 Research Article
Research Article
Synthesis and Self-Assembly of a Novel Cyclic Hexapeptide and Octapeptide
Shuang Guo, Pengxiang Ge, Mindong Chen* and Ge Cheng
School of Environmental Science and Engineering, Nanjing University of information Science & Technology, China
Mindong Chen, Jiangsu key laboratory of Atmospheric Environment Monitoring and Pollution Control; Collaborative Innovation Center of Atmospheric Environment and Equipment Technology; School of Environmental Science and Engineering, Nanjing University of information Science & Technology; Nanjing, 210044, China.
Received Date: April 12, 2021; Published Date: April 21, 2021
Abstract
In recent decades, cyclic peptides have attracted great attention due to their unique properties. In this paper, novel cyclic hexapeptide (6) and octapeptide (9) were designed and synthesized using liquid phase synthesis methodology. They were characterized by FT-IR, 1HNMR spectroscopy, LC-MS, scanning electron microscopy (SEM) and atomic-force microscopy (AFM). We have used these cyclic peptides as novel building blocks to generate porous materials by self-assembly.
Keywords: Cyclic hexapeptide; Cyclic octapeptide; Self-assembly
Mini Review
Chemists have isolated and identified a large array of cyclic peptide compounds from nature, particularly from plants and marine organisms, since the first one having biological activity, the cyclic peptide antibiotic gramicidin S, was discovered in 1974 [1]. Recently, there has been a number of excellent works disclosed regarding synthetic cyclic peptides [2,3]. Cyclic peptides may be classified as homodetic cyclopeptides, wherein the ring is composed of standard peptide bonds, and heterodetic cyclopeptides in which one or several links are not peptidic. A characteristic property of cyclic peptides is their stability conferred by the absence of N- and C-termini and a constrained backbone [4,5]. Based on this, cyclic peptides play an increasingly important role in bio simulation [6], drug design [7-9], nanophase materials [10], biosensors [11], catalysis, material science [12-23] and bone tissue engineering [24,25].
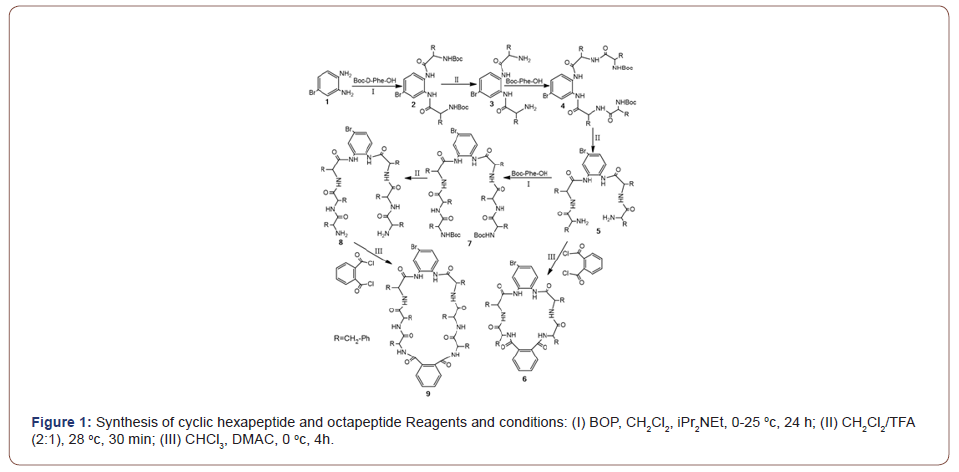
In this paper, we present some studies in the rapidly developing field of synthesis of cyclic peptides, utilizing self-assembly with conventional as well as some more recently developed initiators. Herein, we report the synthesis of two novel phenylalaninebased cyclic peptides and their self-assembly properties. All reactions were carried out in liquid phase and proceeded with moderate to good yields (Figure 1) [18]. Dimethylacetamide (DMAC) was used as solvent and also as the catalyst for cyclization. We used benzotriazole-1-yloxytris (dimethylamine) phosphonium hexafluorophosphate (BOP) as the condensing agent and trifluoroacetic acid (TFA) as the deprotecting reagent.
Compound 2 was prepared by condensation of 4-bromo- 1,2-benzenediamine and Boc-D-Phe-OH under nitrogen in good yield (68%) and, following deprotection with 30% TFA in DCM gave compound 3 in a yield of 78% after simple recrystallization. Compound 3 was then repeatedly condensed and deprotected by similar procedures to afford 5 and 8 in acceptable overall yields (92%, 29%). The desired cyclic hexapeptide 6 was synthesized by treating the corresponding precursor 5 with 1,2-benzenedicarbonyl chloride under mild conditions in a moderate yield (29%), and the same procedure gave cyclic octapeptide 9 in a yield of 21% [26]. The structures of compounds 2-9 were characterized by FT-IR, 1H NMR spectroscopy and LC- MS. The final product 6 showed molecular ions at 904, 906 and 9 at 1198 and 1200, corresponding to [M+Na]+Br79-Br81 respectively. Resonances in the 1H NMR spectrum could be fully assigned and the 1H NMR and FT-IR spectra of 2-8 were also in agreement with the proposed structures. Partial assignments are given in the experimental section [26].
