 Mini Review
Mini Review
Study of Nanocomposites of Silicon Structures for use in Mechatronics
Stefan Kartunov*
Department of Mechanical and Precision Engineering, Technical University of Gabrovo, Bulgaria
Stefan Kartunov, Department of Mechanical and Precision Engineering, Technical University of Gabrovo, Bulgaria.
Received Date: February 22, 2020; Published Date: March 04, 2020
Abstract
This article analyzes the developments on the subject and draws conclusions for the study 1722/М - 2017 at the UZNIT of TU - Gabrovo BG. Most metals are highly chemically reactive, especially at nanoscale, without having properties that can be easily used with TiO2 composites. As such, most of the metals below will be considered metal oxides of precious metals. In addition, the most common oxide TiO2 will be discussed, as other oxides are probably unstable and thus do not form stable composites.
Keywords: Nanocomposites; Silicon structures; Titanium dioxide
Introduction
A detailed consideration of all precious metals is given in study 1722/М-2017 at the UZNIT of Technical University-Gabrovo and [1]. Only palladium, platinum, silver and gold are considered here.
Technical Requirements
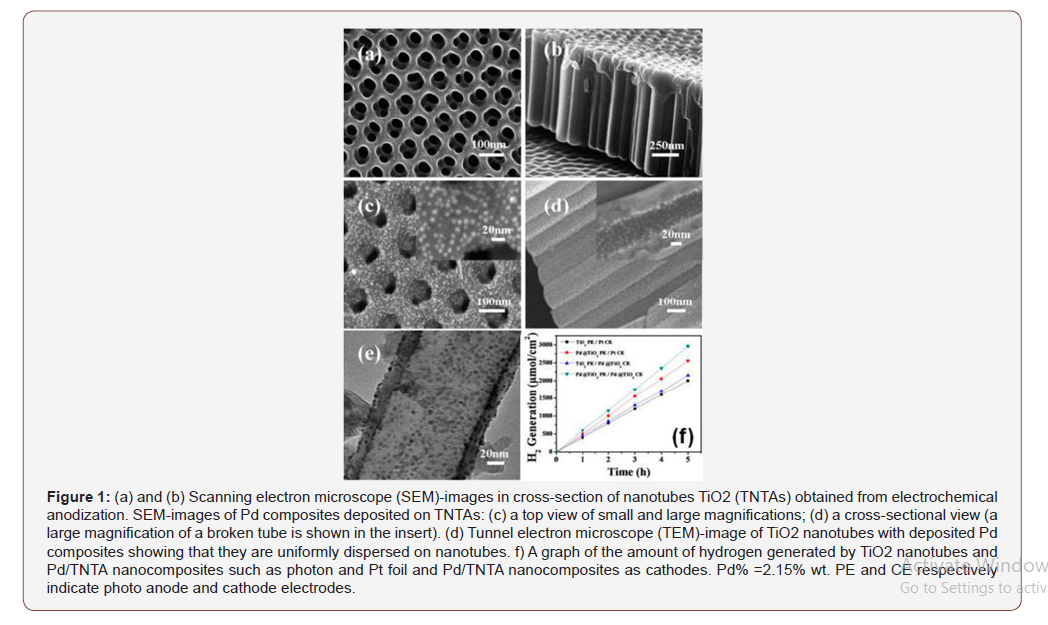
The development of Pd/TiO2-based palladium composites on visible light radiation was studied by Mohapatra. TiO2 nanoparticles were synthesized by Ti foil anodization followed by PdCl2 functionalization and subsequent H2/Ar calcining to crystallize TiO2 and converting the Pd salt to pure Pd. The synthesized composite with optimized 1.25wt % Pd showed significant photocatalytic improvement compared to naked TiO2 nanotubes. A nitrogen-dosed Pd/ TiO2-active to the visible light composite is also prepared. Prepared TiO2 nanotube matrices through a three-stage anodization of Ti foil, followed by calcination and hydrothermal reduction of Pd-nanoparticles on the crystal TiO2 nanotubes in the presence of polyvinylpyrrolidone (PVP) and Na I allow for controlling the PVP-concentration and the hydrothermal reaction time. Figures 1a and 1b show a Scanning electron microscope (SEM)-image of a preconditioned nanotube matrix, and Figures 1c and 1d show the mass after hydrothermal deposition of Pd. Figure 1 shows a Tunnel elec tron microscope (TEM)-image of the nanotube with clearly placed Pd-nanoparticles. No such silicon matrix composites are known.
