 Mini Review
Mini Review
Biobased Glycerol Hyperbranched Poly(Ester)s of Precise Structure as Plasticizers for Poly (Vinyl Chloride)
Bob A Howell* and Simone T Lazar
Science of Advanced Materials, Central Michigan University, Mt. Pleasant, MI 48859-0001, USA
Bob A Howell, Science of Advanced Materials, Center for Applications in Polymer Science, Department of Chemistry and Biochemistry, Central Michigan University, Mt. Pleasant, MI 48859-0001, USA.
Received Date: August 25, 2021; Published Date: October 04, 2021
Abstract
Hyperbranched poly(ester)s from the renewable, abundantly-available, biomonomers, glycerol and adipic acid, may be prepared under conditions that permit control of molecular weight, avoid gelation, and ensure the presence of hydroxyl endgroups. Capping the endgroups as esters provides materials that are thermally stable, fluid at room temperature, fully compatible with a PVC matrix and provide effective plasticization of PVC at low levels of incorporation. Because of low toxicity and migratory potential, these materials represent attractive alternatives to traditional toxic plasticizers for PVC.
Introduction
Poly(vinyl chloride) [PVC] is the third highest volume polymer produced worldwide. It has a unique set of properties that makes it useful in both rigid and flexible formulations. Aging, corroding, contamination-shedding metal water systems are being replaced with durable, noncorroding rigid PVC piping around the world. Flexible PVC is a tough material with relatively low gas permeability widely used in items from medical tubing to toys.
Despite its usefulness today, PVC was not an immediate commercial success. PVC was first synthesized in 1872 but was not considered to be a useful material [1]. The presence of polar carbonchlorine bonds makes PVC subject to strong interchain interactions. Consequently, it is a rigid material and not amenable to processing. It was not until the discovery by Waldo Semon at the B.F. Goodrich Company in 1926 that PVC could be plasticized to make it workable that it became a useful polymer [2].
In general, effective plasticizers must contain a polar group to make them compatible with the polymer and a nonpolar component to increase free volume within the polymer [3-5]. For this reason, esters early became popular plasticizers. The most common have been esters of phthalic acid. This acid is obtained by oxidation of o-xylene, the least valuable of the mixed xylenes from the reforming of naphtha [6]. Numerous phthalate esters may function as effective PVC plasticizers. However, the most prominent has been di(2-ethylhexyl)phthalate [DEHP]. The alcohol, 2-ethylhexanol, is obtained from butyraldehyde, a byproduct of hydroformylation of propylene. Adol condensation of butyraldehyde followed by hydrogenation affords the alcohol. Thus, DEHP may be readily obtained from two inexpensive materials. Further, it is an effective plasticizer for PVC. Huge volumes of plasticizers are required for PVC processing. Often plasticizer incorporation accounts for 40-60 of flexible PVC. Approximately, 85% of all plasticizer production has traditionally been used in PVC formulation. DEHP alone has accounted for greater than 60% of usage.
Although phthalates, principally DEHP, have been effective PVC plasticizers that are readily available at modest cost, their use is now being restricted. These materials readily migrate from the polymer matrix. This gives rise to two problems, negative health effects and flammability [7-9]. Phthalate plasticizers are widely distributed in the environment and human populations around the world have been exposed to these compounds, particularly DEHP. Exposure to phthalates disrupts endocrine function and leads to the development of various diseases affecting several organs, including the liver, kidneys, heart, lungs, and the reproductive tract [8-11]. These materials may no longer be used in PVC intended for the construction of medical devices or toys for young children. Limitations on the use of phthalate plasticizers has spurred the development of new efficient, nontoxic, nonmigrating plasticizing additives for PVC [12,13]. Those derived from biomaterials are particularly attractive. The precursors to these plasticizers are renewable, independent of petroleum production, generally nontoxic, and often biodegradable [14-22].
