 Research Article
Research Article
Eco-Friendly System for Fire Suppressant Use in Confined Spaces
Amanda A Soares1, Luã TT de Melo1, Pedro H dos S S Teixeira1, Giovanni N M de Souza1, Renata S de O Buzzati1, Emerson P Fernandes1, Ângelo R de Oliveira2, Antônio M M S Lameirão3, Victor S Cruz3, Lucas M Souza3, Mabelle C M da Rocha3, Patrícia S de O Patrício1* and Patterson P de Souza1*
1Department of Chemistry, Centro Federal de Educação Tecnológica de Minas Gerais, CEFET-MG, Av. Amazonas 5253, 30421-169, BH-MG, Brazil
2Department of Electroeletronics, Centro Federal de Educação Tecnológica de Minas Gerais, CEFET-MG, R. José Péres, 558, 36700-000, Leopoldina – MG, Brazil
3Light Serviços de Eletricidade S/A, Marechal Floriano 168, Centro, RJ-Rio de Janeiro, Brazil
Patrícia S de O Patrício, Patterson P de Souza, Department of Chemistry, Centro Federal de Educação Tecnológica de Minas Gerais, CEFET-MG, Av. Amazonas 5253, 30421-169, BH-MG, Brazil.
Received Date: May 28, 2025; Published Date: June 05, 2025
Abstract
A fire-suppressant system was developed by incorporating bicarbonate and carbonate salts into the porous structure of Light Expanded Clay Aggregate (LECA), creating a composite material suitable for fire suppression in confined spaces. The system was applied inside armored energy meter boxes, which are common targets of arson and cause significant damage to power infrastructure. Upon heating, the incorporated salts decomposed and released CO₂, while the LECA provided thermal insulation, jointly extinguishing the fire and protecting internal components. The material’s structure and performance were confirmed through Scanning Electron Microscopy (SEM), Thermogravimetric Analysis (TGA), and Gas Chromatography with Barrier Discharge Ionization Detection (GC-BID), followed by full-scale fire suppression testing. The results confirmed the effectiveness of the composite system. The use of low-cost, sustainable materials enables scalable production with a favorable cost-benefit ratio. The incorporation method also shows potential for broader applications in fire safety systems for other confined environments.
Keywords: Fire suppressants; Light expanded clay; Carbon dioxide; Carbonate salts; Confined spaces
Introduction
Most traditional fire suppressants contain chemical agents that, while effective, pose toxic risks to occupants and the environment and are challenging to remove after use. In confined spaces such as vehicles, equipment rooms, storage areas, silos, or armored energy meter boxes, these systems can produce harmful residues and toxic gases, posing a high risk of contamination. Additionally, certain salt-based suppressants are unsuitable for environments prone to corrosive processes. Therefore, investigating eco-friendly solutions using non-toxic agents presents a critical challenge. The development of alternative systems capable of maintaining fire suppression efficacy while minimizing environmental impact and meeting safety requirements, as well as evaluating the economic feasibility of their implementation compared to conventional methods, is highly desirable and remains underexplored in literature.
Numerous studies have assessed the efficiency of various substances as fire suppressants. Following the 1989 Montreal Protocol, which banned Halon 1301 (bromotrifluoromethane, CF₃Br), the search for clean yet effective suppressant alternatives intensified [1]. Researchers have revisited new and previously known fire-suppressive compounds, and this field remains active today. Sodium bicarbonate (NaHCO₃), for example, was already being studied as an explosion suppressant in grain silos before the Montreal Protocol [2,3] and has been revisited in post-Protocol research, continuing to be studied in recent years [4,5]. Other notable studies include those on inert gases [6], the mineral struvite (in both synthetic form and recovered from wastewater) [7, 8], and alkali metal chlorides [9,10]. Halogenated organic compounds with low boiling points have also been explored [11,12], along with zeolites [13]; recent research has expanded to innovative materials such as Dry Water [14,15], hydrogels [16], and ecological retardants like banana pseudostem sap, a byproduct of agricultural waste [17].
In the context of other uses, Light Expanded Clay Aggregates (LECAs) represent a promising addition to fire protection strategies due to their highly porous, chemically stable structures. Composed of a variety of oxides – such as those of aluminum, iron, magnesium, titanium and manganese – these materials may also contain varying amounts of alkali and alkaline earth metals, as well as particles from other minerals, including non-crystalline forms [18-21]. LECAs are produced through the heat treatment of natural clay in rotary kilns and their porous structure forms as the temperature approaches 1000-1200ºC [19,21,22]. Some clay compounds melt while others chemically decompose, releasing gases trapped in the sintered mass. This expansion can increase the volume by up to seven times, and because the gases remain enclosed during the molten phase, the porous structure remains intact upon cooling [19]. Several academic works have focused on characterizing these aggregates [19-21,23]. Furthermore, LECAs can be sourced from industrial waste [22,24-26], enhancing their relevance as sustainable materials.
A wide range of chemical compounds, individually or in combination, is observed to achieve suppressive or retardant effects. Following this approach, the proposed combination consists of LECAs as a support material, modified by incorporating a suppressant/retardant salt within its porous internal structure and on its surface. Several key characteristics must be prioritized when selecting the ionic compounds for that incorporation. First, the presence of carbonate ions (CO₃²⁻), bicarbonate ions (HCO₃⁻), or other “carbonated” ions (e.g., acetate ions [27], C₂H₃O₂⁻) is essential. Upon exposure to heat, decompose, releasing carbon dioxide (CO₂) and water. Regarding thermal stability, bicarbonates decompose at lower temperatures (between 100 and 200ºC), converting into carbonates. In contrast, carbonates decompose only at temperatures above 800ºC, ultimately forming their respective oxide [28]. These decomposition processes are illustrated in Equation 1 and Equation 2, using potassium salts as examples.
