 Review Article
Review Article
Application of Hydrogel in Tissue Engineering: Process of Bone Regeneration and Fracture Healing
Shahirin Shahida*
Department of Mechatronics Engineering, Kyungsung University, Republic of Korea
Shahirin Shahida, Department of Mechatronics Engineering, Kyungsung University, 309 Suyeong-ro, Nam-gu, Busan-48434, Republic of Korea.
Received Date: May 26, 2021; Published Date: June 08, 2021
Abstract
Nowadays, the wide-reaching prevalence of bone disorders and conditions has been increasing very rapidly. So, the clinical need for efficacious solutions for the bone regeneration process is unanimously recognized. Attributable to the constantly expanding technological and scientific advances in medical science, it turns into an even supplementary serious problem with the increasing expectations of both surgeons and patients. Nowadays the bone regeneration treatment needs progressively more bone diseases, such as bone fracture, infections, tumors, and bone loss. The bone is a nano-material that is comprised of organic collagen and inorganic; mainly the nano-hydroxyapatite components with a tiered structure vacillating from nano-scale to macroscale. In contemplation of the earnest shortcomings in the traditional bone healing processes, nano-materials provide some new strategies in the bone healing and regeneration process. The nano-structured scaffolds are providing a nearer structural sustenance approach to the instinctive bone architecture for the tissue cells and adjust the cell growth, distinction, and relocation, which causes the development of functional bone tissues. The bone regeneration process is a complex, well-structured functional process of the bone composition process, it can be observed through the usual healing process of the bone fractures, and it is involved in the relentless transformation through adult life. However, there are more complex clinical conditions in the bone regeneration process. Currently, there is repletion of several approaches to enhance the impaired or insufficient bone-regeneration process, in this process implementation of bioactive materials in bone tissue engineering gained tremendous attention among scientists all over the world. Bioactive materials such as hydrogel are using for the enhancement of bone fracture repairs, different studies showed that it is possible to overcome the current limitations of the bone regeneration processes. The development of hydrogel scaffolds for bone regeneration has highlighted the challenge that load-bearing hydrogels need to be biocompatible while achieving high strength. Several researchers have been stated to improve the toughness of hydrogels, but achieving high toughness and biocompatibility simultaneously remains a challenge.
This article focused on reviewing the application of hydrogel for bone tissue engineering and regeneration. Furthermore, there some challenges about future research on the application of biomaterials for bone regeneration are described in the conclusion and perspectives part.
Keywords: Bone Regeneration; Bone Tissue Engineering; Hydrogel; Bioactive Materials; Biomaterials
Introduction
The bone is the core structural form of the body and it is convoluted for inorganic homeostasis and the shield of internal organs of the body. Most of the familiar bone diseases can be listed as follows: tumors, infections, cancer, and in the case of older people; aging is the main reason for bone disease. Another major cause of bone disease is a fracture, the fracture can occur in several parts of the human body such as the skull, spinal, nasal, femur, radius, tibia, and ankle are mostly resulting from distressing processes. This distress can destroy the normal bone healing ability consequential in structural and functional abnormalities [1-4].
The bone damage occurs as a consequence of disease or injury repeatedly and hazardous surgical interventions concerning the encouragement of the amalgamation of implants onto the bone to influence the proper regeneration process. According to the previous researches, it is shown that in the United States of America, in 2025; the age-related fractures among the older peoples are expected to increase by over 3 million fractures; in 2005 it was 2.1 million, which is merely taking into account that the aging population at high risk. Similarly, in Europe, it is assessed that from 2010 to 2025, within fifteen years the number of bone fractures will be rise to 28% each year, equally with an absolute rate of increasing the injuries from 3.5 million to 4.5 million [5- 7]. For the aging population, musculoskeletal disease is leading to rises in the costs of healthcare, attenuates the productivity rate in workplaces, and it also lessens the quality of life. In this condition, there is an urgent need for the development of the most efficient, regenerative, and attuned treatments for bone injuries [8-11].
However, the regeneration process of bone tissue is the most complicated task, which is demanding the harmonious interaction of cells, cellular sustenance scaffolds, bioactive growth factor substances, and the body’s prevailing tissue. There are several approaches for bone-healing or regeneration-related treatments have already been investigated; each of the procedures has its inimitable benefits and drawbacks. At the interface, the bioactivity of bone tissue scaffold transplants depends on the interaction of constituent molecules, stem cells, and osteoblastic-progenitor cells [12,13].
Recently, hydrogels have been gained vast attention in bone tissue engineering (BTE) as scaffolds for the healing and regeneration process, because of their cross-linked hydrated 3-D porous structure that attains a high concentration of hydrophilic groups with an attraction for water and consummate of absorbing a significant amount of water. It can compete with the substantial properties of extracellular matrix (ECM) for signaling, transportation of nutrients, and homeostasis, as well as to support cell growths, proliferation, and depiction. Moreover, in load-bearing tissues such as bone, tendon, ligament, and cartilage, etc., the hydrogels are substantially restraining their application because of the insufficient mechanical properties [14-18].
In the group of several hydrogels with defined properties, natural and synthetic polymers are also employed. They have a great concentration because of the gelatin at body temperature, which grants the large implementation including the carrying cells, thermolabile medicines, bioactive particles, and can fill into the slightly irregular-shaped bone defects. Natural polymers validate the more significant benefits over synthetic polymers as well as the high biocompatibility and structural similarity with natural bone components [19-22]. This paper aims to review the uses of hydrogel for bone regeneration in Bone Tissue Engineering (BTE).
The Bone
The bone is a stiff body tissue containing the cells entrenched in numerous hard intercellular materials with two vital components of this material, one is collagen and the rest is calcium phosphate. Distinguish bone from other hard tissues such as chitin, coating, and shell which provides the proper mechanical strength and structural support to the body. It can be classified into two parts; cortical or dense, and trabecular or porous bone, both of these subclasses have a composed 3-dimensional architecture with high structural complexity as shown in Figure 1. Based on the architectural arrangement, the structures of bone contain three levels as follows; macro, micro, and nano-level [22,23].
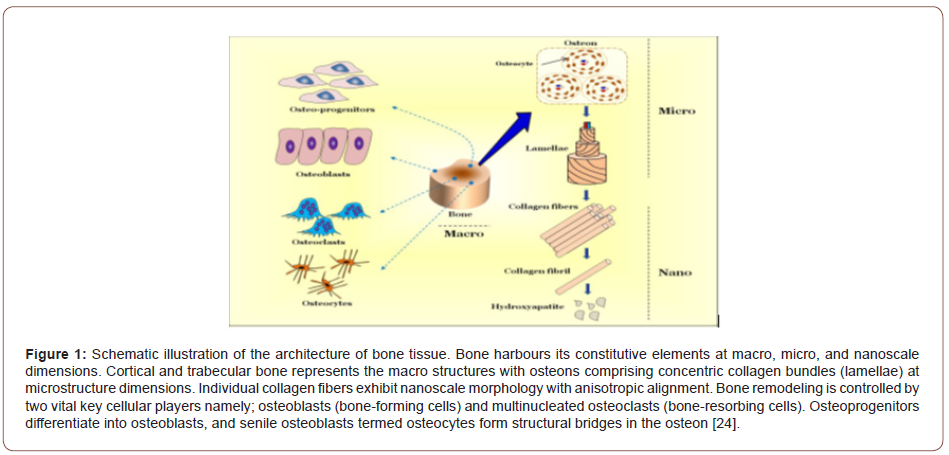
Bone anatomy and the biological structure of bone
Anatomically, the bone is a stiff and compressed tissue that is mainly composed of three main components; these are the periosteum, cortex, and medulla. The periosteum surrounds the exterior surface of bones except in the region of articular cartilage and between the bone and the adjacent soft tissues, it serves as a transitional region. The cortex is contained in under the periosteum and is thicker along surfaces that can carry more load such as the shaft of long bones. The inner layer of bones, blood vessels, nerves, and the hemopoietic or fatty bone marrows are represented by the medulla [24-26].
For mechanical support and osteo-regeneration, the bone substitutes are mainly serving as combined functions, which involve three vital biological properties; these are osteo-conduction, osteoinduction, and osteogenesis. The osteo-conduction refers to the ability to support the attachment of osteoblast and osteoprogenitor cells and allow the migration and ingrowth of these cells within the 3-dimensional architecture of the graft [27,28]. The osteo-induction characterizes that the graft can induce the primeval, uniform, and pluripotential cells to evolve into the bone-forming cell extraction, by which osteogenesis is induced. Osteogenesis means the osteodifferentiation and subsequently new bone formation by donor cells that are originated from either the host or grafts [29-31].
The extracellular matrix (ECM) of the bone is a biphasic system, in which one-third is composed of organic matter, mostly type I collagen fibers, and the remaining two-thirds consist of inorganic matter or bone salt, such as hydroxyapatite-like calcium phosphates. Three cell types osteoblasts, osteocytes, and osteoclasts work in concordance to form an amalgamated bone organism. Osteoblasts are the main functional cells of bone formation and are responsible for the synthesis, secretion, and mineralization of the bone matrix. Which are from mesenchymal stem cells (MSCs), or progenitor cells, from the tenacious portion of bone marrow and cover the surface of bone seams, forming a protein-like mixture called osteoid, this mainly contains polymerized collagen chains, and later it is mineralized into bone, mediated by the deposition of calcium and phosphate.
