 Research Article
Research Article
Sensing Capacitors Based on Thin Films of Unsaturated Polyester Resin/Carbon Nano-Tubes Nano-Composites
Efrat Hochma and Moshe Narkis*
Chemical Engineering Faculty, Technion-IIT, Israel
Moshe Narkis, Chemical Engineering Faculty, Technion- IIT, Haifa, Israel.
Received Date: August 03, 2021; Published Date: September 16, 2021
Abstract
VOCs (volatile organic compounds) are associated with emissions from industrial processes, transportation and the use of organic solvents that are partially toxic or carcinogenic. They tend to evaporate easily at room temperature. When exposed to volatile analytes, absorption of the chemical into the polymer film may alter its permittivity and effective volume, resulting in changes in the capacitance and resistance respectively of the sensor elements. Since the discovery of carbon nano-tubes (CNTs) numerous studies have focused on the development of novel futuristic materials by combining CNTs with polymers to achieve their complimentary properties. The lack of published information regarding systems comprising un saturated polyester resin (UPR) and CNTs composite materials, especially UPR/CNTs based chemo-capacity sensors, promotes the development of novel hybrid nano-material thin film sensing units. CNTs high aspect ratio provides the composites with better electric and mechanical performance than the other candidates such as carbon black. Although highly selective sensors do exist for a limited number of analytes, such gas sensors are highly affected by contaminants and humidity. In addition, these sensors contain a high weight percentage of conducting filler and the use spectra of amorphous polymers is limited. In the present study, chemical capacity sensors based on nano-composite thin films of unsaturated polyester resin (UPR in 40wt% of styrene)/carbon nano-tubes (only 0.05wt% of Multi Wall-CNTs) are developed and investigated under AC voltage and standard environment conditions (ambient air, at room temperature and atmospheric pressure). The purpose is to detect and quantify THF under these conditions for the first time. The change in both resistance and capacitance at low and high frequencies (between 500Hz to10KHz), due to different volumes of the exposed THF (10μl, 20μl and 30μl) and THF droplet (source) - sensing unit height variations (1mm,2mm and 3mm) were studied.
Introduction
Gas sensors development, for chemical, bio-chemical and biological species real time detection for a variety of applications, is a highly devolving field. These detection applications are mainly among the security, environment monitoring and medical industries. Volatile organic compounds (VOCs), which evaporate easily at room temperature, are linked to materials, associated with metabolic and/or pathophysiologic processes, as well as homeland security and environmental care [1-7]. High sensitivity, selectivity and repeatability are essential characteristics for a good sensor production as well as appropriate detection limits, low cost, and tolerance for a variety of chemical background demands [8,9]. CNTbased composites have been reported to exhibit gas sensitivity to several gases, including vapors of organic solvents. Their electrical response is believed to be related to modification of their conductive network path due to the swelling effect in polymers thin films and/or charge transfer resulting from the interaction of adsorbed analyte molecules with the sensing unit. Examples of such cases are seen in semi-conducting CNTs, conducting polymers and CNTs/polymer nano-composites [10-13]. The polarity of analyte molecules dominates the interaction with the sensors that are based on electronic transduction [1,14]. Polar compounds are usually directly detected through charge exchange between the analyte and the sensing unit [8,14-18]. This charge transfer might also provide swelling of a polymer film due to electrostatic repulsion. Non-polar compounds can be detected through steric interaction, which might alter the charge carrier transference [14-18].
In the nano-composite chemo-capacity sensors composed of unsaturated polyester resin (UPR)/carbon nano-tubes (MWCNTs), the polyester matrix is the acceptor, it’s electronegative pull enables the CNTs to form p-type semi-conductors, hence create hole conductivity [19,20]. Additionally, oxygen absorption to the CNTs in ambient air also contributes to their p-type nature [21]. The tetrahydrofuran (THF) VOC is considered as electron donor (possesses a loan pair of electrons), thus, adding electrons to the conduction band. These electrons can return to the valence band through recombination if a vacancy exists, in order to obtain minimum energy. however, this process takes time [19]. The amount of charged species and the number of electrons in the conduction band contribute to the conductivity. Frequency is what moves them in the band at a certain velocity. CNTs conductivity is influenced by frequency, σ(w). The higher the frequency the higher the conductivity [22]. Thus, applying AC voltage on dielectric chemosensor which contains CNTs influences its conductivity, in addition to exposure to the analytes. Therefore, these competing responses (analyte vs. frequency effect) need to be considered while analyzing the sensor performance.