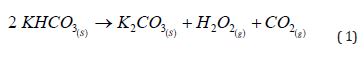
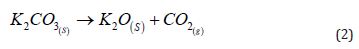
A second essential requirement is the solubility of these salts in water. This parameter is crucial because LECAs enable efficient adsorption of salts through immersion in a saturated or high-concentration saline solution due to their high porosity. This process involves immersing the LECAs in a saline bath, a high-concentration solution of the target salt, within a controlled pH range. This behavior creates an efficient material that combines LECA’s natural thermal insulation with the fire suppression properties of CO₂. Additionally, the metals present in the salts, such as sodium or potassium, function as scavengers for the radicals that propagate flames [10,29].
Application Context
A relevant application of this apparatus is fire prevention in armored energy boxes, designed to house critical electronic devices (complex confined environments), a common problem faced by electric utility companies using these devices. When the fire reaches these boxes, the resulting damage is significant, compromising equipment integrity and leading to high replacement and repair costs. Such fires may originate intentionally or accidentally due to overheating or electrical failures. Therefore, this study focused on developing a solution that met flame suppression requirements, was also quick and simple to produce from basic, affordable materials, and easy to install and manage in these environments. Beyond flame suppression, the materials must inhibit thermal conduction within the confined space to protect electronic components, often made of thermoplastic materials that can melt and deform under heat, further fueling the flames. While these plastics are highly flammable, ceramic materials, such as clay minerals, are known for their excellent thermal insulation properties, making them suitable for this application. Additionally, the CO₂ released from the incorporated salts is a key agent in fire extinguishers for electrical fires or flammable liquids, as it is a non-conductive and leaves no residue that could damage electrical components [30].
Materials and Methods
Production of Modified LECAs and Evaluation of Materials
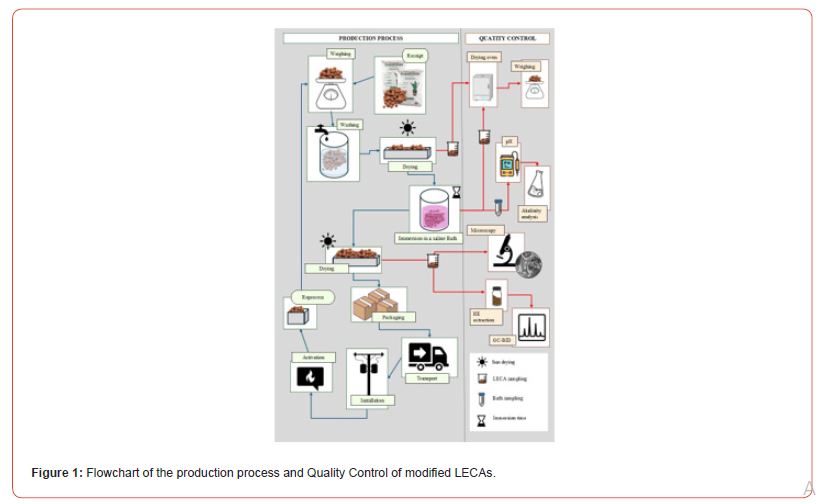
LECAs of size 3222 (22 to 32 mm in diameter) were sourced from the supplier Cinexpan to produce the suppressant system on a pilot scale. Saline baths were prepared using commercial potassium carbonate with 96-98% purity, purchased from Sulfal Química LTDA. However, the purity of commercial products should be evaluated, as they are often of lower quality. For this purpose, Fourier transform infrared spectroscopy (FTIR) was used in the 4000-400 cm-1 spectral range. To evaluate the salt’s degradation profile, a system Thermogravimetric Analysis (TGA) [31] using DTG-60H (Shimadzu) was applied under nitrogen atmosphere with flow rate of 50 mL.min-1, temperature from 25 ºC to 900 ºC, and heating rate of 10 ºC.min-1. To acidify the baths, concentrated solutions of 2 mol/L were prepared using 37% hydrochloric acid P.A. purchased from Neon Reagentes Analíticos. For the laboratory-scale tests, potassium bicarbonate P.A. and potassium carbonate P.A. were purchased from Neon Reagentes Analíticos and sodium bicarbonate P.A. from Synth Reagentes Analíticos. Figure 1 illustrates the simplified life cycle of the developed product, encompassing production, quality control, installation, activation, and reprocessing.
Evaluation of Materials and Validation of salt Incorporation
The first characteristic evaluated for the LECAs was their ability to absorb solutions. This property was assessed through a water absorption test, following procedures outlined in standards such as ASTM C127, ASTM C128 and the European standard EN 13055/ Annex D [32,33]. The test involves immersing the dry aggregate in water for a specified period, which can range from a few hours to several months. After immersion, the aggregate is weighed, and the final mass is compared to the initial dry mass to determine the absorption rate.
For this study, the test procedure was adapted to identify the minimum immersion time required to achieve maximum absorption capacity of the internal pores. A known mass of LECAs was first dried in an air-circulating oven (7lab Laboratory Equipment) at 110 °C to eliminate all volatiles, achieving a constant mass. Subsequently, the LECAs were immersed in the solution and weighed hourly to track the increase in mass on a semi-analytical balance with a resolution of 0.001 grams (BEL Engineering). After completing the tests, the modified LECAs were dried again at 110 °C to remove all absorbed/adsorbed water, and the final mass was calculated by difference. This process of mass difference analysis is referred to as Gravimetric Analysis. Throughout the tests, variations in parameters such as pH, volume, agitation, and concentration were explored to evaluate their effects on the mass results.