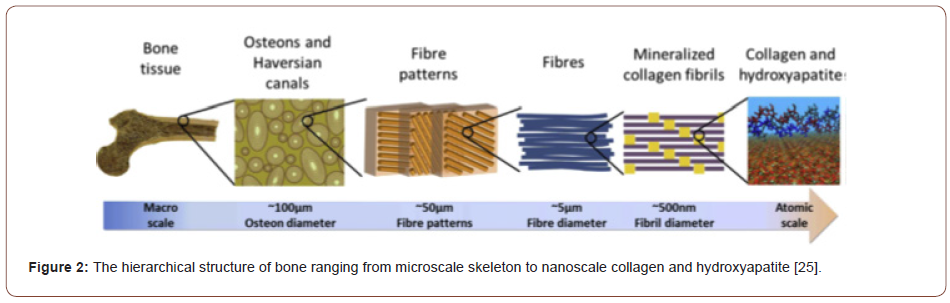
Besides, the corresponding hormones also produce by osteoblasts that promote surrounding bone formation. Osteocytes are dormant post-synthetic osteoblasts that migrated to the ECM matrix of bone. Those are connecting with osteoblasts and other cells and playing a significant role in mineral homeostasis. The third type of cells, osteoclasts, are originated from hematopoietic stem cells in the non-adherent portion of marrow and are primarily inclined to bone resorption through discharging several matters. The synchronized contacts among osteoblasts and osteoclasts retain the normal bone mass and aid in the final bone remodeling [3,32,33]. These properties are mainly attributed to the outstanding hierarchical architecture, which is composed of the soft collagen protein and stiffer apatite mineral, as shown in Figure 2 [25,34].
Defects of bone
The bone defect is one of the most common orthopedic problems and threatens human health. A bone defect is a lack of bone where it should normally occur. The majority of the bone defects, damages, and injuries can be categorized into the following parts: fractures, old age, infection, cancer, and hereditary diseases. Bone fractures are considering a major cause of bone injuries and they can happen in several parts of the human body, as well as the skull, radius, tibia, femur, spinal, nasal, and ankle, and are mostly caused by traumatic procedures. Bone loss and osteoporosis are ensuing as a result of aging are also considered as the sources of bone damage, and which can usually increase the risk of bone fractures as well. Generally, autografts, allografts, or xenografts are used to treat the bone defects that occurred due to incidents such as fractures, diseases mainly osteoporosis and osteosarcoma, or surgeries. In a specific area, infections such as osteomyelitis are often manifested by pain, redness, and fever, and if not properly treated, it may lead to irreversible trauma, such as amputation [2,35-37].
Additionally, tumors can induce significant bone fractures, remodeling, and anaemia, and can even threaten human life under severe circumstances [38]. While surgery is commonly employed in bone cancer treatment, it often drives to the generation of defects in the treatment site. Hereditary diseases, for example, hereditary bone marrow failure syndromes and hereditary multiple exostoses (HME) can also evoke bone injuries, and usually need surgical resection or stem cell transplantation. All of the situations stated above can cause bone defects to an extent, to boost bone regeneration and to recover normal bone function, the critical size of bone self-healing capability, extraneous intervention is required. It is necessary to be addressed and deeply investigated each of these categories of defects and needs to develop new technologies to effectively treat bone damage [39,40].
Bone healing
Enhancement of life expectancy in the last decades and the future has required the development of strategies to strike the increase of unconsolidated bone fractures. Bone tissue repair and regeneration technique is a dynamic process that starts with proliferation and migration of osteogenesis cells, the reconstruction of bone with differentiation of osteoprogenitor cells, and bone ECM formation is finally apprehended. The scaffold materials which load different growth factors and drugs have achieved immense advancement in bone tissue engineering [36,41].
The various factors affecting repairing can also be applied to classify bone healing, the extent of tissue loss is one of them. Hence, the bone repairing process can be categorized into two parts: primary or direct bone healing and secondary or indirect bone healing. The primary or direct bone healing mainly happens when the gap of fracture is less than 0.1 mm and the fracture site is rigidly alleviating. As stated in previous research findings, it is proposed that the bone gap in this method is filled directly by uninterrupted ossification and subsequent Haversian remodeling, with the absence of cartilaginous or connective tissue. The formation of callus is also concealed. However, due to the lack of neither histological evidence nor clinical cases the concept of direct continuous bone formation is still controversial [37-39].
The most common form of the bone healing process is secondary or indirect bone healing and it ensues when the edges of bone fracture are lower than twice the diameter of the injured bone. The multi-events such as bone remodeling, blood clotting, inflammatory response, fibrocartilage callus formation, intramembranous, and endochondral ossification are implicated in the secondary or indirect bone fracture healing process. Specific to the major metabolic activity, recruiting stem cell differentiation, and retardation with chondrocyte, the anabolism into a bone fracture is activated firstly in the form of increasing bone volume apoptosis. The anabolic activity continues in a prolonged phase is dominated by catabolic activity. The diminution of callus tissue volume is the symbol of this activity. The catabolic phase reaches the final period when the vascular bed increases and the vascular flow rate returns to pre-injury level [37,42-44]. The biological events and activities, as well as the cells involved in typical bone fracture healing at various phases, are illustrated in Figure 3.
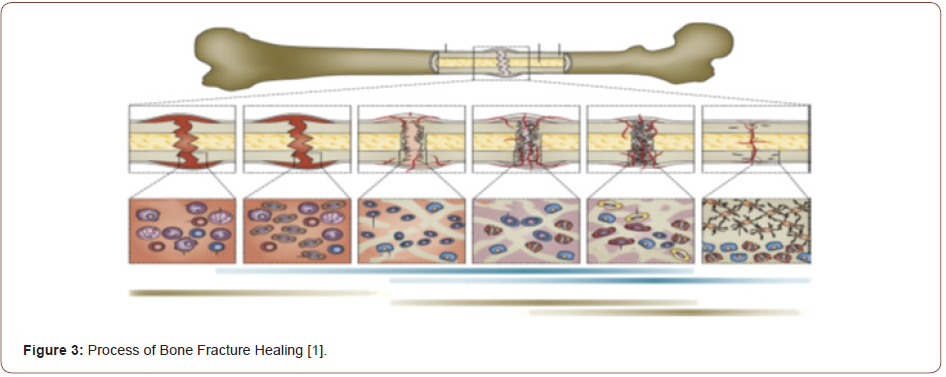
Bone Regeneration Process
The bone regeneration process is one of the well-orchestrated, complex physiological processes of bone formation during normal fracture healing. It is comprised of a well-orchestrated series of biological proceedings of bone generation and conduction, as well as several cell types and intracellular and extracellular molecularsignaling pathways, through an ascertainable mundane and spatial sequence, to optimize skeletal repair and restore skeletal function [45,46].
However, there are many intricate clinical conditions in which bone regeneration is needed, such as for skeletal reconstruction of large bone defects formed by bone fracture, aging, infection, and skeletal abnormalities. In the clinical condition, bone regeneration is the most common form of fracture healing, during which the pathway of normal fatal skeletogenesis, as well as intramembranous and endochondral ossification, is recapitulated [46,47].
Currently, there is a surfeit of different strategies to enhance the impaired or inadequate bone-regeneration process, including the autologous bone graft, free fibula vascularized graft, allograft implanting, and use of growth factors, osteoconductive scaffolds, osteoprogenitor cells, and distraction osteogenesis. Improved strategies in terms of tissue engineering to the enhancement of bone repair are under intense investigation, to overcome the limitations of the current bone regeneration process, to produce bone-graft that is as equal to the normal bone as possible, to accelerate the overall bone regeneration procedure, or even to deal with systemic conditions, such as skeletal disorders and osteoporosis [45,48].
Necessity of effective bone repair strategies: economic and social aspects
Bone and joint degenerative and inflammatory problems influence millions of people over the world. Musculoskeletal conditions, namely joint pathologies, fractures related to osteoporosis, serious injuries, back pain, and different types of bone diseases and disablement are among the most common causes of hundreds of millions of people worldwide suffering severe longterm pain and becoming physically handicapped or crippled. It has been reported that over 100 million Europeans suffer chronic musculoskeletal pain, whereas in the USA musculoskeletal difficulties impact over 40 million people aged 45 years or older and are projected to affect more than 60 million persons, or 22% of the population, by the year 2030. While mortality from the abovementioned conditions is low, they have a major effect on disability, medical costs, and patient quality of life [41,43,49].
It has been reported that back pain and arthritis are the two most common causes of pain worldwide. The most common problem is low-back pain, approximately 4-33% of the population are affecting. Arthritis is the most common cause of pain in older people and women, which is a pathology that includes damage to and inflammation of the joints. It has been estimated previously that approximately 18% of women and 10% of men aged over 60 years are affected by osteoarthritis while rheumatoid arthritis, in developed countries at the age of 45-46 years, that is a more severe disease, affects 0.3-1% of the general population and is more prevalent among women. Additionally, it has been estimated that approximately 40% of arthritic adults suffer from osteoarthritis of the knee, 80% of people with osteoarthritis have the constraint of movement and 25% cannot execute their major daily activities [50,51].
Another most common problems affecting contemporary society are Osteoporosis and particularly fractures which are caused by this illness. Osteoporotic fractures primarily result from low BMD (bone mass density). It has been defined as a condition in which BMD is 2.5 standard deviations or further below the mean seen in young healthy subjects. However, the microstructural alterations in bone, especially of trabecular bone, also contribute substantially by increasing trabecular brittleness. This fragility is translated into an increase in the vertebra, wrist, and hip fractures [52,53].
In the USA only the prevalence of osteoporosis is estimated to increase from 10 million to more than 14 million people by 2020. This is a significant increase with a high risk of falls and fractures in the population [54]. It is expected that over 50 years of age, 40% of all women will suffer from an osteoporotic fracture and the fractures related to osteoporosis have almost doubled in number in the last decade. Although it is estimated that 30% of all hip fractures occur in men, osteoporosis is less prevalent in men than in women. Additionally, the investigation showed that the fracture-related morbidity rate for men is higher than for women [55]. Osteoporosis is a functional abnormality and an important clinical syndrome in the case of arthritis and other musculoskeletal diseases, which is leading to many problems concerning the quality of life [56].
To consider the economic aspects, the costs of bone regeneration strategies are commonly classified into three categories: indirect costs, direct costs, and intangible costs. Expenditure for medical care and related items is included indirect costs, for example; diagnostic tests, prescription, physician visits, and over-the-counter medications, hospital stays, aids and devices, and outpatient surgical processes are included in this expenditure. Indirect costs are including work disability, sick leave, including these subsequent from lost function in one’s usual activity, or reduced productivity associated with a reduction in work hours. Several studies have shown the significant effect of musculoskeletal conditions on employment. Based on the specific condition, the direct costs and indirect costs of musculoskeletal conditions may equal, or even exceed. Intangible costs are related to increased pain, loss of function, and reduced quality of life [57].