In the development of organic devices which comprises dielectric materials, the following considerations need to be taken into account: A dielectric material is an electrical insulator that can be polarized only by an applied electric field. Whenever a dielectric is placed under an external field, electric charges slightly shift from average equilibrium positions cause dielectric polarization [23,24]. The total electric displacement field is defined as the sum of the electric field established if there was not a dielectric in the capacitor and the polarization field within the material. Thus, polarization is related to the external field E by the complex dielectric constant and therefore correlated with capacitance, respectively. Under AC voltage, a different frequency range results in a different polarization mechanism [24,25].
Although different materials have been used to form the sensing film of a chemo-capacitor, non-conducting polymers are considered as the most advantageous to be developed for chemo-capacitor sensors [26]. A polymer can be readily deposited using drop-cast, inkjet or lithography [27,28]. The absorption of the analyte by the film can be accompanied by changes in its dielectric properties, such as modification of its dielectric constant and hence its capacitance. Besides, changes in the thickness of the sensing film might also alter its intrinsic capacitance through density changes. Meaning, the effective polymer volume and density of dipole moments may decrease as a result of a swelling effect, and thereby lower the sensor response. However, incorporating conducting particles in the polymer has shown an increase in the signal amplitude of the chemo-capacitor response [29]. This leads to a measurable change in capacitance between the electrodes.
Chemo-capacitors are advantageous due to their noncomplex moving parts. The results demonstrate that it is possible to increase the sensor selectivity using a low A.C. frequency response rather than D.C. resistance change [30,31].
Moreover, experiments have shown that chemo-capacitors, coated with a polymer layer of thickness larger than half the periodicity of the electrodes for a target analyte with a dielectric constant less than that of the polymer, display a decreased capacitance. Additionally, for an analyte dielectric constant larger than that of the polymer the capacitance would increase [32]. Hence, the smaller the spatial geometry that can be fabricated, the thinner the sensing film can be for full utilization.
For a layer thickness less than half of the periodicity of the electrodes, the capacitance would always increase regardless of the analyte dielectric constant. This is probably due to the increased polymer/analyte volume within the field-line region, exhibiting a larger dielectric constant than that of the air it has displaced [33].
This behavior was also verified before using simulations by these authors [34] and suggested a criterion for distinguishing between different analytes.
Chemo-capacity sensors do not rely on the changes in conductivity of the material and ideally are non-dissipative in power. Therefore, it is the favored direction to pursue in the development of low-power sensors.
On the other hand, the possibility to detect desired analytes according to changes in both resistance and capacitance constitutes a significant advantage and a highly reliable performance. To conclude, the following abilities of the present chemosensing capacitor were demonstrated:
1. Identification and quantification of the THF vapor in the
range of 500Hz to 10KHz under standard conditions (ambient
air, at room temperature and atmospheric pressure).
2. Measurement of the distance between the THF source and
the sensing unit.
3. High sensitivity within time response to changes in
temporal exposures and in THF properties, causing a rapid and
fully reversable response upon exposure to analyte vapor.
4. Inexpensive fabrication, leading to more feasible
industrial application.
These results can lead to the development of cost-effective, lightweight, low powered, noninvasive sensing devices highly suitable for applications in the environmental, security analysis and monitoring industries.