The effectiveness of salt incorporation was evaluated using three approaches. The first one involved directly assessing the insertion of salts into the internal structure of LECAs using Scanning Electron Microscopy (SEM) using an SSX-550 instrument (Shimadzu).
The second approach evaluated the effectiveness of carbon dioxide release and indirectly determined the mass of the carbonated ions incorporated during the process. Gas Chromatography with Barrier Discharge Detection (GC-BID) was employed for this purpose, with sample preparation using static Headspace extraction (HE). Typically, vials with volumes ranging from 5 to 22 mL, equipped with screw caps and septa for injection, are used for this analysis [34]. The vial was then heated and homogenized at 60°C for 10 minutes to ensure rapid establishment of equilibrium between the phases and to ensure that all carbon dioxide generated transitions into the gas phase [35-38]. After this period, 3 mL of the gas phase is extracted. This sample is injected into a gas sampler (MGS-2030 Shimadzu [39]) connected to a GC-BID (Shimadzu Nexis 2030), equipped with a RESTEK Rt-Q-Bond column (30 m, 0.53 mm internal diameter). The analysis is conducted with a helium flow rate of 3.5 mL/min, a column temperature of 40°C, an injector temperature of 200°C, and a detector temperature of 180°C. A blank analysis was first conducted, following the same sample preparation procedure but using LECAs that had not been immersed in the saline bath. This blank test was essential to verify the detectability of CO₂, both from the air inside the vial where the reaction occurs and any CO₂ present within the LECA’s interstices.
For analysis, the LECA sample is first ground to a fine powder to facilitate accurate weighing and transfer into a 20 mL vial. A powdered LECA (~1g) was placed into the vial, which is then sealed tightly. Using a syringe through the vial’s septum, 2 mL of 2 mol/L sulfuric acid is added to immerse the sample completely. The sulfuric acid reacts with the bicarbonate and carbonate in the LECA, producing carbon dioxide that will move into the gas phase, as described in Equations (5) and (6). Additionally, the acid maintains a low pH in the liquid phase, promoting the complete conversion of the bicarbonate and carbonate into carbon dioxide.
Performance Tests
field test was conducted using 10 Kg of potassium bicarbonate- modified LECAs inside an armored power box. The test simulated a fire outbreak for 15 minutes in a controlled environment. The test setup introduced an ignition source located at the bottom of the structure, specifically at the opening corresponding to the outlet point of the boxes. The ignition source used was a piece of fabric soaked in fuel, intended to simulate an open flame ignition scenario. Temperature measurements were taken using an infrared thermometer aimed at the external surface of the boxes throughout the exposure period. The electrical circuits were not energized during the initial simulation test. However, in the second stage, conducted under real operating conditions, the circuits were kept energized.
Results and Discussion
Production of Modified LECAs
The characteristic behavior of bicarbonates fulfills the first requirement, which is the release of CO2 shortly after exposure to heat, which means bicarbonates provide a “faster” response, requiring a lower temperature and shorter heating period before CO₂ release begins. If a variation in the release temperature is needed, this can be achieved by adjusting the bicarbonate and carbonate concentrations. Therefore, a crucial step involved studying the chemical equilibria and species in the saline baths. This understanding enabled the optimization of these baths to maximize the amount of bicarbonate incorporated per gram of LECA.
Equations (3) to (9) [40] illustrate the main equilibrium to consider. Equation 3 represents the production of a saline bath from the dissolution of solid potassium carbonate (K₂CO₃(s)). Due to the equilibrium established between the carbonate anion and water (Equation (4)), bicarbonate and carbonic acid (H₂CO₃(aq)) are formed in the aqueous medium
(Equations (5) and (6)), resulting in a basic pH of 11 to 12 after dissolution. Carbonic acid is an unstable species that decomposes into CO₂ and water (Equation (7)). Conversely, CO₂ can equilibrate with water to regenerate carbonic acid, bicarbonate, and carbonate. For the saline bath produced from solid potassium bicarbonate (KHCO₃ (s)), as illustrated in Equation (8), the resulting equilibria are similar, though the final pH is approximately 8.5. In this case, carbonate ions are also formed (Equation (9)), but in smaller quantities. This underscores the critical role of pH in determining the proportions of each species in the solution.
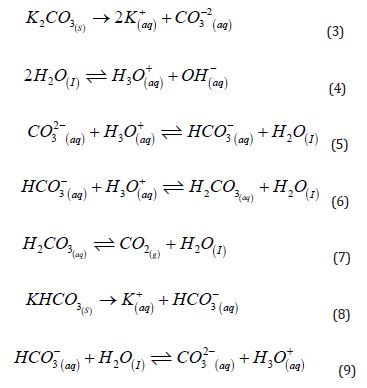
When potassium carbonate is used to prepare a saline bath with a pH between 11 and 12, the solution predominantly contains carbonate ions, around 85%. On the other hand, a bath made from potassium bicarbonate, with a final pH of around 8.5, yields a bicarbonate fraction exceeding 90%, which is ideal for incorporation into LECAs to achieve a rapid thermal response. Therefore, producing a fast-responding aqueous bicarbonate solution from carbonate requires an additional step to adjust the pH to a range between 8.0 and 8.5 using an acidic solution.