Across fracture types, in the USA, it has been estimated that the total cost of incident fractures will rise to US$228 billion for 2016-2025, during 2006-2015 which was about US$209 billion. For pelvic fractures the largest changes are predicted, where between 2005 and 2025 the rate increases by 56% and costs are predicted to rise by 60%. By race/ethnicity, among the non-white population, the fractures and costs rate will increase proportionally to 21% and 19% in 2025; in 2005 the increasing rate was 14% and 12% respectively [58].
A significant outcome of musculoskeletal diseases is a disability, including limitation of the activities of daily living, reduction in leisure and chronic pain, community activities, and psychological problems, including depression, anxiety, and decreased general health are the limitations associated with musculoskeletal diseases. The economic impact of musculoskeletal disorders and chronic pain related to them not only involves the direct, indirect, and immaterial costs earlier mentioned, they also have a deep effect on the economic burden related to work absenteeism and lower performance. It has been estimated that the impact of arthritis on lost productive work time amounted to US$7.11 billion, but 66% of this attributed to the 38% of workers with pain-related disabilities [59].
Moreover, musculoskeletal conditions are the most usual medical cause of long-term sickness absence. In the UK, for example, 3000 people go on to the incapacity benefit scheme every week and approximately 300 never return to work. In Germany, musculoskeletal conditions cost employers US$30.8 million and are considered to be the largest single contributor to lost productivity. Based on a previous study, in France there have been nearly 2000 professionals are suffering from severe pain among them there are approximately 50% of them suffered musculoskeletal-related pain; 9days/year was the average number of sick days resulting from their disability [44,60].
From the socio-economical perspective, it is expected that as the average age of the population rises, the influence of musculoskeletal conditions on society will also enhance. It is also predicted that the number of annual fractures and costs will rise by 50% in 2025. The factors which are more relevant to the patient such as gender, race/ethnicity, age, and lifestyle also affect strongly the result of the disease. The disability associated with certain musculoskeletal conditions, for example; arthritis, the most common musculoskeletal condition, osteoporosis, and its related fractures and defects substantially influences society; most of the time, the individuals’ quality of life is substantially affected. In the worst cases, the individual is not able to have a fully autonomous lifestyle [56-58,61].
Current treatments for bone regeneration
Over thousands of years, the reconstruction and regeneration of significant skeletal defects have amazed mankind. The current treatment for bone regeneration is harvesting autologous grafts from other positions in the body. Autologous grafts are harvested primarily from the patient’s iliac crest or other locations, namely the distal femur, ribs, proximal tibia, intramedullary canal and transplantation into the massive fractures, or the transplantation of allografts, which have many obstacles, such as donor‐site morbidity, limited tissue supply, infection, and poor integration. The clinically approved therapies are autografts, which reveal the biological characteristics of osteogenesis, osteoconduction, and osteoinduction [62-64].
Successfully treating bone fractures requires the displaced bone to be adjusted and fixed to the normal state usually with metallic scaffolds to facilitate correct bone healing. The amalgamation between materials and the natural tissue is greatly influenced bone regeneration. While avoiding an inadvertent immune response, the Materials possessing superiority in biocompatibility and biodegradability can stably fix the damaged bone and promote bone tissue growth. In general, the metal implants such as bone nails have good mechanical strength in treating defects in weightbearing bones, containing tibia, femur, and spine [65].
Nevertheless, the metallic scaffolds are bioinert and cannot fully incorporate with surrounding tissues. Compared to other commonly used bioactive and osteoconductive biomaterials such as calcium phosphate (CaP), the main constituent of bone tissues, were later developed to accelerate the bone healing process. As an example, metal grafts wrapped with CaP have been found to not only repair the displaced bone but also allow integration with nearby tissue. Considering its biodegradability, protein-binding affinity, and osteoconductivity, a CaP bone graft alternate is thought to be a perfect substitute for bone treatments, and there have been various attempts in CPC grafts. Due to the capability to form apatite and for the resorbability, Dicalcium phosphate anhydrous (DCPA) (monetite) and Dicalcium phosphate anhydrous (DCP) (brushite) are considered as another group of biomaterials that is a crucial parameter for the generation of hard tissue. The potential use of monetite as a biomaterial for bone tissue regeneration has recently been highlighted by a series of in vitro, animal, and human studies [66].
Despite that, it is biological implementation is restrained and can only be applied to non-weight comportment defects, due to poor water-soluble resistance, limited mechanical strength, and uncertain degradation rate and curing time. Hydroxyapatite shows good biocompatibility and can firmly combine with the natural bone. On the other hand, its low mechanical strength, poor toughness, and complication in controlling pore size and permeability limit its success in bone regeneration [3,65-67].
Hydrogels
A three-dimensional network of polymers made of natural or synthetic materials possessing a high degree of flexibility due to large water content is called hydrogels. The hydrogel is made of predominantly water, which is mixed with compounds to thicken the substance and add to its healing abilities. Hydrogels are a popular treatment for particularly dry wounds, such as burns, radiation damage, and certain partial- or full-thickness wounds. They’re also beneficial for ensuring the wound bed stays moist enough to prevent pain during dressing changes and promote proper healing [68,69].
Hydrogels are a sub-category of natural and synthetic polymers. The hydrogel is a 3-dimensional network of hydrophilic polymers that increase in water and maintain a large number of water while maintaining the structure due to chemical or physical crosslinking of individual polymer chains. By explanation, water should compose at least 10% of the total volume or weight for a material to be a hydrogel. Hydrogels also possess a degree of flexibility very similar to natural tissue because of their substantial water content. The hydrophilicity of the network is owing to the presence of hydrophilic groups such as -NH2, -COOH, -OH, -CONH2, - CONH -, and -SO3H.
Hydrogels undergo a substantial volume phase conversion or gel-sol phase transition in response to definite physical and chemical impulses. The physical stimuli include temperature, magnetic and electric fields, light intensity, solvent composition, and pressure, while the chemical or biochemical stimuli include pH, ions, and specific chemical compositions. As a biomaterial, hydrogel plays an important role in biomedical applications. Hydrogels have great potential to produce various types of products for wound care, hygiene products, tissue engineering, and drug delivery.
Nevertheless, in several cases such deformational modifications are reversible; consequently, the hydrogels are competent of returning to their primary state after a reaction as soon as the trigger is removed. The response of hydrogels to external incentives is mostly determined by the nature of the monomer, pendant chains, charge density, and the degree of cross-linkage. The magnitude of response is also directly proportional to the applied external stimulus. There are several research papers, reviews, and monographs focused on the synthesis, properties, and applications of hydrogels [70,71].
Hydrogel-Based Bone Regeneration Process
The use of hydrogels for bone regeneration is recommended because of their similarity with Extra Cellular Matrix (ECM) constituents, eventual biodegradability, and the possibility to produce porous scaffolds, and their capability to deliver bioactive molecules and cells. Hydrogels are also appealing injectable systems due to controlled viscosity, loading and release of bioactive factors, and capacity for in situ physical or chemical crosslinking.
The application of hydrogels for the bone regeneration process is more recent. Now, after some years of intensive research, there are various initiatives to use hydrogels for bone regeneration process approaches including porous scaffolds, bioactive membranes, and injectable bone filler. To advance better bone regeneration, hydrogel-based drugs and cell delivery have emerged as potential solutions in tissue engineering and regenerative medicine. They can provide a natural hydrophilic 3-dimensional(3D) environment tributary to cell continuation and assist new bone development. Furthermore, hydrogels can be customized to secure the required geometry for implantation or injection, and the degradation rate and porosity or release profile can be easily restrained by modifying the crosslinking technique and degree [72,73].
Theoretically, optimized hydrogel formulations for bone regeneration demand to meet the following requirements:
1. Noncytotoxic and nonimmunogenic to prevent causing
inflammatory response;
2. Osteoinductive, osteoconductive, osteogenic, as well as
osteocompatible for improved bone regeneration;
3. Mimic the natural ECM to the greatest degree to facilitate
propagation, cell adhesion, and eventually osteogenic
distinction at implant site;
4. Degradable by endogenous enzymes or hydrolysis,
synchronizing with new bone ingrowth to make sufficient
space for new bone formation;
5. Mechanical strength and structural stability that can be
used in treating load-bearing defects and impede denaturation
during sterilization;
6. Suitable pore size and interconnected porosity that can
be optimized via modifying the concentration and diversity of
polymers and crosslinkers to improve cell interaction, control
the release of embedded bioactive components, and allow the
exchange of nutrients, oxygen, and metabolic waste within the
hydrogels;
7. Injectable capability with patient compliance to reduce
the pain and simplify the administration process [3,74].
Hydrogels in particular have emerged as useful scaffolding biomaterials as they most closely resemble the natural tissues. Hydrogels are emerging candidates for applications in cartilage regeneration. Hydrogels are 3-dimensional hydrophilic polymer networks made up of water-soluble polymers, crosslinked by either covalent bonds or physical methods. Cell matrix adhesion to hydrogel is an important interaction that regulates stem cell survival, self-renewal, and differentiation. Depending on their physical structure and chemical composition, hydrogels can preserve a compositional and mechanical similarity with the native extracellular matrix of cartilage. These properties are necessary for controlling cell response, differentiation, and functional tissue regeneration [75].
Types of hydrogels employed in bone regeneration process
Hydrogels are an enticing scaffold material due to their structures are similar to the extracellular matrix (ECM) of many tissues, they can often be processed under relatively mild conditions, and they may be delivered in a negligibly intrusive manner [76]. Hydrogels are used for ternary purposes in tissue engineering applications. They may be used as agents for wadding vacuous spaces, carriers for the delivery of bioactive molecules, and 3D structures that act as a support for cells and help the formation of an ideal tissue. In general, hydrogels can be categorized by their preparation methods, sources, crosslinking properties, delivery method, degradability, and so on. According to sources, hydrogels are classified into two types: a) natural materials and b) synthetic materials. Both synthetic and naturally derived materials can be used to form hydrogels for bone regeneration [76,77].
This review will concentrate on different natural and synthetic hydrogel systems that have attracted interest in the scientific world.