Fabrication Method
The fabricated sensors were tested electrically using the instrument: HIOKI 3532 LCR HITESTER, impedance analyzer. Both resistance and capacitance were measured before, during and after exposure to THF vapors. This was done continuously under AC voltage of 1V. Subsequent exposure cycles (2-3) were performed. The influence of THF vapor (boiling point 66°C) on the UPR/ MWCNTs chemo-resistors was measured under frequencies of 500Hz and 10KHz. For each frequency, different liquid volume of THF were drop casted on a glass (10μl THF, 20μl THF and 30μl THF) below the chemo-resistor, at different heights each time (1mm, 2mm, 3mm). The direct mixing of nano-MWCNTs within a low viscosity (318 cp) UPR matrix was performed, using a high shear mixer, is the core of this study. Finding the ideal mixing conditions is essential in order to minimize styrene evaporation, which crosslinks the sensing composite. In addition, using a low viscosity matrix, eases film preparation using the spin coating technique, in the case of direct filler mixing (without the use of a solvent and its evaporation which might harm the uniformity of the dispersed filler), as performed in this study. The dielectric semi-conductor samples were spin coated on a metal oxide p-type semi-conductor- MOS capacitor. Calibration curves of relative resistance (ΔR= R-Rb /Rb) and relative capacitance (ΔCp= Cp-Cp,b /Cp,b) with time, frequency, and volume were prepared and analyzed, where Rb is the baseline resistance and Cp is the baseline capacitance (Figures 1&2 and Tables 1&2). Moreover, examination of possible mechanisms of THF/chemo-capacity sensor interactions with respect to changes in both R and Cp were hypothesized and conducted in order to try and further understand the electrical response obtained from the sensors in the exposure experiments.
Table 1: Resistance statistical analysis (average an deviations) of measurements taken for each frequency with its respected volumes and heights
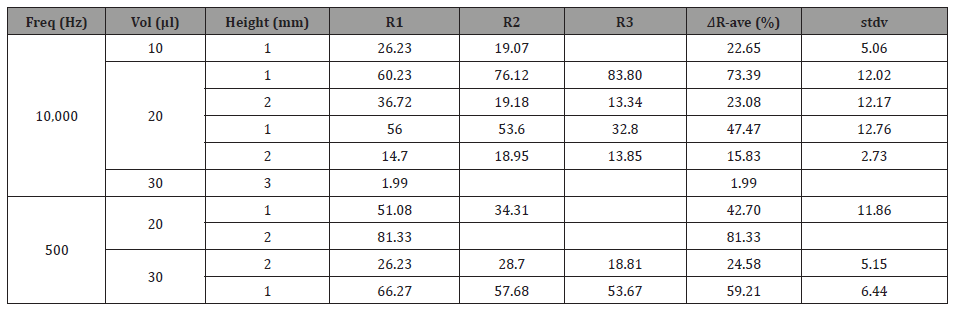
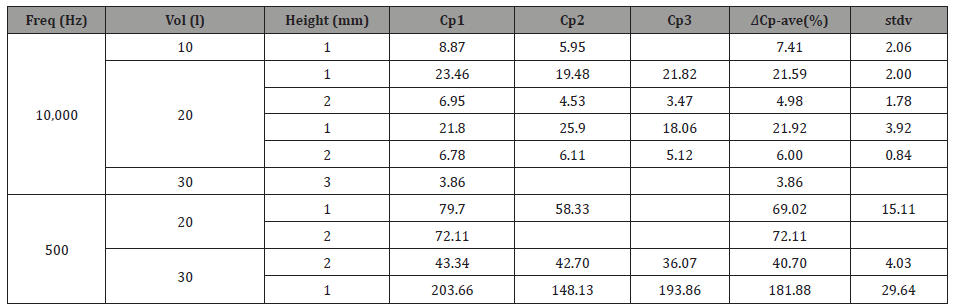
Table 2: Capacitance statistical analysis (average an deviations) of measurements taken for each frequency with its respected volumes and heights.