Photocatalytic production of Pt/TiO2 composites consistently shows increasing growth with the advancement of nanoscale synthesis and controllable/tunable properties of nanomaterials. These composites allow optimization of parameters such as morphology, crystalline phase, crystallinity, porosity and surface area, each of which can alter the photoactivity of the composition. Improving photocatalytic activity with the introduction of Pt is typically attributed to the formation of a Schottky barrier at the metal- TiO2 interface. This happens because the work function of Pt (~ 5,36-5,63 eV) is greater than that of TiO2 (~ 4,6-4,7 eV), so that electrons are transferred at Pt and holes Localized in TiO2 media to improve photocatalytic efficiency. In order to improve the Pt/TiO2 composites, steps have been taken to optimize the interaction between Pt and TiO2 from Kandiel, effectively effecting the surface area and crystal structure of TiO2 in the resulting Pt/TiO2 composite and demonstrating that although the large Area is useful, increasing crystallinity is preferential. Increasing photocatalytic activity is due to the reduction of site defects that act as charge recombination centers when crystallinity increases.
Due to their low price compared to other precious metals, intensive localized surface plasma resonance (LSPR) and easy shape control, silver nanomaterials are used in TiO2 composites to a considerable extent. Silver is widely used with TiO2 to produce composites to decompose photocatalytic organic molecules, DSS-composites, photoactive bactericides, photochromic materials and other applications. Although silver cannot recombine H+ atoms for hydrogen production, based on its slightly larger function, ~ 4.7 and 4.6 eV for Ag and TiO2, respectively still can attract photogenerated electrons of TiO2 and thus improve the separation of the charge. Many reports show improved UV-photocatalytic degradation of organic pollutants from Ag/ TiO2 composites compared to pure TiO2. Although the precise nature of LSPR-resonance on improved photocatalytic activity has not been fully investigated, it improves the photocatalytic activity of TiO2 and the generation of photoelectrochemical currents. In the Awazu study, direct contact between Ag and TiO2 is not necessary for photocatalytic amplification, which suggests that the reason is the increase of the Ag LSPR-resonance electromagnetic field.
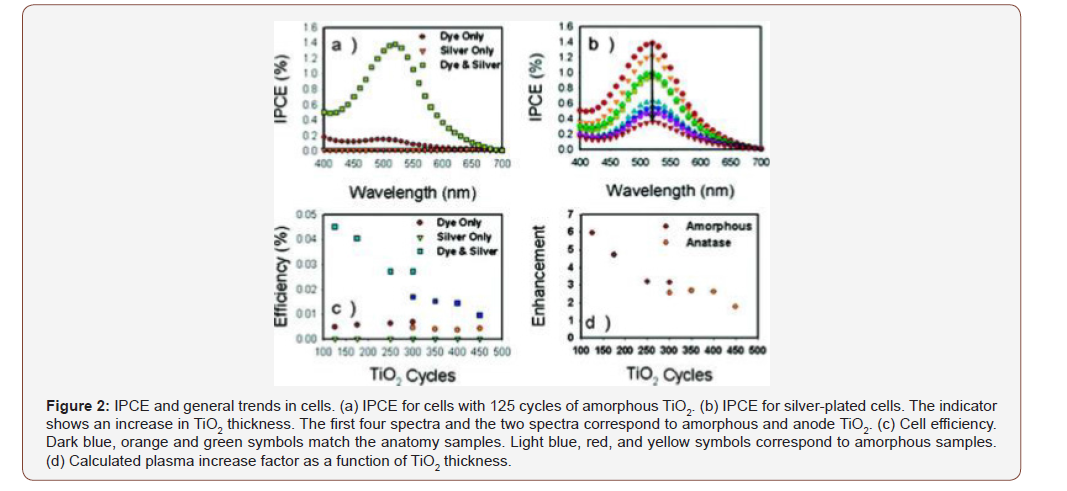
In this study, silver nanoparticles are embedded in SiO2 layers of varying thickness, followed by a TiO2 layer, which is of interest to the topic. It is shown that when the SiO2 layer is thinner, photocatalytic degradation increases even without contact with TiO2. The system consists of Ag nanoparticles, coated with different TiO2 thickening particles. As shown in Figure 2b, TiO2’s finest coatings give the greatest improvement in IPCEs, due to the larger electromagnetic field attributed to silver nanoparticles. This in turn leads to greater cellular efficacy as shown in Figure 2c.
In order to better investigate the effects of the metal- TiO2 interaction in the Ag/ TiO2 composite, core Ag/TiO2 structures were synthesized. The original core composites were manufactured by Liz-Marzan and others, but the Ag/TiO2 composite nuclear coatings specifically made for interaction studies were first synthesized and tested by Hirakawa and Kamat. Reversible charge and dilution of the Ag core of photocurrent electrons from the TiO2 shell is observed, and when the composite is irradiated, the electrons are moved to the Ag core because the holes generated in TiO2 are extracted from ethanol. Details can be seen in study 1722/M-2017.