Results and Discussion
Glycerol is a trifunctional alcohol with great potential for the generation of useful, low-cost plasticizers for PVC. Although challenges for isolation and purification remain, glycerol is generated in huge volume as a byproduct of biodiesel production [23]. Simple esters of glycerol may be prepared from monofunctional carboxylic acids and are effective plasticizers for PVC [23]. However, the greatest potential for the development of effective, nontoxic, nonmigrating glycerol-derived plasticizers lies, with hyperbranched oligomers. Hyperbranched glycerol poly(ester)s may function as effective plasticizers and display low potential for migration from a polymer matrix into which they have been incorporated [24]. The outstanding effectiveness of these poly(ester)s as plasticizers largely arises from the presence of atomic-scale free-volume cavities [25-27].
The great potential of hyperbranched poly(ester)s generated from glycerol and a bioderived dicarboxylic acid has long been recognized. However, the development of useful materials has been hampered by the lack of a reliable method for preparing welldefined polymers while avoiding gelation. The condensation of glycerol with a diacid is complicated in several ways. The reactivity of the three glycerol hydroxyl groups is not the same (the primary hydroxyls are approximately 1.4 times as reactive in esterification as is the secondary hydroxyl). Further, the reactivity of remaining hydroxyl groups is impacted by initial esterification. Traditional gelation theory is inadequate for predicting the ratio of monomers (ratio of reactive functional groups) that will permit reaction to high monomer conversion without gelation and with the assurance of a single kind of endgroup functionality [27-34]. The preparation of glycerol hyperbranched polymers has generally been approached empirically--varying monomer concentration, reaction times and temperature, or extent of reaction [35-39]. For the generation of functional plasticizers, this approach is inadequate. By limiting the extent of reaction, nongelling polymers may be obtained. However, these materials contain both hydroxyl and carboxyl endgroups and will gel upon storage. Empirical methods do not permit polymerization to a high degree of monomer conversion without gelation or the targeting of molecular weight, degree of branching or endpoint identity based on initial reactant stoichiometry. A more rational approach is required [40-43]. Using the Martin- Smith model for the determination of initial monomer ratios (ratio of reactive functional groups), polymerization may be carried out of high degrees of monomer conversion without gelation. The materials generated have well-defined structure and a single type of endgroup. This approach is based on Macosko-Miller statistical probability and accounts for both differences in functional group reactivity and changes in reactivity as a consequence of partial substitution [43].
Hyperbranched poly(ester)s were prepared from two renewable biomaterials, glycerol and adipic acid, under conditions to avoid gelation and ensure the presence of hydroxyl endgroups. A polymer with molar mass of 1250 g/mol, a dispersity of 2.9 and a degree of branching of 30% was chosen for further modification to generate plasticizers. A series of ester end capped polymers was generated from the hydroxyl terminal hyperbranched material by treatment with 2-ethylhexanoyl chloride (HBPE-E), octanoyl chloride (HBPE-O) and benzoyl chloride (HBPE-B) [44]. For evaluation as plasticizers, these oligomers were blended with PVC at 5 and 15 parts-per-hundred (phr). The effectiveness of plasticization was reflected in a reduction of the PVC glass transition temperature (Tg) as determined using differential scanning calorimetry (DSC) or dynamic mechanical analysis (DMA) [4]. Results are presented in Table 1. Unplasticized PVC and PVC plasticized with DEHP are included for comparison.
Table 1: Effectiveness of Ester Endcapped Hyperbranched Glycerol/Adipic Acid Oligomers as Plasticizers for PVC.