Results and Discussion
For 10KHz: The 30μl THF vapor source at 1mm height displayed a lower change in both ΔR and ΔCp, compared to the 20μl THF vapor source under the same conditions (Figure 1). This result can be explained as follows; the 30μl THF droplet provides a better wetting of the substrate (glass). As a result, the THF vapor molecules are out of focus relative to the chemo-sensor target area. Meaning, more vapor molecules are scattered around and do not “contribute” to the swelling effect. Moreover, less electron donors means less contribution to polarization. This can probably explain why a lower change in both R and Cp is shown. For the lowest volume measured (10μl), in the same height of 1mm, the lowest change in both R and Cp was obtained. It is expected that the lowest amount of THF provides the lowest change. These results are consistent and complement each other
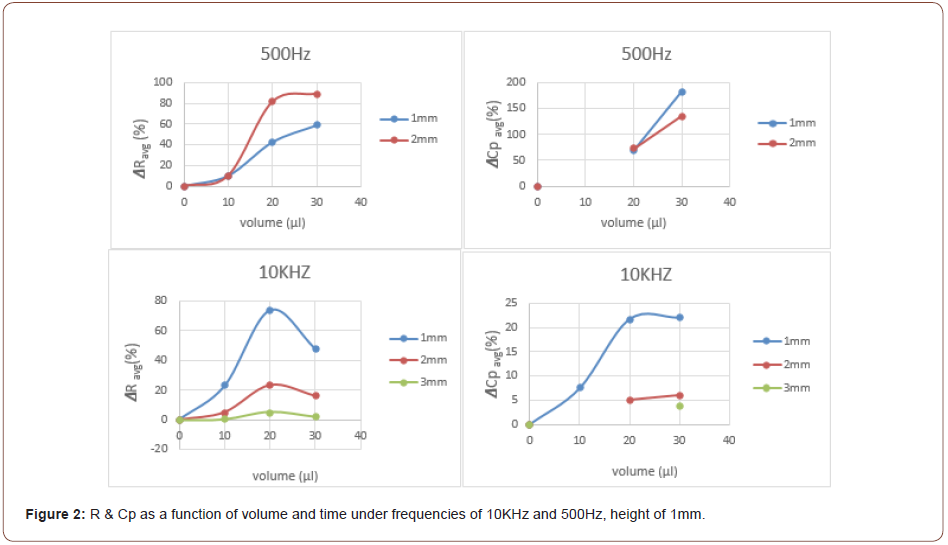
The developed chemo-sensing capacitor in 1mm distance from a 30μl THF droplet vapor source displayed a higher change in both relative resistance and capacity (ΔR/Rb and ΔCp/Cp,b) compared to 2mm height under the same conditions (Figure 2). This result is probably due to the swelling effect in the polymer and a change in the polarization of the dielectric sensing unit for the change in R and Cp, respectively. Polar analyte adsorption is usually detected directly through charge exchange between the sensing material and the analyte. This charge transfer might alter the distances between the polar functional groups of the sensing material, due to electrostatic repulsion, which can also result in the swelling of the polymer in this case. THF is a “good solvent” for both styrene (which cross-links the polyester) and polyester matrices. Therefore, the polymer-solvent interaction is more preferred than the polymer-polymer interaction. This preference, when carried out, causes the polymer chains to be less packed and swell the polymer matrix. This swelling causes a modification in the CNTs conductive network, which might alter the charge carrier transport and even harm the electrons mobility. Thus, a decrease in conductivity and corresponding increase in resistivity can be observed. The THF pentagonal ring contains one oxygen which has a lone pair of electrons. Thus, the THF molecules act as an electron donor. This interaction can cause a shift of equilibrium state, in polarization of the dielectric sensor and it thereby contributes to an increase in capacitance. For 1mm height, THF molecules have less diffusion distance to pass to reach the device. Thus, statistically more vapor molecules will interact with the chemo-sensor via van der Waals interactions, compared to the case of 2mm height. Therefore, the change in both R and Cp in the case of 1mm height is more significant. Moreover, less diffusion limitations increase the probability of interaction between the analyte and the sensing device. Thus, a low time response and a high expressed swelling effect will be displayed. The same trend was observed in the case of 30μl and 3mm height. As the distance between the vapor source and the sensor increase, the change decreases. It should be noted that the 2mm height, is a case of mixed diffusion and kinetics, where the 3mm height is a case of dominant diffusion. The direct correlation between concentration and response time in the Fick’s 2nd law of diffusion theorem explains the phenomenon mentioned above.