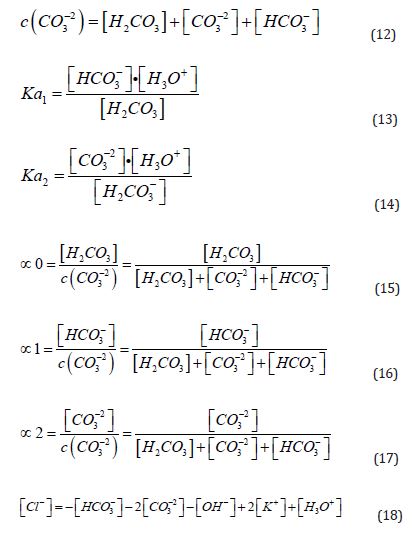
The calculations for the fractions of each species (Equations (15), (16), and (17)) are based on the pH value (Equation (11)), mass balance (Equation (12)), charge balance (Equation 18, using hydrochloric acid for pH adjustment), and the law of mass action governing this system (Equations (13) and (14)). The relevant pKa values (the negative logarithm of the acidity constant, Ka, as shown in Equation (10)) are also applied. For this system, the first pKa₁ is 6.35, corresponding to the deprotonation of carbonic acid, and the second pKa₂ is 10.33, associated with the deprotonation of bicarbonate [40].
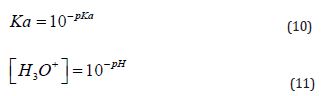
To calculate the fractions of carbonic acid (∝₀, Equation (21)), bicarbonate (∝₁, Equation (22)), and carbonate (∝₂, Equation (23)) based solely on the pH and pKa values. This approach allows calculating the theoretical concentrations of species within the saline bath and the amount of hydrochloric acid required for pH adjustment.
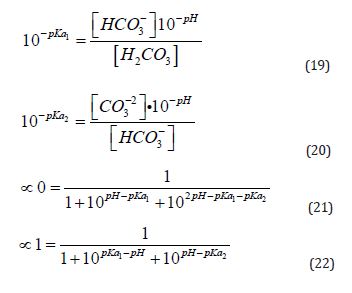
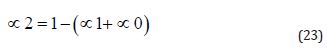
Selection of Salts and Absorption Tests
The first salts selected for testing were sodium carbonate, potassium carbonate, potassium bicarbonate, and sodium bicarbonate. As previously mentioned, the second criteria for selection were decomposition temperatures and solubility. However, an essential third criterion was cost, as one of the objectives is to enable largescale production with a good cost-benefit ratio. Some information on the selected salts is provided in Table 1. The average cost of commercial products is lower than that of analytical grade (P.A.) products, which typically have a purity above 98%. The FTIR technique was employed to validate the use of commercial reagents. Figure 2 shows the spectra obtained for potassium carbonate in both its commercial and analytical grade versions. We can observe that the spectra of both salts, commercial and P.A. overlap, ensuring they have the same composition. The FTIR spectrum exhibits typical bands of K2CO3 for both substances analyzed. The bands observed in the spectrum are associated with the carbonate group and hydroxyl groups present in the water structure. The bands observed between 3500 and 3300cm⁻¹ correspond to the stretching vibrations of the O-H bond due to the presence of water in the sample. More intense bands are identified in the region between 1400–1500cm⁻¹, associated with the asymmetric stretching of the CO32− ion, which is characteristic of carbonates. The ion’s out-ofplane deformation and angular deformation modes are observed between 890–870cm⁻¹ and 740–680cm⁻¹.
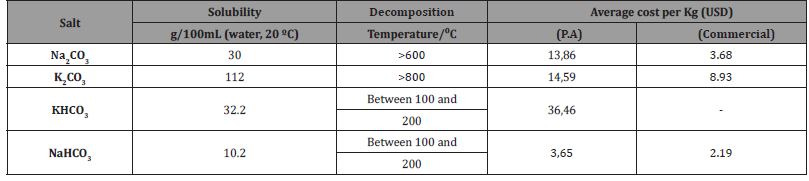
Regarding cost, sodium bicarbonate is the cheapest product; however, it has lower solubility, which affects the amount of salt that can be dissolved in solution—only 10 g per 100 mL. In contrast, potassium carbonate has much higher solubility, with 112 grams per 100 mL of solution.
Another method used for characterizing the salts was TGA. The obtained curves allow for the evaluation of the decomposition profile of the salts as the temperature gradually increases, making it possible to identify when the mass loss of interest occurs. Figure 3 shows the curve for potassium bicarbonate, showing an initial drop related to water loss, followed by a gradual loss starting around 170°C, indicating the onset of carbon dioxide release. Small losses are observed below 200°C for the carbonate salts, which suggests the loss of water molecules. After these events, further losses occur only above 700°C, corroborating the data already presented in Table 1.