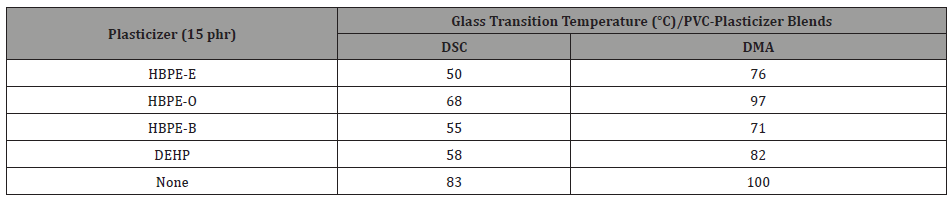
As may be noted all the oligomeric poly(ester)s are effective plasticizers for PVC. Two, HBPE-E and HBPE-B, are fully as effective as DEHP. All are liquid at room temperature are fully compatible with PVC. They offer great potential as nontoxic, nonmigrating replacements for phthalates as plasticizers for PVC.
Conclusion
Hyperbranched poly(ester)s from renewable biomonomers, glycerol and adipic acid, may be prepared using standard, costeffective techniques under conditions that permit control of molecular weight, avoid gelation, and ensure the presence of hydroxyl endgroups. The terminal endgroups may be converted to esters of varied structure to afford materials that are fluid at room temperature, thermally stable, fully compatible with a PVC matrix, provide effective plasticization of PVC at low levels of incorporation, and display low migratory potential. The ease of production from inexpensive, abundant biomaterials coupled with effectiveness and nontoxicity make these materials attractive candidates for commercial development as replacement for phthalates as PVC plasticizers.
Acknowledgment
None.
Conflict of Interest
No conflict of interest.
References
- E Baumann (1872) About some vinyl compounds. European Journal of Organic Chemistry 163(3): 308-322.
- HA Sarvetnick (1969) Poly(vinyl chloride), Van Nostrand Reinhold, New York, NY.
- VA Pereira, AC Fonseca, CSMF Costa, A Ramalho, JFJ Coelho, et al. (2020) End-capped Biobased Saturated Polyesters as Effective Plasticizers for PVC. Polym Test 85: 106406.
- PA Daniels (2009) A Brief Overview of Theories for PVC Plasticization and Methods Used to Evaluate PVC-Plasticizer Interaction. J Vinyl Addit. Technol 15(4): 219-223.
- HC Erythropel, S Shipley, A Bormann, JA Nicell, N Marie, et al. (2016) Designing Green Plasticizers: Influence of Molecule Geometry and Alkyl Chain Length on the Plasticizing Effectiveness of Diester Plasticizers in PVC Blends. Polymer 89: 18-27.
- HA Wittcoff, BG Reuben, JS Plotkin (2012) Industrial Organic Chemicals, 3rd Edition John Wiley & Sons, New York, NY.
- BA Howell, A Alrubayyi, EA Ostrander (2019) Thermal Properties of Charring Plasticizers from the Biobased Alcohols, Pentaerythritol and 3,5-Dihydroxybenzoic Acid. J Therm Anal Calorim 138(25): 2661-2668.
- JA Tickner, T Schettler, T Guidotti, M McCally, M Rossi (2001) Health Risks Posed by Use of Di(2-ethylhexyl)phthalate (DEHP) in PVC Medical Devices: A Critical Review. Amer J Ind Med 39(1): 100-111.
- F Chiellini, M Ferri, A Morelli, L Dipaola, G Latini (2013) Perspectives on Alternatives to Phthalate Plasticized Poly(vinyl chloride) in Medical Device Applications. Prog Polym Sci 38(7): 1067-1088.
- G Latini, A Verrotti, C De Felice (2004) Di-2-ethylhexyl phthalate and Endocrine Disruption: A Review. Current Drug Targets--Immune, Endocrine and Metabolic Disorders 4(1): 37-40.
- M Preciados, C Yoo, D Roy (2016) Estrogenic Endocrine Disrupting Chemicals Influencing NRF1 Regulated Gene Networks in the Development of Complex Human Brain Diseases. Int J Mol Sci 17(12): 2086.
- C Gao, X Zhang, J Sun, Z Yuan, S Han, et al. (2018) Poly(hexane succinate) Plasticizer for Poly(vinyl chloride) with a High Efficiency, Nontoxicity and Improved Migration Resistance. J Appl Polym Sci 135(25): 46388.