Natural materials: Hydrogels can be formed by two types of sources: firstly, from natural materials including natural proteins such as fibrin, fibroin, collagen, and gelatin, and secondly, from polysaccharides such as chitosan, hyaluronan, and alginate. The natural polymers are either constituents or are equivalent to the natural ECM, it has good biocompatibility, providing mechanical stability and structural integrity, low immune respond, and cytotoxicity, and can promote cell adhesion, proliferation, and new tissue regeneration. They can be absorbed using metabolic degradation or enzyme-controlled degradation [78].
Several types of research have been conducted based on natural materials for the bone regeneration process. Lindsey et al. [79] worked on the bone defect in the dorsal nasal bone of rats, they used collagen gel in the bone defect. There have been significant changes observed after six weeks, there was a thin bone layer on the surface of the defect, while the healing area of the rats unless collagen gel filling were less than 7%, stating that collagen gel has a positive effect on the repair of the nasal cavity defect. Patterson et al. [80] stated bone morphogenetic protein (BMP)-2 plastered hyaluronic acid (HA) gel to the cranial defect site of rats, and 75e100% of the BMP was discharged within the first 24 h. Combination of HA gel BMP promoted higher bone formation in the defected area of rats than the treatment without HA gel. Different advantageous mechanical properties of the material are required for the embed in the clinical operation; though, hydrogels made of natural polymers are usually associated with poor mechanical strength and only can be applied to non-weight- bearing sites. Physical or chemical methods such as functional, crosslinking, or copolymerization are applied to enhance the internal precise functional groups, hydrogen bonding, and electrostatic interaction of the natural materials can increase the materials’ bioactivity, strength, and toughness, thereby expanding the scope of their clinical use. Mredha et al. [82] worked to develop a strong and toughened dual network (DN) hydrogel, in which physically chemically crosslinked anisotropic swimming bladder collagen (SBC) fibril is the first network, and neutral, biocompatible poly (N, N0- two methacrylamide) (PDMAAm) is the second network. The experimental results show that the new DN hydrogel enhanced the stability of the gel and the strength of the binding to the bone. Kim et al. [83] researched to design a bionic system for local delivery of drugs made from hyaluronic acid (HA) and vinyl phosphonic acid (VPAc) cross-linked biomineralized hydrogels. By regulating the mineralization degree, crosslinking density, and ionic strength, the system could control the water content, speed of drug release, degradation rate, and could successfully deliver the protein drugs that would promote bone repair and regeneration [81].
Synthetic materials: Hydrogels that are used in the bone healing and regeneration process can be made of biodegradable polymer materials, such as polyvinyl alcohol (PVA), polyacrylamide (PAM), polyethylene glycol (PEG), Sanya methyl carbonate, poly (lactic acid), and its copolymers, and so on [84]. Unlike natural materials, synthetic polymers have fundamental structural units, so the properties of polymers such as degradation time, porosity, and mechanical properties can be amended for specific applications. Synthetic polymers have reliable material sources and a long shelf-life, so they can be manufactured in large quantities without the risk of immunogenicity. Hydrogels obtained from synthetic polymers are propitious carriers for delivering growth factors, active proteins, and drugs to bone tissue [85,87].
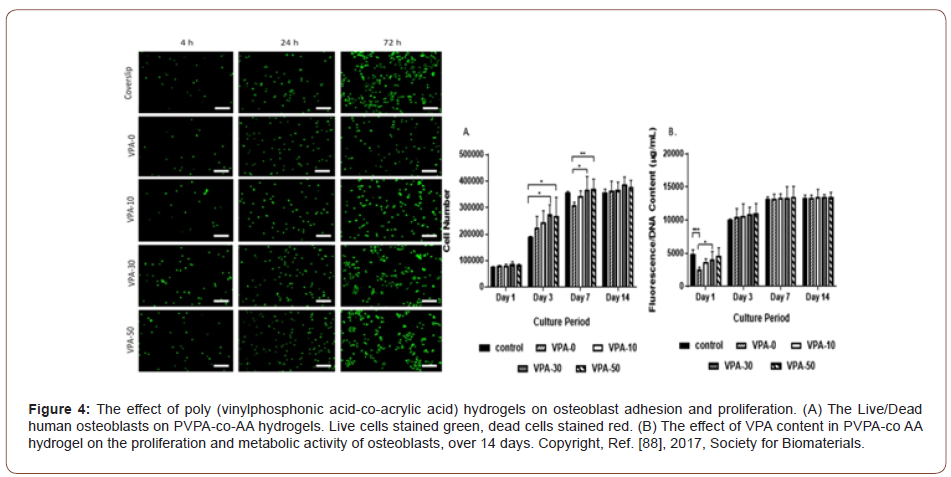
Many researchers and scientists are focused on synthetic materials to developed scaffolds for implementation in the bone healing and regeneration process. Lee et al. [86], utilized new hydrogels composed of adipic acid dihydrazide and poly- (aldehyde guluronate)- PAG as an alternative of alginate hydrogels as cell carriers to embed primary rat cranial osteoblasts into the backbone defect in mice. The experimental process was observed for nine weeks long, nine weeks later the mineralized bone tissue formed at the defect. Synthetic polymers have comprehensive mechanical stiffness and controllable degradation rate. It is stated that the pendant cyclic ester amendment of PCL can inflect the slow drug release. The degradation of amphiphilic PCL-PEG-PCL hydrogel resulting from the strong hydrophobicity and crystallinity of PCL sections. Dey et al. [88] observed that the composition of synthetic copolymers affects the structure and properties of the gels. The formulation method of poly-(vinyl phosphonic acid-co-acrylic acid) (PVPA-co-AA) employed as a bone graft substitute, found that increasing PVPA content formed hydrogels with great swelling capacities, high porosities, and adjustable mechanical and cell adhesion properties. Figure 4 shows the effect of poly (vinylphosphonic acid-co-acrylic acid). Although synthetic materials have the aforementioned advantages, their success is limited by their own innately poor biological activity, acid by-products, and other shortcomings. Hence, synthetic materials can be conjugated with biological and chemical entities to ameliorate the extensive properties of hydrogels. Thoma et al.[90] worked on PEG hydrogels; they divided PEG hydrogels into six groups, conforming to the density of the gel such as physical modification and the effect of polyethylene glycol (PEG) hydrogels amended with the sequence of RGD (chemical modification). Each group was ingrained onto six loci of rabbit skulls. The observation period was six weeks long; after six weeks of observation, they found that chemical or physical amendment had a substantial impact on PEG hydrogel matrix stability, degradation time, and integration into the surrounding hard tissue and soft tissues [88-90].
Injectable hydrogel for bone regeneration process
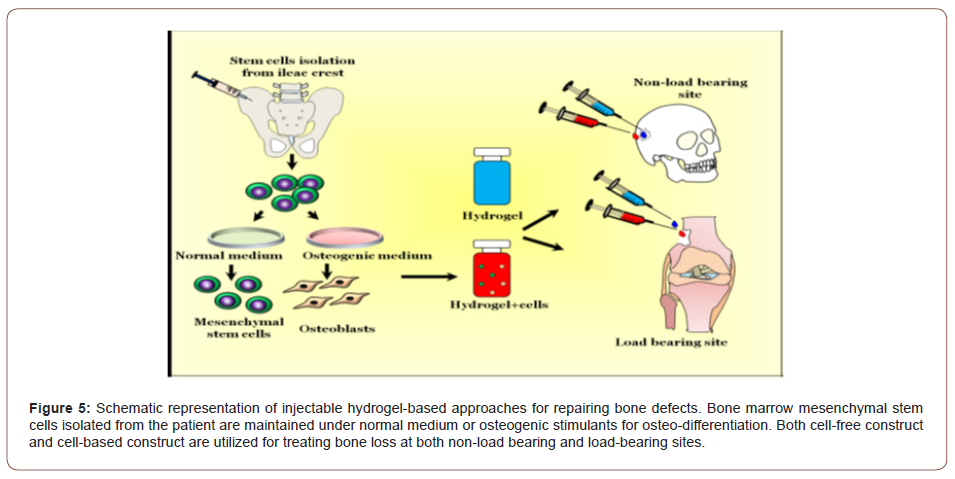
Hydrogels can absorb various folds of water into their structures without disintegrating therefore mimicking the natural tissue environment [4]. Over the past few years, injectable in situ forming hydrogels has been broadly researched for tissue engineering and orthopedic applications. Unlike the use of a pre-fabricated scaffold which required medical implantation, hydrogels can be injected into the defect site to confront any geometrical defects and with insignificantly interfering procedures [7,8,41-43]. Hydrogels are exceptional platforms of biomaterials that are used to fill the bone defects in non-load bearing sites, which do not require high mechanical strength. Furthermore, the injectable hydrogels are used to fortify the injured bone tissue and act as a carrier for delivering therapeutic agents and cells. Figure 5 represents the schematic representation of injectable hydrogel. Injectable hydrogels attain the site of the defect and deliver plastered biomolecules or cells and then form a gel through transitional characteristics when a response to a stimulus changes the physical or chemical properties [78-80].
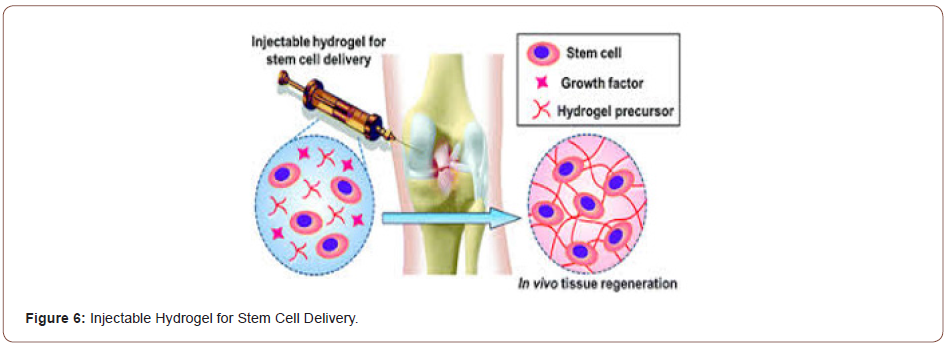
Nowadays, bone defects have become one of the leading causes of morbidity and disability among elderly people around the world. Even though autografting is observed as the gold standard for bone healing and regeneration process, it is restricted by the donorsite morbidity and uncertain adverse effects. Thus, bone tissue engineering has enticed significant attention from researchers as a propitious strategy for healing bone defects unless the constraints and drawbacks of utilizing either bone allografts, autografts, or xenografts. Recently, several injectable hydrogels with good moldability and 3D forms have been widely investigated for use in bone tissue engineering. In bone tissue engineering, various hydrogels have served as a scaffold material or are used to deliver osteogenic drugs. An ideal hydrogel should be injectable so that it can be used to treat a bone defect noninvasively and fill abnormallyshaped tissue defects. It should also be biocompatible and able to release osteogenic drugs over an extended time [81] (Figure 6).
