (Algeria) Nov. 2017.
- Tedjar IBA2007 Conference, Kylan Villa, Shenzhen (China), 16-20 November 2007
- Tedjar International Battery Seminar, Annecy (France) September 1998
- P Zhang, T Yokoyama, O Itabashi, TM Suzuki, K Inoue (1998) Hydrometallurgical process for recovery of metal values from spent lithium-ion secondary batteries. Hydrometallurgy 47( 2-3): 259-271.
- J Xu, HR. Thomas, RW Francis, KR Lum J. Power Sources, vol. 177, no. 2, pp. 512–527, 2008.
- Zou, E. Gratz, D. Apelian, and Y. Wang, Green Chem., vol. 15, no. 5, pp. 1183–1191, 2013.
- Huang, Z. Pan, X. Su, and L. An, J. Power Sources, vol. 399, no. August, pp. 274–286, 2018.
- European Battery Directive EU 06/66, 26 Sept. 2006.
- Pilot International Seminar on Batteries , Fort Lauderdale, FL (USA) March 2017
- Tedjar Invited Conference, E-Mobility and Circular Economy, Tokyo (Japan) 1-3 July 2019
- Solvay US Patent 2018219256
- AMELI Project N° 265910: Call for sustainable automotive electrochemical storage applications
- Meatza, A. Moretti, S. Passerini, , F. Tedjar, EU TRANS, Vienna, 04-2018
- CYCLADE Project N° 1282C0425 Granted by French Environment Agency (ADEME) to RECUPYL (July 2013).
- Tedjar, Circular Economy on Batteries Conference (Keynote speaker) , Goteborg, Sweden 26/09/2018
- Tedjar, Battery and Storage Energy 3(2020) 116-118, march 2020.
-
Shahid Ali Khan, Bagh Ali, Yufeng Nie, Liaqat Ali. Analysis of Fly Ash and Paramagnetic Nanoparticles with Hybrid Base Fluid Due to Applied Magnetic Dipole in a Stretching Sheet with Momentum Slip Condition: FEM Approach. Mod Concept Material Sci. 3(1): 2020. MCMS. MS.ID.000554.
-
Materials, Urban Mine, Battery recycling, Lithium, Metals, Energy, Batteries, Natural resources, Chemistry, Composition, Sustainability
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