- H Miao, H Zhao, P Jiang (2017) Poly(vinyl chloride) Films Plasticized with Novel Poly-Nadic-Anhydride Polyester Plasticizers. J Vinyl Addit Technol 23(4): 321-328.
- JP Harmon, R Otter (2018) Green Chemistry and the Search for New Plasticizers. ACS Sustainable Chem Eng 6(2): 2078-2085.
- X Zhang, Y Li, JM Hankett, Z Chen (2015) The Molecular Interfacial Structure and Plasticizer Migration Behavior of Green Plasticized Poly(vinyl chloride). Phys Chem Chem Phys 17: 4472-4482.
- S Kumar (2019) Recent Developments of Biobased Plasticizers and their Effect on Mechanical and Thermal Properties of Poly(vinyl chloride): A Review. Ind Eng Chem Res 58(27): 11659-11672.
- P Jia, H Xia, K Tang, Y Zhou (2018) Plasticizers Derived from Biomass Resources: A Short Review. Polymers 10(12): 1303.
- Z Zhang, PP Jiang, D Liu, S Feng, P Zhang, et al. (2021) Research Progress of Novel Biobased Plasticizers and their Applications in Poly(vinyl chloride). J Mater Sci 56: 10155-10182.
- Y Hao, A Tian, J Zhu, J Fan, Y Yang (2020) Synthesis and Evaluation of Biobased Plasticizers from 5-Hydroxymethyl-2-furancarboxylic Acid for Poly(vinyl chloride). Ind Eng Chem Res 59(40): 18290-18297.
- Z He, Y Lu, C Lin, H Jia, H Wu, et al. (2020) Designing Antimigration Furan-based Plasticizers and their Plasticization Properties in Poly(vinyl chloride) Blends. Polym Test 91: 106793.
- BA Howell, ST Lazar (2018) Biobased Plasticizers from Carbohydrate-derived 2,5-bis-(Hydroxymethyl)furan. Ind Eng Chem Res 58(3): 1222-1228.
- BA Howell, W Sun (2018) Biobased Plasticizers from Tartaric Acid, an Abundantly Available, Renewable Material. Ind Eng Chem Res 57(45): 15234-152342.
- OYS Palacios, PCN Rincon, JP Corriou, MC Pardo, C Fonteix (2014) Low-molecular-weight Glycerol Esters as Plasticizers for Poly(vinyl chloride). J Vinyl Addit Technol 20(2): 65-71.
- BA Howell, ST Lazar (2019) Biobased Plasticizers from Glycerol Adipic Acid Hyperbranched Poly(ester)s. Ind Eng Chem Res 58(37): 17227-17234.
- W Gong, Y Mai, Y Zhou, N Qi, B Wang, et al. (2005) Effect of the Degree of Branching on Atomic-scale Free Volume in Hyperbranched Poly[3-ethyl-3(hydroxymethyl)]oxetane. A Positron Study. Macromolecules 38(23): 9644-9649.
- Z Sun, X Mi, Y Yu, W Shi, A Feng, et al. (2021) All-PVC Flexible Poly(vinyl chloride): Nonmigratory Star-Poly(vinyl chloride) as Plasticizers for PVC by RAFT Polymerization. Macromolecules 54(11): 5022-5032.
- Dumitrascu, A Sarkar, J Chai, T Zhang, RA Bubeck, et al. (2018) Thermal Properties of Hyperbranched Poly(ester)s. J Therm Anal Calorim 131: 273-280.
- PJ Flory (1952) Molecular Size Distribution in Three-dimensional Polymers. VI. Branched Polymers Containing A-R-BF-1 Type Units. J Am Chem Soc 74(11): 2718-2723.
- WH Stockmayer (1943) Theory of Molecular Size Distribution and Gel Formation in Branched-chain Polymers. J Chem Phys 11: 45.