For 500Hz: The developed chemo-sensor at 1mm distance from 30μl THF vapor source, displayed a higher change in both resistance and capacitance compared to 20μl under the same conditions (Figure 1). Lower frequency provides a lower excitation rate of charge carriers which contributes to the electrical conductivity. Thus, the low excitation rate and the low mobility of the charge carriers require a higher amount of THF vapor in order to see a significant increase in both resistance and capacitance. Moreover, the 1mm height provides the lowest distance between the analyte and the sensing unit, therefore, the kinetics of the system plays a more significant role (compared to diffusion) and is influenced by the concentration of THF. With respect to capacitance, exposure of the dielectric sensing unit to the THF vapor (electron donor) leads to the formation of a new vertical electric field (as a result of a new potential difference formed) which causes a higher total external electric field to be formed. An additional vector component in the perpendicular direction to the applied electric field is the reason for this. Exposure to a higher concentration of electron donors leads to a higher external field that increases polarization and therefore the capacitance, respectively.
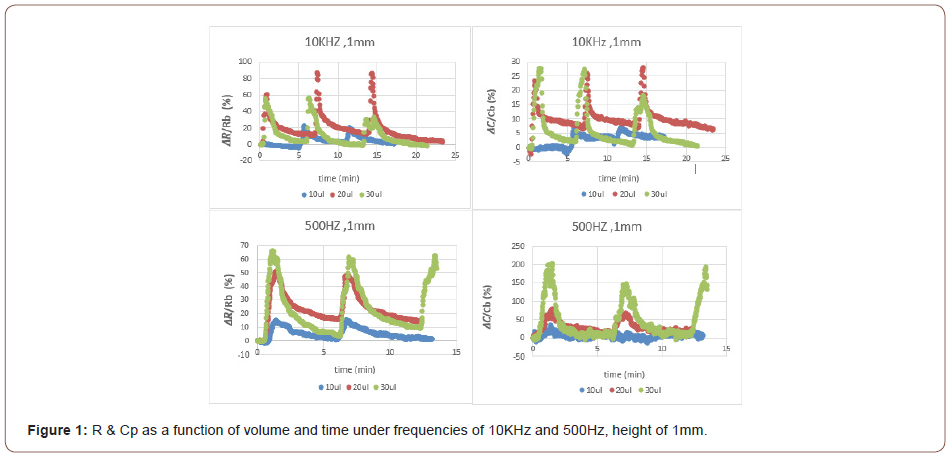
Another optional explanation to the present result 𝛥R(30μl)>𝛥R(20μl) is as follows: For a p-type CNTs conducting network, meaning holes conductivity, exposure to THF vapor(possesses a loan pair of electrons, thus perform as electron donor) results in a decrease in conductivity. The reason for this is the electrostatic attraction between the CNTs conducting holes to THF free electrons which leads to lower hole conduction. Therefore, as the THF vapor concentration increases, the probability to “trap” a hole and reduce conductivity increases. This can explain the present chemo-sensor result for 500Hz: 𝛥R(30μl)>𝛥R(20μl). The developed sensing device at 2mm distance from the 20μl THF vapor source displayed a higher change in resistance compared to the 1mm height under the same conditions (Figure 2). The reason for this result is probably due to the presence of the THF source (droplet) in the “field of vision” of the sensing device. At 2mm height, the probable presence of THF vapor molecules in the sensor’s “field of vision” encourages higher interaction and kinetic performance of the system, hence, it enables a higher change in resistance
Conclusion
The present UPR/MWCNT chemo-capacity sensor is able to identify and quantify THF vapor in the range of 500Hz to 10KHz under standard conditions (ambient air, at room temperature and atmospheric pressure). The response upon exposure to the analyte vapor was rapid and fully reversible. According to the change obtained in both resistance and capacitance caused by exposure to THF, both the volume of THF source and the distance between the source and the device can be ascertained. It was found that the relaxation time depends on the frequency, height and volume. In addition, it also has been shown that the signals width of both R and Cp depends on exposure time, volume of THF, and the average of repeated measurements. Considering that the proportion of CNTs in the composite is only 0.05wt%, the total capacitance is mainly due to the polymer matrix.