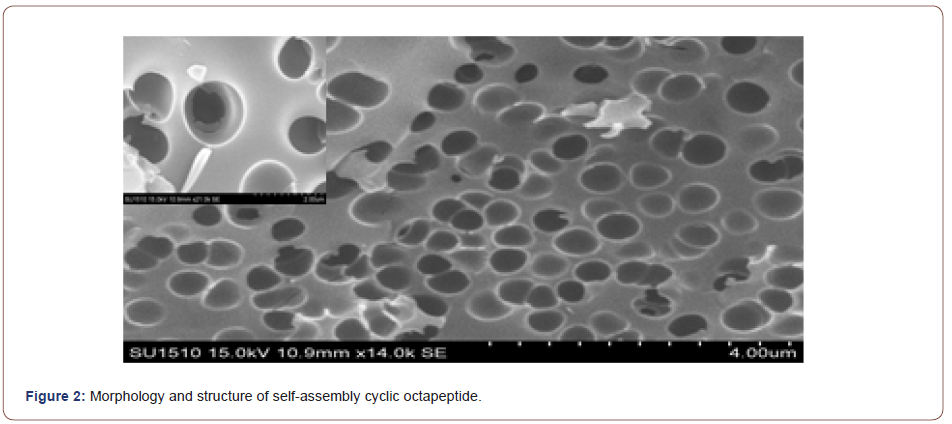
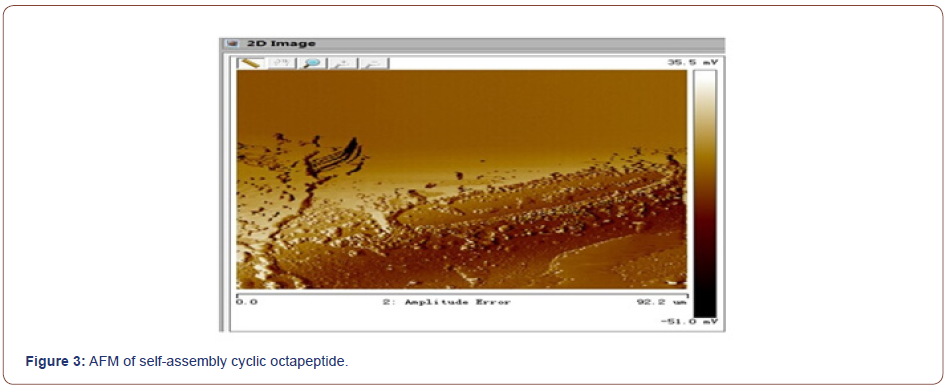
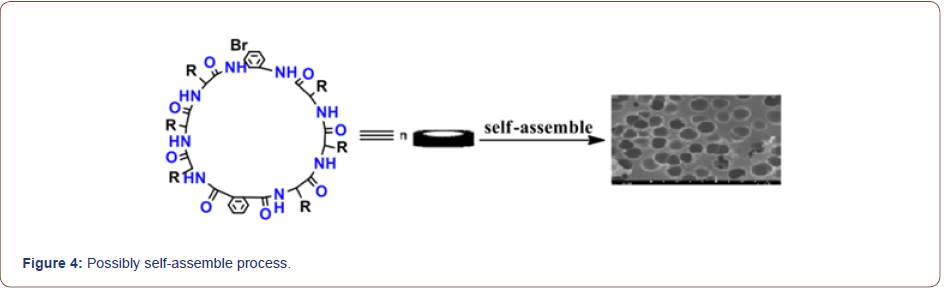
Self-assembly studies were performed by dissolving 10 mg of the cyclic peptide in 1 mL neat trifluoroacetic acid (TFA) and placing the resulting solution in an open 2 mL Eppendorf tube. The Eppendorf tube was then floated in 30 mL of double-distilled (dd) H2O in a 45-mL conical tube. The two samples were allowed to equilibrate for 48-72 h, after which the Append or f tube was removed and found to contain a heavy suspension of microcrystals. The crystals were recovered quantitatively by centrifugation and washing with ddH2O or by dialyzing against ddH2O to remove any remaining TFA. FT-IR spectroscopy indicated that TFA did not participate in the crystal lattice and the morphology and structure of the self-assembled cyclic peptide 9 were characterized by scanning electron microscopy (Figure 2, 3). The SEM images indicated that the self-assembled material derived from cyclic octapeptide 9 was porous and sheet-like. The AFM image showed that average diameter of the holes was 0.90μm, with an average depth of about 1.5~2.0μm. Possibly self-assemble process was shown in Figure 4.