Among the biomaterials used for preparing injectable hydrogels, alginate is one of the most investigated biomaterials employed in bone tissue engineering. Several types of research have been conducted on alginate-based injectable materials. Matsuno et al [82] have developed a novel injectable 3-dimensional hydrogel for bone tissue engineering that utilizes β-tricalcium phosphate beads and alginate as a scaffold. Mesenchymal stem cells 3-dimensional cultured through the hydrogel has been ingrained subcutaneously for in vivo experiments and has indicated that the scaffold can advantageously sustain osteogenic differentiation. A significant investigation has been conducted into using hydrogels for bone regeneration. Controlled drug release remains a challenge for hydrogels because they are usually inclined to a burst release of drugs through the first 24-48 h of treatment. Hydrogels are repeatedly combined with other materials to form composite scaffolds for bone regeneration. To induce a composite scaffold, PLLA/PCL nano yarns were suspended in a collagen hydrogel that showed advantages for in vitro distinction of human MSCs [83]. In another study, a nanohydroxyapatite-reinforced chitosan hydrogel was used to stimulate osteogenic differentiation in vitro and bone regeneration in vivo. These represent a small sampling of the many composite hydrogels being evaluated for applications of bone tissue engineering. Han et al. [84] have developed an injectable hybrid hydrogel by incorporating calcium silicate into an alginate solution. In the 30s to 10min, this hydrogel undergoes internal in situ gelling when calcium ions are released from calcium silicate with the introduction of D-gluconic acid δ-lactone. Furthermore, the hydrogel effectively promotes the adhesion, proliferation, and differentiation of osteogenic and angiogenic cells. To enhance the mechanical characteristics and mineralization of the scaffold in bone tissue engineering, inorganic materials are usually introduced with hybrid hydrogels.
Chitosan is another frequently used biomaterial for interdisciplinary injectable hydrogels in bone tissue engineering. Dessi et al. [85] have efficiently developed a thermosensitive chitosan-based hydrogel cross-linked with β-glycerophosphate and propped by physical interactions with β-tricalcium phosphate. The hydrogel simulates natural bone tissue and supports cellular activity and undergoes a sol-gel transition at physiological temperature with typical rheological characteristics. Meanwhile, owing to the properties of collagen, this hydrogel improves cell adhesion and proliferation.
However, incorporation of collagen into the chitosan or β-glycerophosphate system to synthesize injectable chitosan or β-glycerophosphate collagen-based hydrogel scaffold for bone healing and regeneration process in tissue engineering. Recently, injectable alginate or hydroxyapatite (HA) hydrogel scaffold, blended with gelatin microspheres (GMs), has been reported by many researchers. The blended hydrogel, HA, and GMs efficiently enhance the mechanical properties of the scaffold, thus demonstrating that the HA and GMs double-integrated alginatebased hydrogel has a suitable physical performance and bioactive properties. Thus, the hydrogel shows great potential for local treatment of pathologies involving bone defects [86-88].
Hydrogel Based Growth Factor and Stem Cell Delivery
The materials for hydrogel-based growth factor and stem cell delivery for bone regeneration can be divided according to the different substances encapsulated. The hydrogel matrix must maintain the biological activity of the delivered materials to provide extended efficient concentrations at the treatment site at a controlled release rate. Growth factors are naturally occurring proteins that are an essential component in promoting bone repair and osteogenesis. A newer technique to stimulate bone regeneration involves the use of undifferentiated stem cells that will stimulate natural bone formation. In this article, two types of delivery systems: growth factor delivery and stem cell delivery will be discussed.
Growth factor delivery
Naturally occurring bone healing or regeneration is a signaling cascade process, involving cells in the defect site excreting cytokines, growth factors, and pro-inflammatory factors and subsequently recruiting surrounding osteoprogenitor cells to emigrate, proliferate, and discriminate into osteoblasts. With a deeper understanding of the mechanism of bone healing and regeneration process, scientists envisage delivering exogenetic bioactive factors into the defect to accelerate bone repair [89-90]. Currently, the most widely explored in the area of bone healing and regeneration process, vascular endothelial growth factor (VEGF), BMP, insulin-like growth factors (IGF), stromal cell-derived factor-1a (SDF-1a), and fibroblast growth factor (FGF) are used. The variation in a structure identifies their different physiological functions and specific role in directing new bone growth factors. Consequently, successful bone repair requires the researcher to select suitable factors to obtain desired experimental results. Whereas the majority of bioactive factors are proteins, direct delivery may result in enzymatic degradation in the extracellular matrix (ECM) [91-93]. Furthermore, the delivered therapeutics may disseminate to other sites, leading to limited retention at the local site and undesirable adverse effects, such as seditious responses or adipogenic induction. Proper carriers are desperately necessary to impede factors from deactivation, due to hydrogels absorb water, expand, and mimic the natural extracellular matrix (ECM), they are considered as an excellent platform to provide a hydrophilic environment to maintain factors’ activity. An interesting synthetic PEG hydrogel functionalized with a2b1 integrin-specific peptide (GFOGER) was prepared by Shekaran et al. to deliver BMP-2 into murine radial critical-sized defects. The results demonstrated that GFOGER hydrogels can preserve embedded BMP-2, attract osteoprogenitor migration, and promote mechanical bone formation. Many researchers also explored the influence on bone regeneration through providing dual factors, as stated; synthesized CHPOA nanogel system including cholesteryl group and acryloyl group to co-deliver BMP-2 and FGF into mouse calvarial bone defect, leading to the generation of trabecular-like structure [94- 98].
Stem cell delivery
Since the 20th century, researchers are the focus on clinical treatment using stem cell transplantation and have been extensively studied in vascular diseases, plastic surgery, and regenerative medicine. Stem cells are mainly a group of cells with unlimited renewal and differentiation potential, allowing them to differentiate into different cell types, thus laying a solid foundation for their widespread use in various areas. The enhanced expression of the related bone-related genes included in the stem cells further affirmed that the stem cells can differentiate into osteogenic lineage to promote bone repair and regeneration. Stem cells used in bone regeneration mainly include embryonic stem cells (ESCs) and mesenchymal stem cells (MSCs). Even though successful bone regeneration in earlier investigations, ESCs are often necessarily attended by disputable moral and ethical issues; later, investigators began to use MSCs to prevent the dilemma. Mesenchymal stem cells (MSCs) can not only discriminate into osteoblasts but also act as a signal center to initiate host response to the bone injury [99]. MSCs can be divided into ASCs, bone marrow mesenchymal cells (BMSCs), and umbilical cord mesenchymal stem cells (UCMSCs), all of which can be sophisticated and grown in vitro. Because of the minimal invasion, easy accessibility, and low immunogenicity of the above-mentioned kinds of MSCs, ASCs are most widely investigated [100,101]. There have been various investigations regarding immediately injecting stem cells into injured areas. To buffer the irreversible damage to stem cells caused by sudden changes in the external environment after direct injection, many researchers began to employ hydrogels to act as a 3-dimensional medium and deliver stem cells [102]. Zhao et al. [104] researched injectable photocrosslinkable hydrogel microspheres using a microfluidic mixing technique to encapsulate BMSCs. This approach involved a photoinitiator-containing gelatin-methacryloyl chloride (GelMA), that forms droplets by utilizing a microfluidic device and, followed by UV photocrosslinking, produces GelMA hydrogel microspheres. The BMSCs were then encapsulated into the hydrogels and were found to demonstrate excellent bone formation in rabbit femoral ankle, particularly when BMP-2 was added. Huebsch et al. [105] developed interconnected and macroporous hydrogels to avoid cell death and limited control in the duration of injection and improve the communication between cells, it can improve the transplanted hMSC durability. The animal model used nude rats with cranial defects. The bone regeneration analysis conducted 12 weeks after cell transplantation showed obvious bone formation when hydrogel elasticity was fixed at 60 kPa. The interaction between hydrogels and cells is complicated, and many factors are involved in the regulation of stem cell differentiation. It has been stated that MSCs prefer to differentiate into osteogenic lineage when stiffness is fixed at 25e40 kPa [106]. Some researchers suggested that the surface characteristics of hydrogel will largely affect its interaction with cells, thus regulating the fate of the cells, developed a series of materials with various surface charges and hydrophilicities. The results demonstrated that small functional groups added to the hydrogel matrix have a substantial influence on hMSC differentiation with charged phosphate groups impelling osteogenesis and hydrophobic tertbutyl leading to adipogenesis [107,108].
Challenges and Prospects
Although new technologies and approaches in orthopedic surgery have substantially provided to improving musculoskeletal conditions and to improving bone repair during the last decades, there are still some cases where problems related to improper bone healing need to be solved. One of the most important problems is related to fracture non-unions and delayed unions. Successful bone regeneration requiring a coordinated interaction among growth factors, cells, and hydrogels. Even though hydrogels display intrinsic advantages in bone regeneration, there are still various problems that must be resolved. Firstly, when designing hydrogels, biocompatibility should be considered to circumvent possible inflammatory responses [109,110]. Natural polymers like gelatin, collagen, and chitosan are widely considered to be biocompatible, but are limited due to the poor mechanical strength and structural stability and burst release of encapsulated proteins or cells after being delivered to the target site. Synthetic polymers can solve the above-mentioned problems to a definite extent but are accompanied by issues such as inadvertent immune responses and poor degradation and cell attachment. Therefore, the optimization of the polymer concentration, composition, and crosslinking methods still needs to be further studied to better promote bone regeneration [111-114]. Theoretically, the encapsulation of natural stem cells and growth factors in the hydrogel matrix will enhance the rate of new extracellular matrix (ECM) generation. One of the constraints could be rapid hydrogel degradation before the generation of a new extracellular matrix (ECM), thus damaging the mechanical stability and interrupt the process of healing and regeneration of bone defects. Furthermore, proper control of stem cell differentiation is crucial to ensure differentiation at the desired cell lineages and prevent undesirable side effects when delivering stem cells to the defect site. Several studies have also demonstrated that defect sites lack blood vessels; hence, it is feasible to incorporate angiogenic factors into hydrogels for enhanced bone repair and regeneration [115-118].