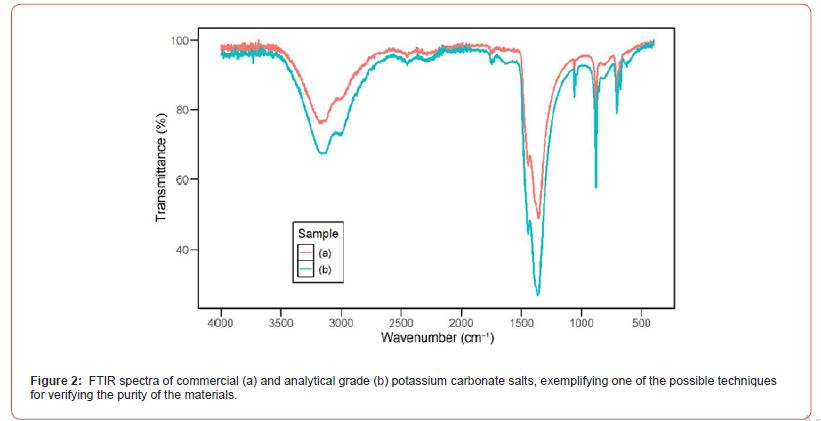
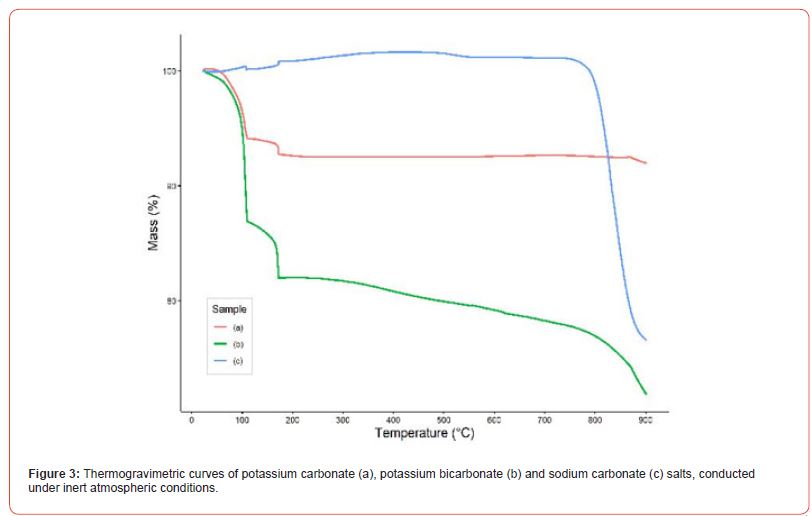
Table 1: Overview of the evaluated salts.
The absorption test results showed an increase in mass absorbed ranging from 12.7% to 18.8% after 5 hours of immersion, as illustrated in Figure 4. The test parameters are detailed in Table 2, with saline baths prepared using potassium carbonate, potassium bicarbonate, and sodium bicarbonate salts. It was observed that the maximum absorption value was reached after 3 hours of immersion and remained constant until the end of the 5 hours. Additionally, it was concluded that variations in bath conditions (such as volume, agitation, concentration, and pH) significantly affect the difference between the initial and final masses, particularly after drying. This difference represents the amount of salt effectively absorbed or adsorbed by the clay support, termed the “aggregated mass,” with results shown in Figure 5. The tests were conducted in duplicate. It was concluded that the factors most significantly affecting the final aggregated mass were agitation, salt solubility in the solution (and consequently its concentration), and pH changes. Agitation, which was maintained constant throughout the test period, may have destabilized the adsorbed layer on the surface of the LECAs. This effect probably hindered the effective accumulation of salt within the pores, leading to a decrease in the final aggregated mass.
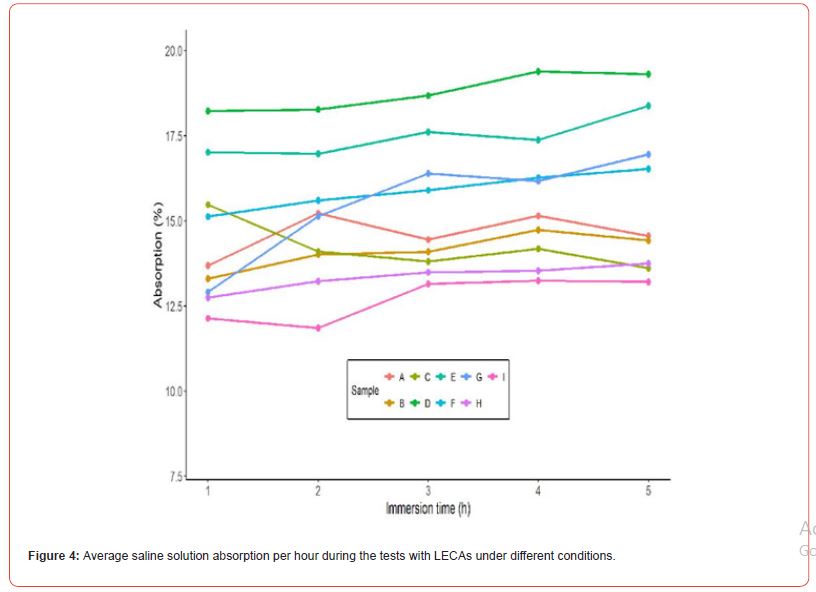
A direct proportional relationship with absorption was inferred regarding the saline bath concentration, as confirmed by tests E, F, H, and I. In test E, with double the concentration compared to test F, approximately double the aggregated mass was observed. Similarly, comparing tests H (with a concentration of 15 g/100 mL) and I (with 10 g/100 mL), a similar proportional relationship was noted. Thus, using salts with higher solubility can ensure greater mass aggregation efficiency during production. Test G involved adjusting the pH of the initial solution using hydrochloric acid, resulting in a final average pH of 8.67, which indicates the conversion of carbonate to bicarbonate, as shown by the obtained alpha values. During this adjustment, the buffering effect of the solution was observed. This effect can be described as the solution’s ability to resist abrupt changes in acidity or alkalinity, maintaining a constant pH despite adding acids or bases. Typically, buffer solutions are prepared from a weak acid and its conjugate base or a weak base and its conjugate acid at a pH close to the pKa [40]. In attempting to adjust the pH of Test G to 8.67, the buffering effect made it difficult to lower the pH, requiring adding a larger volume of acid. This excess acid increased the final volume of the bath, leading to a lower final mass compared to Test F, which was conducted under the same concentration conditions. Additionally, instantaneous acidification during the addition of acid may have caused a slight loss of CO2, shifting the equilibrium towards gas production. However, when comparing the averages of Tests G and H (p=0.157), the means are likely similar, as Test H was conducted under the same concentration conditions but with potassium bicarbonate.