In conclusion, we have reported the liquid phase synthesis of a cyclic hexapeptide and a cyclic octopeptide based on phenylalanine linked with o-phenylenediamine and phthalic acid caps. The advantage of this approach is that the reaction can be performed by simply mixing starting materials, acid and capture agent, giving moderate to good yields under mild conditions. A self-assembled porous material was also obtained in a good yield under mild conditions.
Acknowledgement
This work was supported by the National Science Foundation of China (No. 21976093 and 21976094), and the National Key Research and Development Project (No. 2018YFC0213802).
Conflict of Interest
No conflict of interest.
References
- Ovchinnikov YA, Ivanov VT (1975) Conformational states and biological activity of cyclic peptides. Tetrahedron 31(18): 2177-2209.
- Poteau R, Trinquier G (2005) All-cis Cyclic Peptides. J Am Chem Soc 127(40): 13875-13889.
- Kazmier U, Maier S (1999) Application of the Peptide Claisen Rearrangement toward the Synthesis of Cyclic Peptides. Org Lett 1(11): 1763-1766.
- Trabi M, Craik DJ (2002) Circular proteins — no end in sight. Trends in Biochemical Sciences 27(3): 132-138.
- Wong CTT, Lam HY, Li, X (2014) Effective synthesis of cyclic peptide yunnanin C and analogues via Ser/Thr ligation (STL)-mediated peptide cyclization. Tetrahedron 70(42): 7770-7773.
- Pappo D, Vartanian M, Lang S, Kashman Y (2005) Synthesis of Cyclic Endiamino Peptides. J Am Chem Soc 127(21): 7682-7683.
- Kaur H, Heapy AM, Kowalczyk R, Amso Z, Watson M, et al. (2014) Synthesis and biological evaluation of the osteoblast proliferating cyclic peptides dianthins G and H. Tetrahedron 70(42): 7788-7794.
- Acar H, Srivastava S, Chung EJ, Schnorenberg MR, Barrett JC, et al. (2016) Advanced Drug Delivery Reviews 9: 441-447.
- Qian Z, Liu T, Liu YY, Briesewitz R, Barrios AM, et al. (2013) Efficient delivery of cyclic peptides into mammalian cells with short sequence motifs. ACS Chem Biol 8(2): 423-431.
- Hartgerink JD, Granja JR, Milligan RA, Ghadiri MR (1996) Self-Assembling Peptide Nanotubes. J Am Chem Soc 118(1): 43-50.
- Liu S (2009) Radiolabeled Cyclic RGD Peptides as Integrin αvβ3-Targeted Radiotracers: Maximizing Binding Affinity via Bivalency. Bioconjugate Chem 20(12): 2199-2213.
- Zhao Q, Schafmeister CE (2017) Journal of Heterocyclic Chemistry 49: 51-80.
- Pugliese R, Gelain F (2017) Peptidic Biomaterials: From Self-Assembling to Regenerative Medicine. Trends in Biotechnology 35(2): 145-158.
- Jewel Y, Yoo K, Liu J, Dutta P (2016) Journal of Nanoparticle Research 18: 1-9.
- Danial M, Tran CM, Jolliffe KA, Perrier S (2014) Thermal Gating in Lipid Membranes Using Thermoresponsive Cyclic Peptide–Polymer Conjugates. J Am Chem Soc 136(22): 8018-8026.
- Yuhan Yan, Yuanze Li, Zhiwen Zhang, Xinhao Wang, Yuzhong Niu, et al. (2021) Advances of peptides for antibacterial applications. Colloids and Surfaces B: Biointerfaces 202: 111682.
- Yi Lai, Fenglin Li, Zhifeng Zou, Madiha Saeed, Zhiai Xu, et al. (2021) Bio-inspired amyloid polypeptides: From self-assembly to nanostructure design and biotechnological applications. Applied Materials Today 22: 100966.
- Yi Li, David Stern, Lye Lin Lock, Jason Mills, Shih-Hao Ou, et al. (2019) Emerging biomaterials for downstream manufacturing of therapeutic proteins. Acta Biomaterialia 95: 73-90.
- Naseem Akhtar, Riaz A Khan (2016) Liposomal systems as viable drug delivery technology for skin cancer sites with an outlook on lipid-based delivery vehicles and diagnostic imaging inputs for skin conditions. Progress in Lipid Research 64: 192-230.