Acknowledgment
None.
Conflict of Interest
No conflict of interest.
References
- Tisch U, Haick H (2010) Nanomaterials for Cross-Reactive Sensor Arrays. MRS Bull 35: 797-803.
- Kara P, De la Escosura-Muñiz A, Maltez-da Costa N, Guix M, Ozsoz M, et al. (2010) Aptamers Based Electrochemical Biosensor for Protein Detection Using Carbon Nanotubes Platforms. Biosens Bioelectron 26(4): 1715-1718.
- Sokolov AN, Robertsb ME, Bao Z (2009) Fabrication of Low Cost Electronic Biosensors. Mater Today 12(9): 12-20.
- Roeck F, Barsan N, Weimar U (2008) Electronic Nose: Curent Status and Future. Trends Chem Rev 108(2): 705-725.
- Kauffman DR, Star A (2008) Carbon Nanotube Gas and Vapor Sensors. Angew Chem Int Ed 47(35): 6550-6570.
- Albert KJ, Lewis NS, Schauer CL, Sotzing GA, Stitzel SE, et al. (2000) Cross-reactive Chemical Sensor Arrays. Chem Rev 100(7): 2595-2626.
- Liu ZB, Tian JG, Guo Z, Ren DM, Du F, et al. (2008) Enhanced Optical Limiting Effects in Porphyrin-Covalently Functionalized Single-Walled Carbon Nanotubes. Adv Mater 20(3): 511-515.
- Clements J (1998) Novel, Self-Organizing Materials for Use in Gas Sensor Arrays: Beating The Humidity Problem. Sensors and Actuators B 47: 37-42.
- Rusling JF, Kumar CV, Gutkind JS, Patel V (2010) Measurment of Biomarker Proteins for Point-of-Care Early Detection and Monitoring of Cancer. Analyst 135(10): 2496-2511.
- T Someya, J Small, P Kim, C Nuckolls, JT Yardley (2003) Alcohol Vapor Sensors Based on Single-Walled Carbon Nanotube Field Effect Transistors. Nano Lett 3(7): 877-881.
- B Philip, JK Abraham, A Chandrasekhar, VK Varadan (2003) Carbon Nanotube/PMMA Composite Thin Films for Gas-Sensing Applications. Smart Mater Struct 12(6): 935-939.
- B Zhang, RW Fu, MQ Zhang, XM Dong, PL Lan, et al. (2005) Preparation and Characterization of Gas-Sensitive Composites From Multi-Walled Carbon Nanotubes/Polystyrene. Sens Actuators B 109(2): 323-328.
- JK Abraham, B Philip, A Witchurch, VK Varadan, CC Reddy (2004) A Compact Wireless Gas Sensor Using a Carbon Nanotube/PMMA thin Film Chemresistor. Smart Mater Struct 13(5): 1045-1049.
- Bennett ME, Alexander WA, Lu JW, Troya D, Morris JR (2008) Collisions of Polar and Nonpolar Gases with Hydrogen Bonding and Hydrocarbon Self-Assembled Monolayers. J Phys Chem 112(44): 17272-17280.
- Peng G, Tisch U, Haick H (2009) Detection of Nonpolar Molecules by Means of Carrier Scattering in Random Networks of Carbon Nanotubes: Toward Diagnosis of Diseases via Breath Samples. Nano Lett 9(4): 1362-7368.
- Li B, Sauvé G, Iovu MC, Malika Jeffries-EL, Zhang R, et al. (2006) Volatile Organic Compound Detection Using Nanostructured Copolymers. Nano Lett 6(8): 1598-1602.