A potential interfering factor in Test G, which has not yet been studied, is potassium chloride, a salt formed from the reaction with hydrochloric acid with the carbonate solution. This salt can also accumulate inside LECA, potentially leading to inaccurate results in the gravimetric analysis. However, this issue would not arise in Test H, where the bicarbonate salt is obtained without pH correction. As discussed in the studies by Cao et al. [9] and Shilling, Dlugogorski, & Kennedy [10], potassium chloride may enhance the desired suppression effect. The theoretical alpha ( ) values, shown in Table 2, are related to the pH of the saline bath, as discussed in Equations 21-23. Calculating the theoretical values of bicarbonate/ carbonate concentrations is crucial for determining the efficiency of saline bath production. This efficiency can be assessed by comparing theoretical concentration to the “real” concentration of the bath obtained through alkalinity analysis. This procedure is important as it helps establish the quality control of the bath and its limits of use.
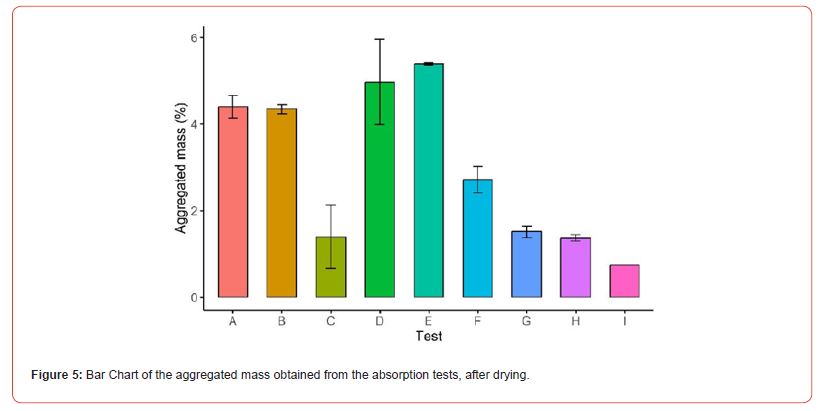
Table 2: Conditions for the saline solutions and absorption experiments.
Validation of Salt Incorporation
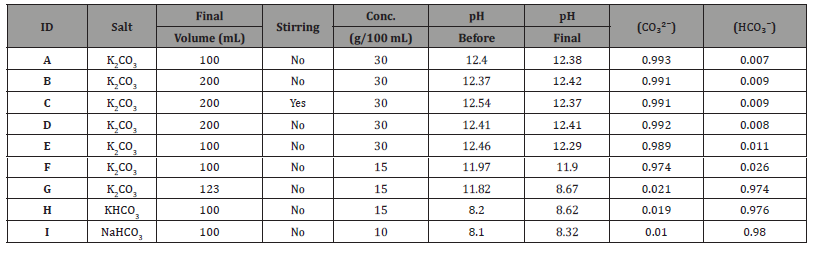
The SEM analysis allows for a comparison of the internal structure of the LECAs before and after the saline bath, demonstrating the effectiveness of the immersion technique. The results shown in Figure 6 reveal that salt crystals within the pores are identifiable. Compared to Figure 7, which depicts the LECAs before immersion in the saline bath, showing empty pores, the effectiveness of the immersion process in incorporating the salts is evident.
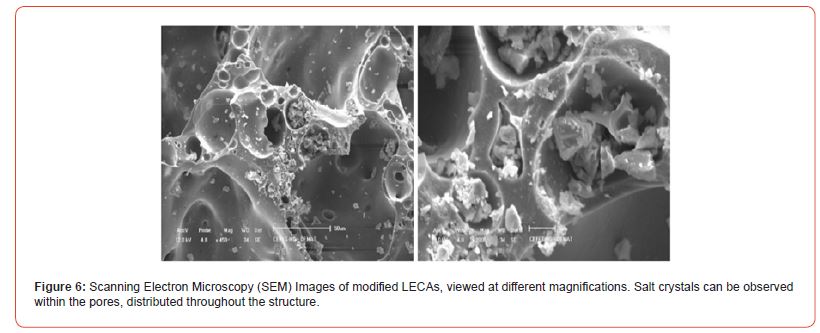

In the approach through chemical analysis, HE is a straightforward extraction method, also known as Equilibrium Headspace, where analytes are transferred from the sample matrix —whether liquid or solid—to the gas phase [34]. “Headspace” refers to the air space above the sample in a sealed vial. Once the analyte has migrated, it can be analyzed, typically coupled with gas chromatography. In HE, both solid and gas phases remain static, and extraction occurs in a single step [34]. Heating or a chemical reaction is usually applied to facilitate the transfer of volatile compounds to the gas phase. However, due to equilibrium, compounds may return to the original matrix, necessitating a waiting period for the system to reach equilibrium before collecting the gas phase sample for analysis. As shown in Figure 08, a small signal was detected for the blank. In contrast, a much stronger signal from one of the production samples was significantly more intense than the blank. This signal was quantified based on the calibration curve obtained using standard CO₂ gas, revealing that 5% of the analyzed aliquot consisted of CO₂ released from the modified LECA.