A significant challenge regarding the uncontrolled release of loaded bioactive growth factors and stem cells, which is directedly related to the hydrogel degradation rate, limits the scope of bone healing and regeneration. Recently, smart hydrogel systems with on-demand delivery ability have become an emergent inventive technology in bone regeneration. Stimuli-flexible hydrogels can sense stimuli in their external environment and make equivalent changes, these stimuli have been extensively employed in biomedicine, tissue engineering, and the immobilization of enzymes. The studied external stimuli mainly include temperature, light, and pH. The development of hydrogels responsive to biochemical stimuli like enzymes, antigens, and ligands has also been explored [59,118,119]. Even though the substantial existent difficulties, containing speedy degradation and burst release, poor integration with low mechanical stability, native cells, and immunogenicity, the development of hydrogel-based bone regeneration holds immense promise for the future treatment of bone-related diseases and defects. With an increased understanding of hydrogels, bone defects, the extracellular matrix (ECM), and their interactions, hydrogels will undoubtedly become a powerful tool for the clinical treatment of bone defects in the future. Under normal conditions, the fracture healing method should be driven by mesenchymal stem cells which emigrate to the injury site and differentiate into osteoblastic cells leading to bone repair. Nevertheless, some fractures do not succeed in healing and become non-unions, which may lead to morbidity and functional disability for patients. In the case of fractures related to osteoporosis, one of the major challenges is the insufficient strength of the bone that must be used to anchor the fixation device. Osteoporosis influences metaphyseal bone and, consequently, most osteoporotic fractures are in the metaphyseal site. This type of fracture is usually processed by internal fixation; however, due to the low bone density, the use of screws to provide a stable fixation is not always possible [56,120-123].
In response to this problem, research and technological development have focused on three approaches to improve surgery results. These contain enhanced anchoring techniques, improved load distribution between the bone and the implants, and the augmentation of the strength of the host bone to improve anchorage. Prosthetic replacing is a fourth technique that is also pertinent to many osteoporotic fractures. Therefore, once more a material able to prompt bone formation to enhance the host bone and ameliorate anchorage is required.
When a section of the bone has died, it does not heal or regenerate voluntarily. Therefore, one method uses to resolve this problem is to remove the dead bone surgically and fill the space with a bone graft that is either taken from the patient or the bone bank. The success rate of this method depends upon the quantity of bone that has died. The utilization of synthetic biomaterials is a potential alternative to the use of autografts and allografts. In cases where osteonecrosis attacks larger areas such as joints, the total joint substitute method must take place [123-127].
Conclusion
Bone fracture is one of the most critical diseases around the world. Different clinical conditions need improvement of bone regeneration either locally or systemically, and several approaches are currently employed to increase or accelerate bone repair, depending on the healing potential and the certain requirements of each case. Research of bone biology has immensely enlarged with the enhanced understanding at the molecular level, resulting in the evolution of many new treatment techniques, with many others predictable in the years to come. Despite that, there are still some drawbacks; in particular, there is still surprisingly little information available about the cellular basis for MSC-mediated fracture repair and bone regeneration in vivo in humans. Furthermore, understanding in this area could be the key to an enhanced and incorporated scheme for skeletal healing and regeneration. Eventually, control of bone regeneration with schemes that mimic the common stream of bone formation will offer successful management of conditions that require improvement of bone regeneration and mitigate their morbidity and cost in the long term. The utilization of hydrogels for bone regeneration is a constantly growing and challenging research field, with still underexplored clinical potential. Hydrogels are attractive materials for various bone regeneration approaches, varies from injectable systems to membranes for guided bone regeneration and smart biofunctionalized scaffolds with biomimetic properties and architecture. The field of hydrogel-based biomaterials is exceedingly wide and various aspects are still to be interpreted despite propitious in vitro or in vivo performance in animal models. Whereas control over the chemistry and characteristics of these scaffolds exists through chemical and physical schemes, a better understanding of cell and matrix interactions is necessary as well as improved fabrication methods allowing to obtain bone biomimetic architecture and structural levels. The nextgeneration hydrogels with enhanced bone-forming capability due to 3-dimensional structure and spatiotemporally optimizing bioactivity are anticipated to prompt bone healing through employment, proliferation, and differentiation of cells.
Recently, there are several types of research are ongoing through all appropriate fields, and it is expected that many bone diseases method secondary to trauma, bone resection due to subtractive surgery, aging, and metabolic or genetic skeletal disorders will be successfully treated with novel bone-regeneration protocols that may address both local and systemic embellishment to optimize outcome.
Acknowledgment
This review paper was not financially supported by any project or institute.
Conflict of Interest
No conflict of interest.
References
- Carrington JL (2005) Aging bone and cartilage: cross-cutting issues. Biochem Biophys Res Commun 328(3): 700-708.
- Bai X, Gao M, Syed S, Zhuang J, Xu X, et al. (2018) Bioactive hydrogels for bone regeneration. Bioact Mater 3(4): 401-417.
- Saravanan S, Vimalraj S, Thanikaivelan P, Banudevi S, Manivasagam G (2019) A review on injectable chitosan/beta glycerophosphate hydrogels for bone tissue regeneration. International Journal of Biological Macromolecules 121: 38-54.
- Lopes D, Martins-Cruz C, Oliveira B, Mano JF (2018) Bone physiology as inspiration for tissue regenerative therapies. Biomaterials 185: 240-275.
- Quarto R, Giannoni P (2016) Bone Tissue Engineering: Past–Present–Future. Methods Mol Biol 1416: 21-33.
- Hernlund E, Svedbom A, Ivergård M, Compston J, Cooper C, et al. (2013) Osteoporosis in the European Union: medical management, epidemiology, and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 8(1): 136.
- Colby SL, Ortman JM (2014) The baby boom cohort in the United States: 2012 to 2060. US Census Bureau.
- O’Neill E, Awale G, Daneshmandi L, Umerah O, Lo KWH (2018) The roles of ions on bone regeneration. Drug Discov Today 23(4): 879-890.
- Yelin E, Weinstein S, King T (2016) The burden of musculoskeletal diseases in the United States. In Seminars in Arthritis and Rheumatism 46: 259.
- Lo KWH, Ulery BD, Ashe KM, Laurencin CT (2012) Studies of bone morphogenetic protein-based surgical repair. Adv Drug Deliv Rev 64(12): 1277-1291.
- Laurencin CT, Nair LS (2015) Regenerative engineering: approaches to limb regeneration and other grand challenges. Regen Eng Transl Med 1(1): 1-3.
- Hunt JA, Chen R, van Veen T, Bryan N (2014) Hydrogels for tissue engineering and regenerative medicine. Journal of Materials Chemistry B 2: 5319-5338.
- Motamedian SR, Hosseinpour S, Ahsaie MG, Khojasteh A (2015) Smart scaffolds in bone tissue engineering: A systematic review of literature. World J Stem Cells 7(3): 657-668.
- Turnbull G, Clarke J, Picard F, Riches P, Jia L, et al. (2018) 3D bioactive composite scaffolds for bone tissue engineering. Bioact Mater 3(3): 278-314.
- Navarro M, Aparicio C, Charles-Harris M, Ginebra MP, Engel E, et al. (2006) Development of a biodegradable composite scaffold for bone tissue engineering: physicochemical, topographical, mechanical, degradation, and biological properties. Ordered Polymeric Nanostructures at Surfaces, pp. 209-231. Springer, Berlin, Heidelberg.
- Drury JL, Mooney DJ (2003) Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 24(24): 4337-4351.
- Fang J, Li P, Lu X, Fang L, Lü X, et al. (2019) A strong, tough, and osteoconductive hydroxyapatite mineralized polyacrylamide/dextran hydrogel for bone tissue regeneration. Acta Biomaterialia 88: 503-513.
- Mehrali M, Thakur A, Pennisi CP, Talebian S, Arpanaei A, et al. (2017) Nanoreinforced Hydrogels for Tissue Engineering: Biomaterials that are Compatible with Load‐Bearing and Electroactive Tissues. Adv Mater 29(8).
- Liu M, Zeng X, Ma C, Yi H, Ali Z, et al. (2017) Injectable hydrogels for cartilage and bone tissue engineering. Bone Res 5: 17014.
- Kim HK, Shim WS, Kim SE, Lee KH, Kang E, et al. (2008) Injectable in situ–forming pH/thermo-sensitive hydrogel for bone tissue engineering. Tissue Eng Part A 15(4): 923-933.
- Vassiliou V, Kalogeropoulou C, Christopoulos C, Solomou E, Leotsinides M, et al. (2007) Combination ibandronate and radiotherapy for the treatment of bone metastases: clinical evaluation and radiologic assessment. Int J Radiat Oncol Biol Phys 67(1): 264-272.
- Florencio-Silva R, Sasso GRDS, Sasso-Cerri E, Simões MJ, Cerri PS (2015) Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed Res Int.
- Saravanan S, Vimalraj S, Thanikaivelan P, Banudevi S, Manivasagam G (2019) A review on injectable chitosan/beta glycerophosphate hydrogels for bone tissue regeneration. International Journal of Biological Macromolecules 121: 38-54.
- Wang W, Yeung KW (2017) Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact Mater 2(4): 224-247.
- Tzelepi V, Tsamandas AC, Zolota V, Scopa CD (2009) Bone anatomy, physiology and function. Bone Metastases, pp. 3-30. Springer, Dordrecht.