- Li B, Santhanam S, Schultz L, Malika Jeffries-EL, Iovu MC, et al. (2007) Inkjet Printed Chemical Sensor Array Based on Polythiophene Conductive Polymers. Sensors and Actuators B: Chemical 123(2): 651-660.
- Li B, Lambeth DN (2008) Chemical Sensing Using Nanostructured Polythiophene Transistors. Nano Lett 8(11): 3563-3567.
- Sze SM, Lee MK (2012) Semiconductor Devices Physics and Technology, 3rd
- Simon M Sze, Kwok K Ng (2006) Physics of Semiconductor Devices, 3rd
- V Derycke, R Martel, J Appenzeller, Ph Avouris (2002) Controlling Doping and Carrier Injection In Carbon Nanotube Transistors. Appl Phys Lett 80: 2773.
- Isaac Aguilar Ventura, Jian Zhou, Gilles Lubineau (2015) Investigating the Inter-Tube Conduction Mechanism. in Polycarbonate Nanocomposites Prepared with Conductive Polymer-Coated Carbon Nanotubes. Nanoscale Res Lett 10: 485.
- Guy Blaise, Daniel Treheux (2010) Physics of Dielectrics. Dielectric Materials for Electrical Engineering.
- Hench LL, West JK (1990) Principles Of Electronic Ceramics. Wiley–Blackwell, USA.
- AA Ward (2016) Dielectric Materials for Advanced Applications.
- Robert Blue, Deepak Uttamchandani (2016) Chemicapacitors as a Versatile Platform for Miniature Gas and Vapor Sensors. Meas Sci Technol 28(2): 022001.
- Balakrisnan B, Patil S, Smela E (2009) Patterning PDMS Using a Combination of Wet and Dry Etching. J Micromech Microeng 19()4: 047002.
- Cho NB, Lim TH, Jeon YM, Gong MS (2008) Humidity Sensors Fabricated With Photo-Curable Electrolyte Inks Using an Ink-Jet Printing Technique and Their Properties Sensors Actuators B 130(2): 594-598.
- NS Lewis (2004) Comparisons Between Mammalian and Artificial Olfaction Based on Arrays of Carbon Black-Polymer Composite Vapor Detectors. Acc Chem Res 37(9): 663-672.
- Fernando Musio, Maria Cristina Ferrara (1997) Low frequency A.C. Response of Polypyrrole Gas Sensors. Sensors and dctuators B 41(1-3): 97-103.
- F Musio, MEH Amrani, KC Persaud (1997) Sensing of Volatile Chemicals: Conductivity and Relative Permittivity Behaviour of Polypyrrole at High Frequency.
- Hierlemann A, Lange D, Hagleitner C, Kerness N, Koll A, et al. (2000) Application-Specific Sensor Systems Based on CMOS Chemical Microsensors Sensors Actuators B: Chemical 70(1-3): 2-11.
- C Hagleitner, A Hierlemann, Lange D, Kummer A, Kerness N, et al. (2001) Smart Single-Chip Gas Sensor Microsystem. Nature 414(6861): 293-296.
- Steiner FP, Hierlemann A, Cornila C, Noetzel G, Bächtold M, et al. (1995) Polymer Coated Capacitive Microintegrated Gas Sensor Proc. Transducers 2: 814-817.
-
Efrat Hochma, Moshe Narkis. Sensing Capacitors Based on Thin Films of Unsaturated Polyester Resin/Carbon Nano-Tubes Nano-Composites. Mod Concept Material Sci. 4(3): 2021. MCMS. MS.ID.000583. DOI: 10.33552/MCMS.2021.04.000583.
-
Capacitors, Thin films, Physics, Carbon nano-tubes, Composites, Polyester resin, Materials, Capacitance, Resistance, Room temperature, Conductivity, Polarization, Dielectric constant, Chemo-capacity, Frequency, Height, Volume
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