The results from the field test are presented in Figures 9 and 10. Figure 9 shows the box before and after the modified LECAs were placed at the bottom, as well as the fire that was started in the wires protruding from the box’s opening, which are the primary targets of criminal activity in real-world scenarios. After the test, the integrity of the internal components was assessed. Following the 15-minute fire test, Figure 10 shows the box’s opening, and on the right is the manual inspection of the internal components and their temperature. Upon opening, a large amount of smoke was released; however, the integrity of the components and their internal temperature were confirmed, with the components remaining cool compared to the external temperature. Preserving the internal components is crucial as it ensures the box’s continued operation. The field operator must verify the continuity of communication between the modules and the central system, normalize operations, and then replace the used batch of modified LECAs.


Conclusion
The eco-friendly system for fire suppressant uses in confined spaces demonstrates significant potential due to its low production cost and versatility in fire suppression applications. A new functionality of modified LECAs is currently being evaluated. Additionally, the production method shows potential for adaptation to other materials with similar application systems.
Salt incorporation into LECAs was successfully validated using the selected analytical techniques. Gravimetry, microscopy, alkalinity measurement and chromatography proved highly effective for quality control as production scales up. Future research should address the impact of various solution equilibria on final mass aggregation to ensure more accurate absorption results. Additionally, the techniques used for material analysis were effective in determining the optimal working temperatures and assessing the use of commercial products, which are more cost-effective and accessible, thereby reducing overall production costs. Further research should explore using mixed carbonate and bicarbonate compositions to achieve different CO2 release profiles during fire events. The evaluation of additional compounds is also in progress. Ongoing studies are investigating the potential for reusing clay-based supports and examining how their size and surface area influence the efficiency of saline solution absorption.
Funding Sourcer
This work was supported by Agência Nacional de Energia Elétrica (ANEEL).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgment
The authors would like to thank the Agência Nacional de Energia Elétrica (ANEEL), which, through the Research, Development, and Innovation Program (PROPDI), enabled the development and achievement of the objectives of the R&D project 00382 0165/2023. The authors also thank Light Serviços de Eletricidade S.A. for data and technical contributions.
Additionally, we extend our thanks to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author Contributions
Amanda Alves Soares: Methodology, experimental work, original draft writing.
Patterson Patrício de Souza/Patrícia S.O. Patricio: Conceptualization, methodology, supervision, writing review.
Emerson Pedroso Fernandes: Methodology, production supervision, writing review.
Luã T.T. Melo / Pedro H.S. Teixeira / Giovanni N. M. de Souza: Experimental work, production.
Antônio Lameirão/Victor Cruz/Lucas Souza/Mabelle Rocha: Conceptualization, full-scale tests results.
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- R E Tapscott, R S Sheinson, V Babushok, M R Nyden, R G Gann (2001) Alternative Fire Suppressant Chemicals: A Research Review with Recommendations - Technical Note 1443.
- M Hertzberg, K L Cashdollar, I Zlochower, D L NG (1985) Inhibition and extinction of explosions in heterogeneous mixtures. Symposium (International) on Combustion 20(1): 1691-1700.
- J Amrogowicz, W Kordylewski (1991) Effectiveness of dust explosion suppression by carbonates and phosphates. Combust Flame 85(3-4): 520-522.
- R Fan, Y Jiang, W Li, C Xiong, R Qiu (2019) Investigation of the physical and chemical effects of fire suppression powder NaHCO3 addition on methane-air flames. Fuel 257: 116048.
- X Chen, Hongming Zhang, Xi Chen, Xuanya Liu, Yi Niu, et al. (2017) Effect of dust explosion suppression by sodium bicarbonate with different granulometric distribution. J Loss Prev Process Ind 49: 905-911.
- J A Senecal (2005) Flame extinguishing in the cup-burner by inert gases. Fire Saf J 40(6): 579-591.
- A H Kim (2021) More than a fertilizer: Wastewater-derived struvite as a high value, sustainable fire retardant. Green Chemistry 23(12): 4510-4523.
- H Guo, M Lukovic, M Mendoza, C M Schleputz, M Griffa, et al. (2019) Bioinspired Struvite Mineralization for Fire-Resistant Wood. ACS Appl Mater Interfaces 11(5): 5427-5434.
- X Cao, J Ren, Y Zhou, Q Wang, X Gao, M Bi, et al. (2015) Suppression of methane/air explosion by ultrafine water mist containing sodium chloride additive. J Hazard Mater 285: 311-318.
- H Shilling, B Z Dlugogorski, Kennedy Eric (1998) Extinction of diffusion flames by ultrafine water mist doped with metal chlorides.
- A G Shmakov, O P Korobeinichev, V M Shvartsberg, S A Yakimov, D A Knyazkov, et al. (2006) Testing Ogranophosphorus, Organofluorine, and Metal-Containing Compounds and Solid-Propellant Gas-Generating Compositions Doped with Phosphorus-Containing Additives as Effective Fire Suppressants. Combust Explos Shock Waves 42(6): 678-687.
- O P Korobeinichev, AG Shmakov, VM Shvartsberg, A A Chernov, S A Yakimov, et al. (2012) Fire suppression by low-volatile chemically active fire suppressants using aerosol technology. Fire Saf J 51: 102-109.
- X Ni, X Wang, S Zhang, M Zhao (2014) Experimental study on the performance of transition metal ions modified zeolite particles in suppressing methane/air coflowing flame on cup burner. J Fire Sci 32(5): 417-430.