- Khan SN, Cammisa Jr FP, Sandhu HS, Diwan AD, Girardi FP, et al. (2005) The biology of bone grafting. J Am Acad Orthop Surg 13(1): 77-86.
- Bauer TW, Muschler GF (2000) Bone graft materials: an overview of the basic science. Clin Orthop Relat Res 371: 10-27.
- Albrektsson T, Johansson C (2001) Osteoinduction, osteoconduction and osseointegration. Eur Spine J 10(Supply 2): S96-S101.
- Wilson-Hench J (1987) Osteoinduction. Progress in Biomedical Engineering 4: 29.
- Roberts TT, Rosenbaum AJ (2012) Bone grafts, bone substitutes and orthobiologics: the bridge between basic science and clinical advancements in fracture healing. Organogenesis 8(4): 114-124.
- Gong T, Xie J, Liao J, Zhang T, Lin S, et al. (2015) Nanomaterials and bone regeneration. Bone Res 3: 15029.
- Bueno EM, Glowacki J (2009) Cell-free and cell-based approaches for bone regeneration. Nat Rev Rheumatol 5(12): 685-697.
- Nair AK, Gautieri A, Chang SW, Buehler MJ (2013) Molecular mechanics of mineralized collagen fibrils in bone. Nat Commun 4: 1724.
- Baroli B (2009) From natural bone grafts to tissue engineering therapeutics: brainstorming on pharmaceutical formulative requirements and challenges. J Pharm Sci 98(4): 1317-1375.
- Lew DP, Waldvogel FA (2004) Osteomyelitis. Lancet 364(9431): 369-379.
- Wijewardena A, Vandervord E, Lajevardi SS, Vandervord J, Jackson CJ (2011) Combination of activated protein C and topical negative pressure rapidly regenerates granulation tissue over exposed bone to heal recalcitrant orthopedic wounds. Int J Low Extrem Wounds 10(3): 146-151.
- Jimenez-Andrade JM, Mantyh WG, Bloom AP, Ferng AS, Geffre CP, et al. (2010) Bone cancer pain. Ann N Y Acad Sci 1198: 173-181.
- Duncan G, McCormick C, Tufaro F (2001) The link between heparan sulfate and hereditary bone disease: finding a function for the EXT family of putative tumor suppressor proteins. J Clin Invest 108(4): 511-516.
- Bizzetto R, Bonfim C, Rocha V, Socié G, Locatelli F, et al. (2011) Outcomes after related and unrelated umbilical cord blood transplantation for hereditary bone marrow failure syndromes other than Fanconi anemia. Haematologica 96(1): 134-141.
- Kumar JP, Lakshmi L, Jyothsna V, Balaji DR, Saravanan S, et al. (2014) Synthesis and characterization of diopside particles and their suitability along with chitosan matrix for bone tissue engineering in vitro and in vivo. J Biomed Nanotechnol 10(6): 970-981.
- Murray CJ, Lopez AD, World Health Organization (1996) The global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020: summary, pp. 41.
- White KP, Harth M (1999) The occurrence and impact of generalized pain. Baillieres Best Pract Res Clin Rheumatol 13(3): 379-389.
- Woolf AD, Pfleger B (2003) Burden of major musculoskeletal conditions. Bull World Health Organ 81(9): 646-656.
- Bates P, Yeo A, Ramachandran M (2018) Bone injury, healing and grafting. In Basic Orthopaedic Sciences, pp. 205-222, CRC Press.
- Einhorn TA (1998) The cell and molecular biology of fracture healing. Clin Orthop Relat Res 355: S7-S21.
- Cho TJ, Gerstenfeld LC, Einhorn TA (2002) Differential temporal expression of members of the transforming growth factor β superfamily during murine fracture healing. J Bone Miner Res 17(3): 513-520.
- Ferguson C, Alpern E, Miclau T, Helms JA (1999) Does adult fracture repair recapitulate embryonic skeletal formation?. Mech Dev 87(1-2): 57-66.
- Woolf AD, Pfleger B (2003) Burden of major musculoskeletal conditions. Bull World Health Organ 81(9): 646-656.
- Elliott AM, Smith BH, Penny KI, Smith WC, Chambers WA (1999) The epidemiology of chronic pain in the community. Lancet 354(9186): 1248-1252.
- Brooks PM (2002) Impact of osteoarthritis on individuals and society: how much disability? Social consequences and health economic implications. Curr Opin Rheumatol 14(5): 573-577.
- Bonjour JP, Schurch MA, Rizzoli R (1996) Nutritional aspects of hip fractures. Bone 18(3 Supply): S139-S144.
- Kanis JA, Melton L 3rd, Christiansen C, Johnston CC, Khaltaev N (1994) The diagnosis of osteoporosis. J Bone Miner Res 9(8): 1137-1141.
- National Osteoporosis Foundation (2002) America's bone health: the state of osteoporosis and low bone mass in our nation. Washington, DC: National Osteoporosis Foundation pp. 1-55.
- Olszynski WP, Davison KS, Adachi JD, Brown JP, Cummings SR, et al. (2004) Osteoporosis in men: epidemiology, diagnosis, prevention, and treatment. Clin Ther 26(1): 15-28.
- Yilmaz F, Sahin F, Ergoz E, Deniz E, Ercalik C, et al. (2008) Quality of life assessments with SF 36 in different musculoskeletal diseases. Clin Rheumatol 27(3): 327-332.
- Blyth FM, March LM, Brnabic AJ, Jorm LR, Williamson M, et al. (2001) Chronic pain in Australia: a prevalence study. Pain 89(2-3): 127-134.
- Burge R, Dawson‐Hughes B, Solomon DH, Wong JB, King A, et al. (2007) Incidence and economic burden of osteoporosis‐related fractures in the United States, 2005–2025. J Bone Miner Res 22(3): 465-475.
- Ricci JA, Stewart WF, Chee E, Leotta C, Foley K, et al, (2005) Pain exacerbation as a major source of lost productive time in US workers with arthritis. Arthritis Rheum 53(5): 673-681.
- Frank AO, Chamberlain MA (2001) Keeping our patients at work: implications for the management of those with rheumatoid arthritis and musculoskeletal conditions. Rheumatology 40(11): 1201-1205.
- Gbureck U, Hölzel T, Klammert U, Würzler K, Müller FA, et al. (2007) Resorbable dicalcium phosphate bone substitutes prepared by 3D powder printing. Advanced Functional Materials 17(18): 3940-3945.
- Griffin KS, Davis KM, McKinley TO, Anglen JO, Chu TMG, et al. (2015) Evolution of bone grafting: bone grafts and tissue engineering strategies for vascularized bone regeneration. Clinical Reviews in Bone and Mineral Metabolism 13: 232-244.
- Laurencin CT, Khan Y, Kofron M, El-Amin S, Botchwey E, et al. (2006) The ABJS Nicolas Andry Award: Tissue engineering of bone and ligament: a 15-year perspective. Clin Orthop Relat Res 447: 221-236.
- Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE (2008) Bone graft harvest using a new intramedullary system. Clin Orthop Relat Res 466(12): 2973-2980.
- Agarwal R, Garcia AJ (2015) Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv Drug Deliv Rev 94: 53-62.
- Gbureck U, Hölzel T, Klammert U, Würzler K, Müller FA, et al. (2007) Resorbable dicalcium phosphate bone substitutes prepared by 3D powder printing. Advanced Functional Materials 17(18): 3940-3945.
- Habraken W, Habibovic P, Epple M, Bohner M (2016) Calcium phosphates in biomedical applications: materials for the future?. Materials Today 19(2): 69-87.
- Ullah F, Othman MBH, Javed F, Ahmad Z, Akil, HM (2015) Classification, processing and application of hydrogels: A review. Mater Sci Eng C Mater Biol Appl57: 414-433.
- Silva AKA, Richard C, Bessodes M, Scherman D, Merten OW (2008) Growth factor delivery approaches in hydrogels. Biomacromolecules 10(1): 9-18.
- Wichterle O, Lim D (1960) Hydrophilic gels for biological use. Nature 185: 117-118.
- Bahram M, Mohseni N, Moghtader M (2016) An introduction to hydrogels and some recent applications. In Emerging Concepts in Analysis and Applications of Hydrogels. IntechOpen.
- Wu G, Feng C, Quan J, Wang Z, Wei W, Zang S, et al. (2018) In situ controlled release of stromal cell-derived factor-1α and antimiR-138 for on-demand cranial bone regeneration. Carbohydr Polym 182: 215-224.
- Silva R, Fabry B, Boccaccini AR (2014) Fibrous protein-based hydrogels for cell encapsulation. Biomaterials 35(25): 6727-6738.
- Lee SH, Shin H (2007) Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Advanced Drug Delivery Reviews 59(4-5): 339-359.
- Sánchez-Téllez DA, Téllez-Jurado L, Rodríguez-Lorenzo LM (2017) Hydrogels for cartilage regeneration, from polysaccharides to hybrids. Polymers (Basel) 9(12): 671.
- Lee KY, Mooney DJ (2001) Hydrogels for tissue engineering. Chem Rev 101(7): 1869-1880.
- Drury JL, Mooney DJ (2003) Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 24(24): 4337-4351.
- Zhao W, Jin X, Cong Y, Liu Y, Fu J (2013) Degradable natural polymer hydrogels for articular cartilage tissue engineering. Journal of Chemical Technology & Biotechnology 88(3): 327-339.
- Lindsey WH, Ogle RC, Morgan RF, Cantrell RW, Sweeney TM (1996) Nasal reconstruction using an osteoconductive collagen gel matrix. Archives of Otolaryngology–Head & Neck Surgery 122(1): 37-40.
- Patterson J, Siew R, Herring SW, Lin AS, Guldberg R, et al. (2010) Hyaluronic acid hydrogels with controlled degradation properties for oriented bone regeneration. Biomaterials 31(26): 6772-6781.
- Guarino V, Caputo T, Altobelli R, Ambrosio L (2015) Degradation properties and metabolic activity of alginate and chitosan polyelectrolytes for drug delivery and tissue engineering applications. AIMS Mater Sci 2(4): 497-502.
- Matsuno T, Hashimoto Y, Adachi S, Omata K, Yoshitaka Y, et al. (2008) Preparation of injectable 3D-formed β-tricalcium phosphate bead/alginate composite for bone tissue engineering. Dent Mater J 27(6): 827-834.