- Vedanjali Gogineni, Mark T Hamann (2018) Marine natural product peptides with therapeutic potential: Chemistry, biosynthesis, and pharmacology. BBA - General Subjects 1862(1): 81-196.
- Marc WT Werten, Gerrit Eggink, Martien A Cohen Stuart, Frits A de Wolf (2019) Production of protein-based polymers in Pichia pastoris. Biotechnology Advances 37(5): 642-666.
- Wensi Zhang, Xiaoqing Yu, Yang Li, Zhiqiang Su, Klaus D Jandt, et al. (2018) Protein-mimetic peptide nanofibers: Motif design, self-assembly synthesis, and sequence-specific biomedical applications. Progress in Polymer Science 80: 94-124.
- Abhijit Ghoraiy, Basudeb Achari, Partha Chattopadhyay (2016) Tetrahedron 72: 3379e3387.
- Visser R, Rico-Llanos GA, Pulkkinen H, Becerra J (2016) Peptides for bone tissue engineering. J Control Release 244(Pt A): 122-135.
- Choi S, Jeong W, Kang SK, Lee M, Kim E, et al. (2012) Differential Self-Assembly Behaviors of Cyclic and Linear Peptides. Biomacromolecules 13(7): 1991-1995.
- General Procedures for Preparation of 7,10,19,22-Tetrabenzyl-2-bromo-5,7,8,10,11,18,19,21,22,24-decahydro- 5,8,11,18,21,24-hexaaza-dibenzo[a,k]cycloeicosene-6,9,12,17,20,23-hexaone (6): 5 (2.25 g, 2.90 mmol) and NaHCO3 (3.00 g, 35.71 mmol) were added to N,N-dimethylacetamide (90 mL) and the mixture stirred at 0 ℃ for 1 h. Then a solution of 1,2-benzenedicarbonyl chloride (0.59 g, 2.91 mmol) in CHCl3 (30 mL) was added slowly, and the mixture stirred for 4h. The reaction was washed with water to remove the DMAC and inorganic material and the organic layer was dried with MgSO4, filtered and concentrated in vacuo. Purification of the crude product by column chromatography (AcOEt/CH2Cl2/PE/n-hexane, 3:5:1:4) afforded 6 as a white solid (0.75 g, 29 %). mp: 150-152 ℃. 1H NMR (300 MHz, DMSO): δ = 1.24 (18H, s), 2.51-3.23 (12H, m), 4.14 (2H, s), 4.66 (2H, s), 4.83 (2H, s), 6.71 (2H, t), 7.06-7.52 (32H, m), 7.74 (1H, s), 8.20 (2H, d), 8.61 (2H, s), 9.75 (2H, d). IR(KBr): 3408, 3309, 2925, 2854, 1648, 1595, 1527, 1454, 1404, 1297 cm-1, LC-MS: 904, 906 [M+Na]+.
- 4,7,10,21,24,27-Hexabenzyl-32-bromo-2,5,8,11,20,23,26,29-octaaza-tricyclo [28.4.0.013,18] tetratriaconta-1(34), 13(18),14,16,30,32-hexaen-3,6,9,12,19,22,25,28-octaone (9): The procedure was carried out as described for 6 with 8 (3.00 g, 2.80 mmol), 1,2-benzenedicarbonyl chloride (0.58 g, 2.80 mmol), NaHCO3 (3.00 g, 35.7 mmol), DMAC (90 mL) and CHCl3 (30 mL). Purification of the crude product by column chromatography (AcOEt/CH2Cl2/PE/n-hexane, 12:5:1:4) and recrystallization from AcOEt/n-hexane gave a white solid (0.70 g, 21 %). mp: 214-216 ℃. 1H NMR (300 MHz, CDCl3): δ = 2.84-3.10 (12H, m), 4.44-4.73 (6H, m), 6.74-7.85 (43H, m), 9.08 (2H, d). IR(KBr): 3293, 2925, 2853, 1654, 1594, 1526, 1497, 1455, 1405, 1295 cm-1. LC-MS: 1198, 1200 [M+Na]+.
-
Shuang Guo, Pengxiang Ge, Mindong Chen, Ge Cheng. Synthesis and Self-Assembly of a Novel Cyclic Hexapeptide and Octapeptide. Mod Concept Material Sci. 3(5): 2021. MCMS. MS.ID.000573. DOI: 10.33552/MCMS.2021.03.000573.
-
Cyclic hexapeptide, Cyclic octapeptide, Self-assembly, Cyclic peptides, Spectroscopy, Structure, Electron microscopy, Material, Liquid phase synthesis, Mild conditions
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