- X Chen, A Fan, B Yuan, Y Sun, Y Zhang, et al. (2019) Renewable biomass gel reinforced core-shell dry water material as novel fire extinguishing agent. J Loss Prev Process Ind 59: 14-22.
- R Fan, Y Jiang, H Jiang (2021) Experimental and theoretical investigation of dry-water containing phosphoric acid for new fire suppressant. J Loss Prev Process Ind 70: 104399.
- G Li (2024) Hydrogel Extinguishants. Nanomaterials 14: 1128.
- S Basak, K K Samanta, S K Chattopadhyay, P Pandit, S Maiti (2016) Green fire-retardant finishing and combined dyeing of proteinous wool fabric. Coloration Technology 132(2): 135-143.
- A Shokri (2020) Using Mn based on lightweight expanded clay aggregate (LECA) as an original catalyst for the removal of NO2 pollutant in aqueous environment. Surfaces and Interfaces 21: 100705.
- A Mladenovič, J S Šuput, V Ducman, A S Škapin (2004) Alkali-silica reactivity of some frequently used lightweight aggregates. Cem Concr Res 34(10): 1809-1816.
- P Tataranni, G M Besemer, V Bortolotti, C Sangiorgi (2018) Preliminary Research on the Physical and Mechanical Properties of Alternative Lightweight Aggregates Produced by Alkali-Activation of Waste Powders. Materials 11(7): 1255.
- A M Rashad (2018) Lightweight expanded clay aggregate as a building material – An overview. Constr Build Mater 170: 757-775.
- A Bayoussef, M Loutou, Y Taha, M Mansori, M Benzaazoua, et al. (2021) Use of clays by-products from phosphate mines for the manufacture of sustainable lightweight aggregates. J Clean Prod 280: 124361.
- A M Rashad (2018) Lightweight expanded clay aggregate as a building material – An overview. Construction and Building Materials 170: 757-775.
- S Jaha, J Carvalheiras, S Mahmoudi, J Labrincha (2024) Production of lightweight expanded aggregates from smectite clay, palygorskite-rich sediment and phosphate sludge. Clay Miner 59(2): 85-99.
- S K Adhikary, D K Ashish (2022) Turning waste expanded polystyrene into lightweight aggregate: Towards sustainable construction industry. Science of the Total Environment 837: 155852.
- C Burbano-Garcia, A Hurtado, Y F Silva, S Delvasto, G Araya-Letelier (2021) Utilization of waste engine oil for expanded clay aggregate production and assessment of its influence on lightweight concrete properties. Constr Build Mater 273: 121677.
- Y Koshiba, Y Hirakawa (2023) Fire-suppression efficiency and extinguishing mechanisms of calcium acetate using heptane cup-burner flames. Fire Mater 47(7): 949-958.
- R L Lehman, J S Gentry, N G Glumac (1998) Thermal stability of potassium carbonate near its melting point. Thermochim Acta 316(1): 1-9.
- Z Jiang, W K Chow, S F Li (2007) Review on additives for new clean fire suppressants. Environ Eng Sci 24(5): 663-674.
- S Rajput, P P Saikia (2018) Fire extinguishing agents: sort and comparison. Int. J. Res. Appl. Sci. Eng. Technol 6: 557-567.
- S W Park, D Sung, B S Choi, J W Lee, Kumazawa Hidehiro (2006) Carbonation Kinetics of Potassium Carbonate by Carbon Dioxide. J Ind Eng Chem 12(4): 522-530.
- B Ok, H Colakoglu (2024) Evaluating the usability of recycled aggregates as fill materials depending on the composition and strength of their grains. Frontiers of Structural and Civil Engineering.
- E Roces, M Muñiz-Menéndez, J González-Galindo, J Estaire (2021) Lightweight expanded clay aggregate properties based on laboratory testing. Constr Build Mater 313: 125486.
- Janusz Pawliszyn, Heather L Lord (2010) Handbook of sample preparation.
- C Q Ni, W Q Xie (2024) Quantifying sodium hypochlorite content in hypochlorite-based disinfectants via phase-conversion headspace technique. J Chromatogr A 1721: 464812.
- W Q Xie, Y X Gong, K X Yu (2018) Simple and accurate method for determining dissolved inorganic carbon in environmental water by reaction headspace gas chromatography. J Sep Sci 41(5): 1091-1095.
- X Wan, C Guo, J Feng, T Yu, X S Chai, et al. (2017) Determination of the Degree of Substitution of Cationic Guar Gum by Headspace-Based Gas Chromatography during Its Synthesis. J Agric Food Chem 65(32): 7012-7016.
- Y Dai, Z H Yu, J B Zhan, X S Chai, S X Zhang, et al. (2017) A pressure-affected headspace-gas chromatography method for determining calcium carbonate content in paper sample. J Chromatogr A 1507: 32-36.
- T Yokoya, T Murata (2017) Application News No. G288 High-Sensitivity Simultaneous Analysis of Inorganic Gases and Light Hydrocarbons using Nexis GC-2030 Dual BID System.
- J N Butler, D R Cogley (1998) Ionic Equilibrium: Solubility and PH Calculations, Revised Edition.
-
Amanda A Soares, Luã TT de Melo, Pedro H dos S S Teixeira, Giovanni N M de Souza and and Patterson P de Souza*. Eco- Friendly System for Fire Suppressant Use in Confined Spaces. Mod Concept Material Sci. 7(2): 2025. MCMS. MS.ID.000657.
-
Fire suppressants, Light expanded clay, Carbon dioxide, Carbonate salts, Confined spaces, Sustainable materials, Montreal Protocol, Hydrogels, Aluminum
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