- WH Stockmayer (1944) Theory of Molecular Size Distribution and Gel Formation in Branched Polymers. II. General Crosslinking. J Chem Phys 12: 125.
- d’Arcy R, Burke J, Tirelli N (2016) Branched Polyesters: Preparative Strategies and Applications. Adv Drug Deliv Rev 107: 60-81.
- M Agach, S Delbaere, S Marinkovic, B Estrine, V Nardello-Rataj (2012) Characterization, Stability and Ecotoxic Properties of Readily Biodegradable Branched Oligoesters Based on Biosourced Succinic Acid and Glycerol. Polym Degrad Stab 97(10): 1956-1963.
- KC Cheng, TH Chuang, TH Tsai, W Guo, WF Su (2008) Model of Hyperbranched Polymers Formed by Monomers A2 and Bg with Endcapping Molecules. Eur Polym J 44(9): 2998-3004.
- M Wrzecionek, P Ruskowski, A Gadomskar-Gajadhur (2021) Mathematically Described Preparation Process of Poly(glycerol succinate) Resins and Elastomers—Meeting Science with Industry. Polym Adv Technol 32(5): 2042-2051.
- JF Stumble, B Bruchmann (2004) Hyperbranched Polyesters Based on Adipic Acid and Glycerol. Macrol Rapid Commun 25(9): 921-924.
- VT Wyatt (2012) Lewis Acid-catalyzed Synthesis of Hyperbranched Polymers Based on Glycerol and Diacids in Toluene. J Am Oil Chem Soc 89(2): 313-319.
- VT Wyatt, GD Strahan (2012) Degree of Branching in Hyperbranched Poly(glycerol-co-diacid)s Synthesized in Toluene. Polymer 4(1): 396-407.
- Y Li, WD Cook, C Moorhoff, WC Huang, QZ Chen (2013) Synthesis, Characterization and Properties of Biocompatible Poly(glycerol sebacate) Prepolymer and Gel. Polym Int 62(4): 534-547.
- Y Yang, W Lu, J Cai, Y Hou, S Ouyang, et al. (2011) Poly(oleic acid-co-glycerol): Comparison of Polymer Structure Resulting from Chemical and Lipase Catalysis. Macromolecules 44(7): 1977-1985.
- BA Howell, T Zhang, PB Smith (2021) Biobased Hyperbranched Poly(ester)s of Precise Structure for the Release of Therapeutics. Medical Research Archives 9(1).
- BA Howell, T Zhang, PB Smith (2019) Hyperbranched Poly(ester)s from Renewable Biomonomers by Design: Nontoxic Platforms for the Delivery of Therapeutic Agents. Sci J Research Rev 2(2): 1-2.
- T Zhang, BA Howell, PB Smith (2017) Rational Synthesis of Hyperbranched Poly(ester)s. Ind Eng Chem Res 56(6): 1661-1670.
- T Zhang, BA Howell, A Dumitrascu, SJ Martin, PB Smith (2014) Synthesis and Characterization of Glycerol-Adipic Acid Hyperbranched Poly(ester)s. Polymer 55(20): 5065-5072.
- BA Howell, ST Lazar (2019) Biobased Plasticizers from Glycerol/Adipic Acid Hyperbranched Poly(ester)s. Ind Eng Chem Res 58(37): 17227-17234.
-
Bob A Howell, Simone T Lazar. Biobased Glycerol Hyperbranched Poly(Ester)s of Precise Structure as Plasticizers for Poly(Vinyl Chloride). Mod Concept Material Sci. 4(3): 2021. MCMS. MS.ID.000587. DOI: 10.33552/MCMS.2021.04.000587.
-
Poly(ester)s, Structure, Plasticizers, Poly(vinyl chloride), Glycerol, Polymer, Material, Esters, Endgroups, Alcohol, Oligomers, Esterification, Biomaterials, Temperature
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