- Mredha MTI, Kitamura N, Nonoyama T, Wada S, Goto K, et al. (2017) Anisotropic tough double network hydrogel from fish collagen and its spontaneous in vivo bonding to bone. Biomaterials 132: 85-95.
- Han Y, Zeng Q, Li H, Chang J (2013) The calcium silicate/alginate composite: Preparation and evaluation of its behavior as bioactive injectable hydrogels. Acta Biomater 9(11): 9107-9117.
- Dessì M, Borzacchiello A, Mohamed TH, Abdel‐Fattah WI, Ambrosio L (2013) Novel biomimetic thermosensitive β‐tricalcium phosphate/chitosan‐based hydrogels for bone tissue engineering. J Biomed Mater Res A 101(10): 2984-2993.
- Kim SY, Park JS (2014) Biomineralized hyaluronic acid/poly (vinylphosphonic acid) hydrogel for bone tissue regeneration. Journal of Applied Polymer Science 131(23).
- Sabir MI, Xu X, Li L (2009) A review on biodegradable polymeric materials for bone tissue engineering applications. Journal of materials science 44(21): 5713-5724.
- Dhandayuthapani B, Yoshida Y, Maekawa T, Kumar DS (2011) Polymeric scaffolds in tissue engineering application: a review. International journal of polymer science.
- Lee KY, Alsberg E, Mooney DJ (2001) Degradable and injectable poly (aldehyde guluronate) hydrogels for bone tissue engineering. J Biomed Mater Res 56(2): 228-233.
- Wang W, Deng L, Liu S, Li X, Zhao X, et al. (2012) Adjustable degradation and drug release of a thermosensitive hydrogel based on a pendant cyclic ether modified poly (ε-caprolactone) and poly (ethylene glycol) co-polymer. Acta Biomater 8(11): 3963-3973.
- Dey RE, Wimpenny I, Gough JE, Watts DC, Budd PM (2018) Poly (vinylphosphonic acid‐co‐acrylic acid) hydrogels: The effect of copolymer composition on osteoblast adhesion and proliferation. J Biomed Mater Res A 106(1): 255-264.
- Dawson E, Mapili G, Erickson K, Taqvi S, Roy K (2008) Biomaterials for stem cell differentiation. Adv Drug Deliv Rev 60(2): 215-228.
- Thoma DS, Weber FE, Bienz SP, Ge Y, Hämmerle CH, et al. (2017) Biodegradation and tissue integration of various polyethylene glycol matrices: a comparative study in rabbits. Clin Oral Implants Res 28(11): e244-e251.
- Saravanan S, Vimalraj S, Thanikaivelan P, Banudevi S, Manivasagam G (2019) A review on injectable chitosan/beta glycerophosphate hydrogels for bone tissue regeneration. International journal of biological macromolecules 121: 38-54.
- Liu M, Zeng X, Ma C, Yi H, Ali Z, et al. (2017) Injectable hydrogels for cartilage and bone tissue engineering. Bone Res 5(1): 1-20.
- Kim HK, Shim WS, Kim SE, Lee KH, Kang E, et al. (2009) Injectable in situ–forming pH/thermo-sensitive hydrogel for bone tissue engineering. Tissue Eng Part A 15(4): 923-933.
- Park SH, Kwon JS, Lee BS, Park JH, Lee BK, et al. (2017) BMP2-modified injectable hydrogel for osteogenic differentiation of human periodontal ligament stem cells. Scientific reports 7(1): 1-15.
- Tan H, Chu CR, Payne KA, Marra KG (2009) Injectable in situ forming biodegradable chitosan–hyaluronic acid-based hydrogels for cartilage tissue engineering. Biomaterials 30(13): 2499-2506.
- Bidarra SJ, Barrias CC, Granja PL (2014) Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomater 10(4): 1646-1662.
- Titorencu I, Georgiana Albu M, Nemecz M, V Jinga V (2017) Natural polymer-cell bioconstructs for bone tissue engineering. Curr Stem Cell Res Ther 12(2): 165-174.
- Liang K, Bae KH, Kurisawa M (2019) Recent advances in the design of injectable hydrogels for stem cell-based therapy. Journal of Materials Chemistry B 7(24): 3775-3791.
- Malafaya PB, Silva GA, Reis RL (2007) Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv Drug Del Rev 59(4-5): 207-233.
- Shinde UP, Yeon B, Jeong B (2013) Recent progress of in situ formed gels for biomedical applications. Progress in polymer science 38(3-4): 672-701.
- Liu H, Liu J, Qi C, Fang Y, Zhang L, et al. (2016) Thermosensitive injectable in-situ forming carboxymethyl chitin hydrogel for three-dimensional cell culture. Acta Biomaterialia 35: 228-237.
- Huebsch N, Lippens E, Lee K, Mehta M, Koshy ST, et al. (2015) Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat Mater 14(12): 1269-1277.
- Zhou Y, Zhao S, Zhang C, Liang K, Li J, et al. (2018) Photopolymerized maleilated chitosan/thiol-terminated poly (vinyl alcohol) hydrogels as potential tissue engineering scaffolds. Carbohydr Polym 184: 383-389.
- Hoffman AS (2012) Hydrogels for biomedical applications. Advanced drug delivery reviews 64: 18-23.
- Sivashanmugam A, Kumar RA, Priya MV, Nair SV, Jayakumar R (2015) An overview of injectable polymeric hydrogels for tissue engineering. European Polymer Journal 72: 543-565.
- Yang JA, Yeom J, Hwang BW, Hoffman AS, Hahn SK (2014) In situ-forming injectable hydrogels for regenerative medicine. Progress in Polymer Science 39(12): 1973-1986.
- Khunmanee S, Jeong Y, Park H (2017) Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J Tissue Eng 8: 2041731417726464.
- Mazaki T, Shiozaki Y, Yamane K, Yoshida A, Nakamura M, et al. (2014) A novel, visible light-induced, rapidly cross-linkable gelatin scaffold for osteochondral tissue engineering. Scientific reports 4(1): 1-10.
- Sood N, Bhardwaj A, Mehta S, Mehta A (2016) Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv 23(3): 748-770.
- Im JS, Yun J, Lim YM, Kim HI, Lee YS (2010) Fluorination of electrospun hydrogel fibers for a controlled release drug delivery system. Acta Biomaterialia 6(1): 102-109.
- Wang L, Wang P, Weir MD, Reynolds MA, Zhao L, et al. (2016) Hydrogel fibers encapsulating human stem cells in an injectable calcium phosphate scaffold for bone tissue engineering. Biomed Mater 11(6): 065008.
- Lee K, Silva EA, Mooney DJ (2011) Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface 8(55): 153-170.
- Tessmar JK, Göpferich AM (2007) Matrices and scaffolds for protein delivery in tissue engineering. Adv Drug Deliv Rev 59(4-5): 274-291.
- Yun YR, Jang JH, Jeon E, Kang W, Lee S, et al. (2012) Administration of growth factors for bone regeneration. Regen Med 7(3): 369-385.
- Bai Z, Guo XH, Tang C, Yue ST, Shi L, et al. (2018) Effects of artesunate on the expressions of insulin-like growth factor-1, osteopontin and C-telopeptides of type II collagen in a rat model of osteoarthritis. Pharmacology 101(1-2): 1-8.
- Street J, Bao M, deGuzman L, Bunting S, Peale FV, et al. (2002) Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A 99(15): 9656-9661.
- Mi L, Liu H, Gao Y, Miao H, Ruan J (2017) Injectable nanoparticles/hydrogels composite as sustained release system with stromal cell-derived factor-1α for calvarial bone regeneration. Int J Biol Macromol 101: 341-347.
- Fujioka-Kobayashi M, Ota MS, Shimoda A, Nakahama KI, Akiyoshi K, et al. (2012) Cholesteryl group-and acryloyl group-bearing pullulan nanogel to deliver BMP2 and FGF18 for bone tissue engineering. Biomaterials 33(30): 7613-7620.
- Zara JN, Siu RK, Zhang X, Shen J, Ngo R, et al. (2011) High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng Part A 17(9-10): 1389-1399.
- Moshaverinia A, Chen C, Akiyama K, Xu X, Chee WW, et al. (2013) Encapsulated dental‐derived mesenchymal stem cells in an injectable and biodegradable scaffold for applications in bone tissue engineering. J Biomed Mater Res A 101(11): 3285-3294.
- Shekaran A, García JR, Clark AY, Kavanaugh TE, Lin AS, et al. (2014) Bone regeneration using an alpha 2 beta 1 integrin-specific hydrogel as a BMP-2 delivery vehicle. Biomaterials 35(21): 5453-5461.
- Shirakura T, Smith C, Hopkins TJJ, Koo Lee YE, Lazaridis F, et al. (2017) Matrix density engineering of hydrogel nanoparticles with simulation-guided synthesis for tuning drug release and cellular uptake. ACS omega 2(7): 3380-3389.
- Holloway JL, Ma H, Rai R, Burdick JA (2014) Modulating hydrogel crosslink density and degradation to control bone morphogenetic protein delivery and in vivo bone formation. J Control Release 191: 63-70.
- He B, Ou Y, Zhou A, Chen S, Zhao W, et al. (2016) Functionalized d-form self-assembling peptide hydrogels for bone regeneration. Drug Des Devel Ther 10: 1379-1388.
- Ni P, Ding Q, Fan M, Liao J, Qian Z, et al. (2014) Injectable thermosensitive PEG–PCL–PEG hydrogel/acellular bone matrix composite for bone regeneration in cranial defects. Biomaterials 35(1): 236-248.
-
Shahirin Shahida. Application of Hydrogel in Tissue Engineering: Process of Bone Regeneration and Fracture Healing. Mod Concept Material Sci. 3(5): 2021. MCMS. MS.ID.000575. DOI: 10.33552/MCMS.2021.03.000575.
-
Bone Regeneration, Bone Tissue Engineering, Hydrogel, Bioactive Materials, Biomaterials, Fracture Healing, Bone disease, Bone defects, Polymers, Hydrogels, Stability
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