Compared to platinum and silver, gold has advantages over each of them. Like silver, gold has an adjustable LSPR-resonance, that can be used to improve photocatalytic activity, but also has a stronger chemical stability similar to platinum. In addition, gold has a high duty function (~ 5.1-5.3 eV). Based on this, the Au/ TiO2 composites are used for many of the same applications as Pt and Ag, with some improvements depending on the case. Typically, Au/ TiO2 composites have a better Ag/TiO2 photoactivity because the higher gold function compared to silver allows better separation of the TiO2 charge. In addition to applications for degradation of organic molecules and hydrogen production, the Au/TiO2 composites are heavily used for CO oxidation, which is of importance to the environment due to its release from combustion of fuels in internal combustion engines. It is known that Au/TiO2 composites effectively convert CO to CO2, even at temperatures below 0 °C. Various factors may influence the activity of CO/CO2 oxidants, the particle size of Ag, but also the TiO2 crystallinity and the method of composing the composite. The deactivation of the composite, due to Au particle sintering, is particularly important because in practical applications such as catalytic converters the composite would be subjected to high temperatures (>750 °C). In order to prevent this deactivation, Lee’s study uses an Au/TiO2 catalyst of methyl-orange that can effectively prevent the sintering of Au nanoparticles by placing a physical TiO2 barrier.
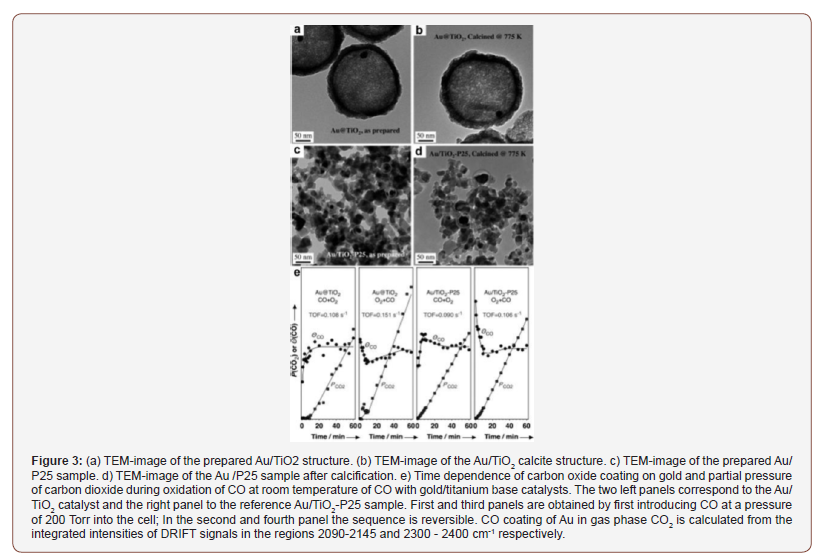
In this study is suggested an Au/TiO2 composite consisting of a nanoparticle of gold (~15nm) in porous TiO2 and is also available. The composite is made by applying the nanoparticles Au first with a SiO2 layer and then coating the TiO2 layer composite. The SiO2 can then be removed from the composite by dissolution with NaOH as shown in Figure 3a. The composite can then be calcined and crystallized TiO2 without alteration of the Au nanoparticles, as shown in Figure 3b. This result is compared with the Au/ TiO2-P25 composite calcification, where Au nanoparticles are synthesized significantly (Figure 3d). All this and the application is the subject of further research.
Conclusion
• No precious metal (Pd) composites of silicon matrix and metal oxides- TiO2 are known.
• In this study, silver nanoparticles are proposed to be embedded in SiO2-layers of varying thickness, followed by a TiO2 layer. It is shown that when the SiO2 layer is thinner, photocatalytic degradation increases even without contact with TiO2.
• An Au/TiO2 composite, consisting of a nanoparticle of gold (~15nm) in porous TiO2 is synthesized. The composite is made by applying the nanoparticles Au first with a SiO2 layer and then coating the TiO2 layer composite. The SiO2 can then be removed from the composite by dissolution with NaOH. The composite can then be calcined and crystallized TiO2 without alteration in the Au nanoparticles. All this is the subject of further research on solar collectors.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
-
Stefan Kartunov. Study of Nanocomposites of Silicon Structures for use in Mechatronics. Mod Concept Material Sci. 2(5): 2020. MCMS.MS.ID.000549.
-
DLVO, Coal, Rheology, Particle size, Energy barrier, Interactions, Surface area, pH, Viscosity, Porosity
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






